Abstract
Purpose
To develop an improved T2 prepared (T2prep) balanced steady-state free-precession (bSSFP) sequence and signal relaxation curve fitting method for myocardial T2 mapping.
Methods
Myocardial T2 mapping is commonly performed by acquisition of multiple T2prep bSSFP images and estimating the voxel-wise T2 values using a 2-parameter fit for relaxation. However, a 2-parameter fit model does not take into account the effect of imaging pulses in a bSSFP sequence or other imperfections in T2prep RF pulses, which may decrease the robustness of T2 mapping. Therefore, we propose a novel T2 mapping sequence that incorporates an additional image acquired with saturation preparation, simulating a very long T2prep echo time. This enables the robust estimation of T2 maps using a 3-parameter fit model, which captures the effect of imaging pulses and other imperfections. Phantom imaging is performed to compare the T2 maps generated using the proposed 3-parameter model to the conventional 2-parameter model, as well as a spin echo reference. In-vivo imaging is performed on eight healthy subjects to compare the different fitting models.
Results
Phantom and in-vivo data show that the T2 values generated by the proposed 3-parameter model fitting do not change with different choices of the T2prep echo times, and are not statistically different than the reference values for the phantom (P = 0.10 with three T2prep echoes). The 2-parameter model exhibits dependence on the choice of T2prep echo times and are significantly different than the reference values (P = 0.01 with three T2prep echoes).
Conclusion
The proposed imaging sequence in combination with a 3-parameter model allows accurate measurement of myocardial T2 values, which is independent of number and duration of T2prep echo times.
Keywords: Quantitative myocardial tissue characterization, myocardial T2 mapping, 3-parameter fit, myocardial inflammation
Introduction
T2-weighted images are commonly used in cardiac MR (CMR) to assess myocardial inflammation and edema in various cardiomyopathies. They have been shown to distinguish acute and chronic myocardial infarction (1), to identify severe transient myocardial ischemia (2), and to predict revascularization needs (3). T2-weighted imaging has also been used in patients with myocarditis (4,5), allograft rejection (6) and Takotsubo cardiomyopathy (7), where T2 values are elevated. The T2-weighted imaging sequences used in these studies rely on turbo spin echo readouts with black-blood preparation (8). However, the T2-weighted imaging sequences suffer from certain limitations (9-11), including qualitative interpretation that is affected by regional differences; myocardial signal variation due to phased-array coil arrays; and difficulty differentiating edema from stagnant sub-endocardial blood.
Quantification of myocardial T2 values (12,13) has been proposed as an alternative to T2-weighted imaging to reduce the variation in assessment. The earlier T2 quantification sequences were based on spin echo/fast spin echo acquisitions (12-15). More recently, T2 mapping techniques (11,16-20) have been proposed, where a number of images are acquired with different T2-weightings, and used to generate a quantitative pixel-wise T2 map based on a spin-spin relaxation model compatible with the acquisition. T2-prepared (21) balanced steady-state free precession (bSSFP) techniques have been utilized for efficient T2 mapping (22). In these techniques, a number of different T2 preparation (T2prep) echo times are used to generate the multiple T2-weighted images (11,16-20). In one such approach, three electrocardiogram (ECG)-triggered single-shot bSSFP images are acquired with three different T2prep times (0 ms, 24 ms, 55 ms) with 2 heart-beat rest periods for signal recovery between each image, using a breath-hold acquisition (11,18). These images are subsequently registered and then fit to a 2-parameter fit model (consisting of the longitudinal magnetization without T2prep and the T2 time) to generate the myocardial T2 maps.
Despite the potential of myocardial T2 mapping for quantitative assessment of myocardial inflammation and edema (18,19,23-26), it has still not replaced T2-weighted techniques in clinical CMR protocols. In terms of the sequence, single-shot bSSFP acquisitions following different T2prep echo times have replaced the spin echo approach. However, there is a lack of data about the robustness of T2 estimation with respect to the choice and number of T2prep echo times when using the conventional sequences and the curve fitting model.
In terms of the curve-fitting model, the 2-parameter fit has been used extensively in the literature, both with spin echo acquisitions (12,14,27) or bSSFP acquisitions with various profile ordering schemes (16,20,22). However, the 2-parameter model ignores the changes in the T1/T2 contrast due to the imaging pulses until the acquisition of the center of k-space when using a bSSFP sequence, with a number of start-up pulses and especially with linear profile ordering. The shortcomings of the 2-parameter model have been noted previously (17,28). In (17), an empirical offset parameter was included to account for the T1 relaxation during the gradient echo sequence. This value was chosen based on Bloch simulations and was fixed for a given set of sequence parameters and physiology characteristics. In (28), a third parameter was empirically included to characterize the deviations from the 2-parameter model in numerical simulations and phantom experiments, although no analytical insight was given regarding the necessity of such a term.
In this work, we propose a sequence and a signal recovery curve fitting model for improved myocardial T2 mapping. We propose a 3-parameter curve fitting model, where the third parameter captures the perturbations in the magnetization curve due to the imaging pulses played between the T2prep and the acquisition of the central k-space line. Our proposed sequence acquires multiple single-shot images with different T2prep echo times, followed by rest periods for magnetization recovery, as well as one saturation-prepared image. The latter captures the effect of imaging pulses on the magnetization curve, and thus improves the estimation of the third parameter. Additionally, to enable efficient free-breathing acquisitions in-vivo, we propose a new NAV-gating scheme that applies T2prep conditionally based on the position of the NAV signal. This eliminates the necessity for rest periods if the NAV signal is outside of the gating window. Phantom experiments and in vivo imaging are performed to evaluate the proposed sequence and the recovery curve fitting model.
Methods
The 2-parameter curve fitting model typically used in myocardial T2 mapping is given by
| [1] |
where t is the T2prep echo time. In this work, we propose to use a 3-parameter model to capture the effect of imaging pulses on the magnetization
| [2] |
The proposed sequence aims to improve the efficiency and accuracy of T2 mapping, while addressing several aspects of this estimation procedure.
Proposed Sequence
Figure 1(a) shows the schematic of the proposed sequence. Multiple single-shot images of the heart are acquired using ECG-triggering, following T2prep (21) of different echo lengths, TET2P. Between each image, a 6 second rest period (with no RF pulses) is applied to allow for full re-growth of the myocardial signal. To improve the estimation of the third parameter (B), the sequence additionally acquires an image, ISAT, directly after a saturation pulse to simulate the effect of a very long T2prep echo time (i.e. T2prep = ∞).
Figure 1.
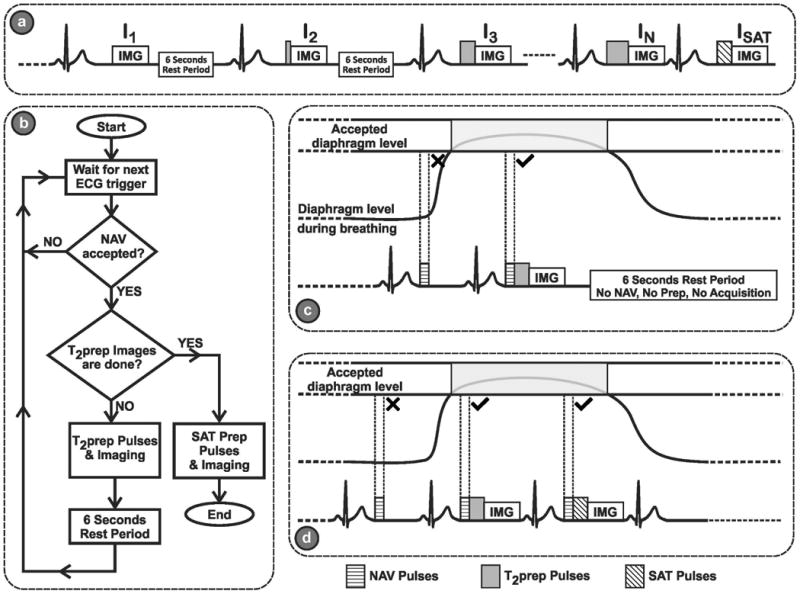
(a) The schematic of the proposed sequence. Multiple single-shot images of the heart are acquired using ECG-triggering, following T2prep of different echo lengths, TET2P. Between each image, a 6 second rest period (with no RF pulses) is applied to allow for full re-growth of the myocardium signal. An image, ISAT, is acquired directly after a saturation pulse to simulate the effect of a very long T2prep echo time (i.e. T2prep = ∞) for improved estimation of the third parameter in the 3-parameter fit. (b) Flowchart for the proposed navigator (NAV)-gated acquisition scheme, The NAV is placed before the T2prep. If the NAV signal preceding the acquisition of the kth image is outside the gating window, no T2prep or imaging pulses are applied, leaving the magnetization undisturbed, and the acquisition of this image is repeated in the next R-R interval. If the NAV signal is within the gating window, the image with the desired T2prep time is acquired, followed by a 6 second rest period for magnetization recovery. (c) An example of the rejection-reacquisition scheme for a T2-prepared image. (d) An example of the acquisition of a saturation-prepared image, which immediately follows the T2-prepared image without any rest periods, and where the NAV is placed before the saturation pulse.
The T2prep itself consists of 90° tip-down pulse, followed by four 180° pulses and ends with 90° tip-up pulse. Both the opening and closing 90° pulses are non-selective hard pulses with a bandwidth of 2.3 kHz, and duration of 0.44 ms. These non-composite short pulses were chosen to minimize any T2* effect that might occur during the pulse. The refocus pulses are weighted in a MLEV opposing phase pairs scheme to compensate for RF pulse shape imperfection (29) and composite refocusing pulses (90°x, 180°y, 90°x) are used to provide second order corrections to variations in B1. The duration of each refocus pulse is 1.75 ms. For ISAT, a composite saturation pulse of bandwidth = 1 kHz, and a total duration of 10 ms is used.
A common issue with T2prep sequences is the effect of B0 and B1 variations of the excitation and refocusing pulses of the T2prep sequence. In the proposed method, this will change the A parameter in Equation [2] for all the images acquired with a non-zero TET2P. However, if TET2P = 0 is acquired with no contrast preparation as in (11), the A parameter for this term will not be affected by these in homogeneity effects, leading to an inconsistency with the other TET2P values. To compensate for this effect, we add a 90°, followed immediately by a -90°, followed by a crusher gradient, for the acquisition of a TET2P = 0, similar to the one proposed in (22). We hypothesize that this compensates for B0 and B1 variations, and removes the bias from the estimation of the T2 parameter that would have been caused by RF flip angle imperfections.
The flowchart for the proposed navigator-gated acquisition scheme is depicted in Figure 1(b). The NAV is placed immediately prior to the T2prep. For the acquisition of the kth image, Ik, if the NAV signal is outside the gating window, no T2prep or imaging pulses are applied, leaving the magnetization undisturbed, and the acquisition of Ik is repeated in the next R-R interval. If the NAV signal is within the gating window, the image with the desired T2prep time is acquired, followed by a 6 second rest period for magnetization recovery. Figure 1(c) shows an example of the rejection-reacquisition scheme for a T2-prepared image. Figure 1(d) also depicts the acquisition of a saturation-prepared (SAT) image, which immediately follows the T2-prepared image without any rest periods, and where the NAV is placed prior to the saturation pulse.
3-parameter Model for T2 Relaxation
We sought to characterize the effect of the bSSFP imaging pulses that are played until the acquisition of central k-space, on the magnetization measured after T2 preparation. When a T2prep scheme (21) with a T2 echo time of TET2P is used, the longitudinal magnetization is given by:
| [3] |
where M0 is the signal at full-recovery. When using a bSSFP readout with n RF pulses, the signal is given by (30)
| [4] |
where α is the flip angle, and where the steady state magnetization, MSS is given by
| [5] |
with E1,2 = e−TR/T1,2 and λ1 = E2 sin2(α/2)+ E1 cos2(α/2). This can be re-written as (31)
| [6] |
Thus, for T2-prepared bSSFP acquisitions, in which several imaging pulses are used before the acquisition of the center of k-space, the T2 relaxation between the different images can be characterized as:
| [7] |
where the parameters, A and B do not depend on the T2prep time, TET2P. However, they are functions of the sequence parameters (flip angle, number of pulses, repetition time, etc). Furthermore, based on Equation [6], we note that B captures the effect of the imaging pulses, when Mstart = 0.
T2 Map Reconstruction
T2 maps are generated by voxel-wise least-squares curve-fitting to the magnitude signal intensity. Both the 2-parameter model in Equation [1] and the 3-parameter model in Equation [2] are utilized. The following curve-fitting methods are performed for the experiments:
The 2-parameter model with various T2prep echo times. This method does not include the SAT image, since the 2-parameter signal model decays to 0 for large T2prep echo times.
The 3-parameter model with various T2prep echo times and with the saturation-prepared image. The SAT image is equivalently characterized as a long (∼2000 ms) T2prep echo time.
The 3-parameter model with various T2prep echo times and without the SAT image.
Numerical Simulations: B1 field inhomogeneity
Numerical simulations were conducted to study the effect of B0 and B1 variations on the estimated T2 values, and to characterize the effect of using the proposed 90°, -90° and crusher gradient preparation during the acquisition of a TET2P = 0 on these T2 estimations. Bloch equation was simulated to consider the effect of spin rotation around Beff instead of B1 during the 90°, -90° and 180° pulses of the T2prep, with
| [8] |
where B0 is the strength of the magnetic field in the z-direction, γ is the gyromagnetic ratio, and B1 and ω are the strength and frequency of the applied RF pulse respectively.
Nominal myocardium T1 and T2 values (i.e. 1200 ms and 50 ms respectively) were assumed for the simulations. The sequence was simulated to acquire T2prep echo times of 0, 25 and 50 ms, as well as the saturation-prepared image. Then, both the 2-parameter and 3-parameter fits were used to estimate the T2 values, and the absolute error from the true T2 value was recorded for different variations of B0 and B1. The simulation was repeated with and without the proposed compensation for TET2P = 0.
Phantom Imaging
All imaging was performed on a 1.5T Philips Achieva (Philips Healthcare, Best, The Netherlands) system using a 32-channel cardiac coil array. Phantom imaging was performed using NiCl2 doped agarose vials, whose T2 and T1 values spanned the ranges of values found in the blood and myocardium. A single-shot ECG-triggered bSSFP sequence with the following parameters was used for the proposed sequence: 2D single-slice, FOV = 240×240 mm2, in-plane resolution = 2.5×2.5 mm2, slice thickness = 8 mm, TR/TE = 2.7 ms/1.35 ms, flip angle = 85°, 10 linear ramp-up pulses, SENSE rate = 2, acquisition window = 138 ms, number of phase encoding lines = 51, linear k-space ordering. A total of 27 different T2prep echo times were used, including TET2P = 0 and TET2P ranging from 25 ms to 150 ms in steps of 5 ms. Additionally one image was acquired after saturation preparation for the 3-parameter fit.
A Carr-Purcell-Meiboom-Gill (CPMG) spin-echo sequence with an echo train length of 32 with TE 10 ms was performed as reference. The scan parameters were: FOV = 240×240 mm2, in-plane resolution = 1.25×1.25 mm2, slice thickness = 4 mm, TR = 6000 ms, flip angle = 90°. Number of averages = 4, reference T2 times were obtained from a 2-parameter model fit to the spin echo signal.
3-parameter vs. 2-parameter Fit: Effect of T2prep Echo Times
We hypothesized that the estimated T2 values would be independent of the T2prep times used to sample the images if the true magnetization model and the curve-fitting model matched. On the other hand, the estimated T2 values would change based on the T2prep times sampled if there was mismatch between the true magnetization model and the curve-fitting model. To test this hypothesis, 2-parameter and 3-parameter models were used to generate T2 maps based on different subsets of images corresponding to different T2prep echo times. The following subsets were used:
TET2P = 0 and n TET2P values starting from 25ms in steps of 5 ms (n from 2 to 26).
TET2P = 0 and n TET2P values starting from 25 ms in steps of 10 ms (n from 2 to 13).
TET2P = 0 and n TET2P values starting from 25 ms in steps of 15 ms (n from 2 to 9).
TET2P = 0 and n TET2P values starting from 25 ms in steps of 20 ms (n from 2 to 7).
TET2P = 0 and n TET2P values starting from 25 ms in steps of 25 ms (n from 2 to 6).
As described previously, the additional SAT image is used with the 3-parameter model, and not used with the 2-parameter model. In order to quantify the effect of using the SAT image in the 3-parameter fit on accuracy and precision, these experiments were also repeated using the 3-parameter model but excluding the SAT image from the curve-fitting process.
Additionally, T2 map estimation was performed using 3 T2prep echoes (0, 25, 50 ms), similar to the ones used in the literature (11,17,18) using a 2-parameter fit. 3-parameter fit was performed using these 3 echoes and the proposed SAT image. 3-parameter fit was also performed using a 4th echo at 90 ms instead of the SAT image. These acquisitions are referred to as the short acquisition with 2-parameter, 3-parameter fit with SAT, and 3-parameter without SAT, respectively.
Effect of the B1 inhomogeneities
To quantify the effect of using the 90°, -90°& crusher gradient preparation during the acquisition of a TET2P = 0 to compensate for any RF pulse imperfection, imaging was performed with and without (i.e. no pulses applied) this correction. The 2-parameter and 3-parameter fits were performed for all echoes for both acquisitions. An additional SAT image was used for the 3-parameter fit as previously described.
Length of the Rest Cycles
Rest cycles are used after the acquisition of each single-shot image to allow for magnetization recovery. Imaging was performed to study the effect of the length of these rest cycles, using rest cycle lengths varying from 0 to 9 seconds in 1 second-steps. Imaging was performed using both 3 echoes (0, 25, 50 ms) and 27 echoes. An additional SAT image was acquired in all cases. The 3-parameter fit was utilized for T2 map reconstruction.
T2 Map Analysis
A region-of-interest (ROI) analysis was performed, where the mean value and standard deviation was recorded for each vial for each calculated T2 map. Accuracy was assessed as the difference between the mean of the vial for the spin echo reference T2 map and the mean of the vial for the given T2 map. Precision was assessed as the standard deviation of the vial for the given T2 map. The null hypotheses that there was no difference in the mean value for a vial in the spin echo reference and in a given T2 map was tested using a paired t-test across all vials. A P value of <0.05 was considered to be significant.
In Vivo Imaging
This portion of the study was approved by the institutional review board and written informed consent was acquired prior to each examination. In a prospective study, eight healthy adult subjects (30.3 ± 17.5 years, range: 22 - 73 years, 4 men) without contraindications to MRI were recruited. For each subject, localizer scouts were acquired to define the mid-ventricular short-axis slice. A two-dimensional spiral NAV echo was positioned on the right hemi-diaphragm, and was used for gating with a 5 mm gating window. A free-breathing single-shot ECG-triggered bSSFP sequence with the following parameters was used for the acquisition of the mid-ventricular short-axis slice: 2D single-slice, FOV = 320×320 mm2, in-plane resolution = 2.5×2.5 mm2, slice thickness = 8 mm, TR/TE = 2.7 ms/1.35 ms, flip angle = 85°, 10 linear ramp-up pulses, SENSE rate = 2, acquisition window = 181 ms, number of phase encoding lines = 67, linear k-space ordering. All acquisitions were performed with 27 images corresponding to different T2prep echo times, including TET2P = 0 and TET2P ranging from 25 ms to 150 ms in steps of 5 ms. An additional SAT image was also acquired for the 3-parameter fit. T2 maps were also generated for the short acquisition configurations. The nominal scan time for these scans, acquiring all 27 T2prep echoes, was 3:10 minutes at 60 heart-beats per min, assuming 100% NAV gating efficiency.
T2 Map Analysis
The acquired images were registered retrospectively using an advanced non-rigid image registration algorithm (32) to compensate for residual in-slice motion. This algorithm simultaneously estimates a non-rigid motion field and intensity variations, and employs an additional regularization term to constrain the deformation field using automatic feature tracking. Voxel-wise curve-fitting was performed, subsequent to registration, to generate T2 maps for the 3-parameter and the 2-parameter models. T2 maps were generated for different subsets of images corresponding to different T2prep echo times, as described for phantom imaging. Epi- and endocardial contours were drawn manually by an experienced blinded reader for each T2 map. The average T2 value and the standard deviation within the septum were recorded.
Finally, a segment-based analysis was performed for the proposed 3-parameter model with the SAT images. Variations in T2 and B/A across different segments were studied to see if the regional variations were due to tissue characteristics or due to sequence parameters, as described by Equation [6]. 6 segments were used in the mid-ventricular LV slice, in accordance with the AHA 16-segment model (33). Segment-based T2 and B/A values were recorded for each subject. These were then averaged over all subjects for each segment. The B/A value was also compared to the value predicted by Equation [6] for the given sequence parameters.
Results
Numerical Simulations
Figure 2 shows the effect of B0 and B1 variations on T2 estimation. Figure 2(a) shows the normalized longitudinal magnetization measured directly after the T2prep sequence. Figure 2(b-d) shows the errors in T2 estimation using 2-parameter fit, and 3-parameter fit, with and without the proposed compensation of 90°, -90° pulses for TET2P = 0. The green area in Figure 2(b) shows valid T2 estimations (within ±5ms) over a ΔB0 range of ±200 Hz and a ΔB1 range of 20%. Using the 3-parameter fit in Figure 2(d) reduced the B0 range to around ±150 Hz but increased the B1 range to nearly 30%. However, when the proposed 90°, -90° was used for TET2P = 0, the B0 range to increased to almost 250 Hz with the same B1 range (Figure 2(e)). This was not the case with the 2-parameter fit in Figure 2(c) where a bias of 10-30 ms was observed in the estimated T2 values for almost the whole range of B0-B1 variations.
Figure 2.
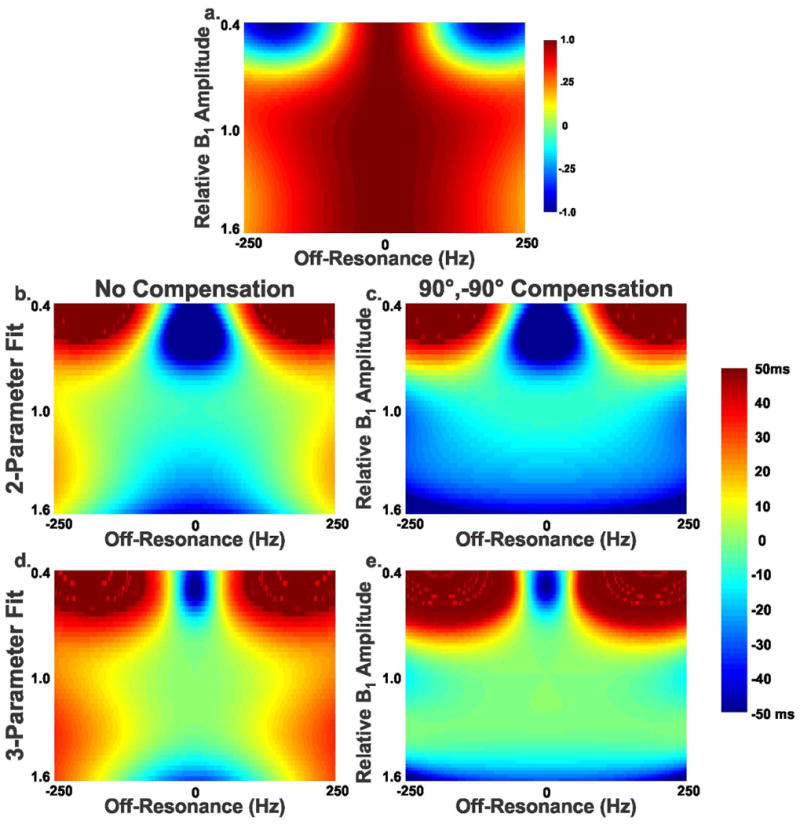
Effect of B0 and B1 variations on T2 estimation using simulation of Bloch equation. Nominal myocardium T1 and T2 values (1200 ms and 50 ms respectively) were used during simulation. The error is reported as the difference between the estimated T2 value and the true T2 value (50 ms). (a) Normalized longitudinal magnetization directly after applying the T2prep pulse, (b) Error in T2 estimation, relative to the used reference T2 value using 2-parameter fit, (c) using 2-parameter fit and the proposed compensation of 90°,-90° pulses for TET2P = 0, (d) using 3-parameter fit, and (e) using 3-parameter fit and the proposed compensation of 90°,-90° pulses for TET2P = 0.
Phantom Imaging
3-parameter vs. 2-parameter Fit
Figure 3 shows the accuracy and precision of the three different fitting approaches (2-parameter, 3-parameter without SAT image and the proposed 3-parameter with SAT image) on various subsets of images corresponding to different T2prep echo times for a vial with a T2 value of 47 ms. The red, green, blue, purple and black points correspond to TET2P = 0 and n TET2P values starting from 25 ms in steps of 5, 10, 15, 20 and 25 ms respectively, and the value of n is depicted on the horizontal axis. The T2 value estimated with the 2-parameter model increased with the number of T2prep echoes. For example, a 2-parameter fit on 9 T2prep echoes resulted in an estimated T2 value of 64 ms, as opposed to an estimate of 55 ms for 5 T2prep echoes, when a 10 ms TET2P spacing was used. The echo spacing also affected the estimated T2 value for the 2-parameter model. For example, using 7 T2prep echo times, a 5 ms TET2P spacing led to a 55 ms T2 estimate, whereas a 25 ms TET2P spacing resulted in the estimation of 63 ms as the T2 value. These exemplify the mismatch between the acquisition and the 2-parameter model. The 3-parameter fit without SAT image converged to the T2 value after 7 T2prep echoes. However, if the number of T2prep echoes was not sufficient, it overestimated the T2 value, with a high level of noise as apparent in the precision measurements. The T2 value estimated using the 3-parameter fit with the SAT image remained almost constant (variation: 2 ms) for different subsets of T2prep echo times. Figure 4 shows examples of the fit for the short acquisition and for 27 T2prep echoes, for the same vial, where the signal in the ROI is averaged prior to fitting. The overestimation of the T2 value using 3 T2prep echoes and the 2-parameter fit were visualized in the under-estimation of the non-zero signal value corresponding to the long T2prep echo time (“T2prep = ∞”). When using 27 echoes, the 3-parameter fit without SAT image matched the behavior of the proposed 3-parameter fit with SAT image, while the 2-parameter fit still overestimated the T2 values.
Figure 3.
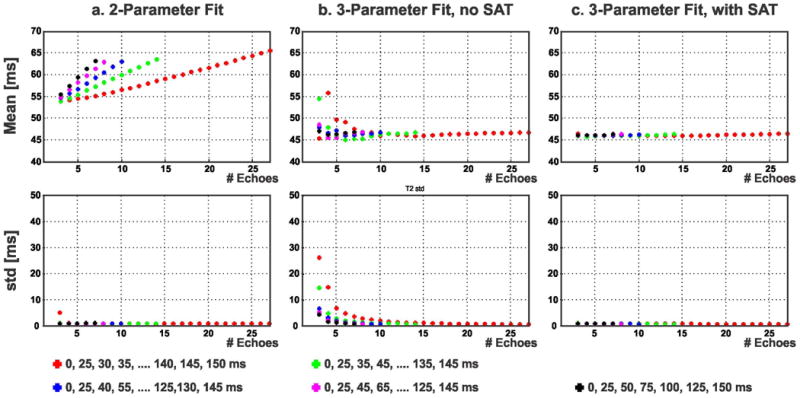
Accuracy and precision of the three different fitting approaches (2-parameter, 3-parameter without saturation-prepared (SAT) image and the proposed 3-parameter with SAT image) on various subsets of images corresponding to different T2prep echo times for a vial with a T2 value of 47 ms. The T2 value estimated with the 2-parameter model (a) shows dependence on the choice and number of T2prep echo times. The 3-parameter fit without SAT image (b) showed large deviations in accuracy and precision for a small number of T2prep echoes, but converged to the T2 value with a large number of T2prep echoes. The T2 value estimated using the proposed 3-parameter fit with the SAT image (c) remained almost constant (variation: 2 ms) for different subsets of T2prep echo times.
Figure 4.
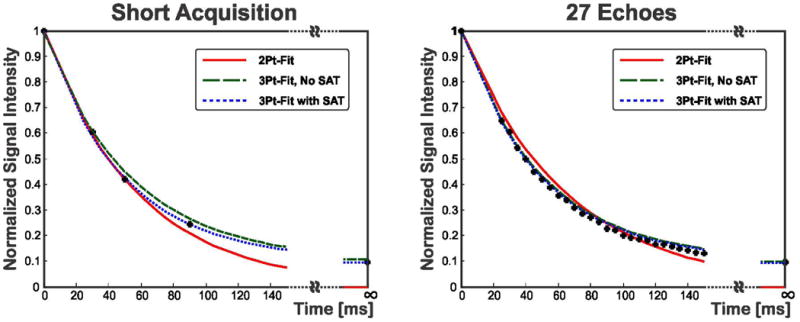
Example of the fit for the short acquisition and using 27 T2prep echoes, for the same vial in Figure 3, where the signal in the region of interest (ROI) is averaged prior to fitting. With the short acquisition, the 2-parameter overestimates the T2 value, as apparent in the under-estimation of the non-zero signal for “T2prep = ∞.” The proposed 3-parameter fit with SAT image fits this signal value well for both 3 and 27 T2prep echoes. With 27 echoes, the 3-parameter fit without SAT image matches the behavior of the proposed 3-parameter fit with SAT image, while the 2-parameter fit still overestimates the T2 values.
Figure 5 depicts the correlation of the different T2 curve fitting methods using the short acquisition or all 27 T2prep echoes, with respect to the spin echo sequence. The proposed 3-parameter fit with SAT image, using the short acquisition or all 27 T2prep echoes, produced T2 values that were not significantly different than the reference values generated using a spin echo acquisition (P = 0.104 and 0.3, respectively). The 3-parameter fit without SAT image showed no significant differences for both the short acquisition and 27 T2prep echoes (P = 0.073 and 0.126 respectively). The conventional 2-parameter fit significantly overestimated the T2 values for both 3 and 27 T2prep echoes (P = 0.013 and 0.005, respectively).
Figure 5.
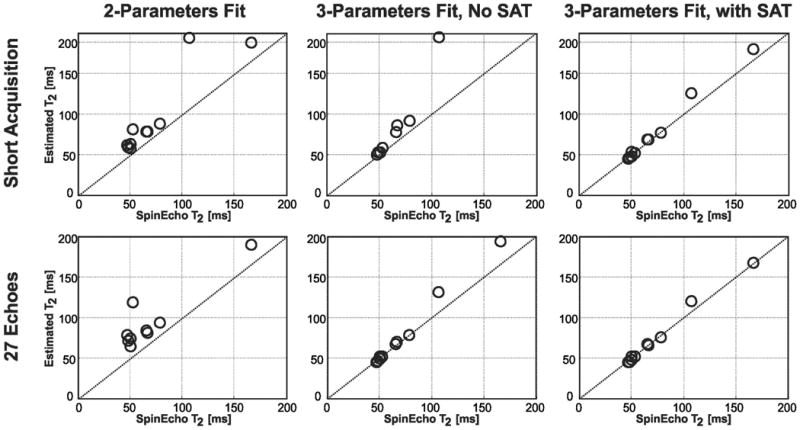
T2 values from different T2 curve fitting methods versus the reference T2 values generated from the spin echo sequence for all vials of the phantom, as well as the identity line. In the upper row, T2 values are estimated using short acquisitions (i.e. 3 (0, 25, 50 ms) samples for the 2-parameter fit, 4 samples (0, 25, 50, 90 ms) for the 3-parameter fit without SAT, and 4 samples (0, 25, 50, ∞ ms) for the 3-parameter fit with SAT. In the lower row, T2 values are estimated using long acquisitions (i.e. all 27 T2prep echoes). The conventional 2-parameter fit significantly overestimates the T2 values for both 3 and 27 T2prep echoes (P = 0.013 and 0.005 respectively). The 3-parameter fit without SAT image results in no significant difference for either the short acquisition echoes (P = 0.073) or with 27 T2prep echoes (P = 0.126). The proposed 3-parameter fit with SAT image, using 3 or 27 T2prep echoes, produces T2 values that are not significantly different than the reference values (P = 0.104 and 0.3 respectively).
Effect of the B1 inhomogeneities
The T2 values using the proposed 3-parameter fit with SAT image were not significantly different than the spin echo values, as described above, when using the proposed RF compensation (P = 0.3). However, the difference was significant without the compensation (P < 0.001). The 2-parameter fit led to significant differences in T2 values, both with and without the compensation (P = 0.005 and 0.010 respectively).
Length of the Rest Cycles
Figure 6 shows the effect of the length of rest cycles. The error in the estimated T2 was the highest for vials with long T1 values due to insufficient magnetization recovery. This error gradually decreased with increasing rest cycles. For the vials with T1 and T2 ranges near the myocardium and for rest cycles ≥ 4s, the error was within 2 ms and 1 ms when using the short acquisition and 27 samples, respectively.
Figure 6.
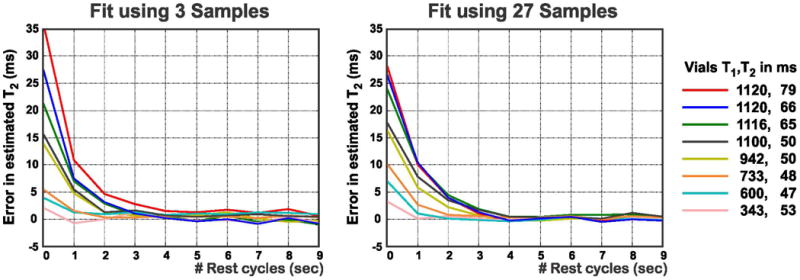
Rest cycles effect on the estimated T2 values. ROIs are placed in vials with different T1 and T2 values. The error is within 2 ms for both acquisitions when using rest cycles of length ≥ 4 s.
In Vivo Imaging
The myocardial T2 mapping sequence was successfully completely in all subjects without complications. The average scan time to acquire all 27 echoes was 3:30 ± 0:10 minutes (range: 3:17 to 3:47 minutes). The difference between the nominal scan time and the actual average scan time is due to the differences in breathing patterns and heart rates of the subjects. Figure 7 shows example T2 maps from a healthy subject, generated using the three different fitting approaches (2-parameter, 3-parameter without SAT image and the proposed 3-parameter with SAT image) with the short acquisition, as well as all 27 T2prep echoes. The myocardial T2 value for the 2-parameter fit increased when using 27 T2prep echoes instead of 3, which was consistent with phantom imaging. For the short acquisitions, the T2 map generated using the 3-parameter fit without SAT image visibly showed more signal inhomogeneity compared to that of the proposed 3-parameter fit with SAT. The quality of the T2 map for the 3-parameter fit without SAT image improved with 27 T2prep echoes. The proposed 3-parameter fit with SAT image led to similar quality myocardial T2 maps with 3 and 27 echoes.
Figure 7.
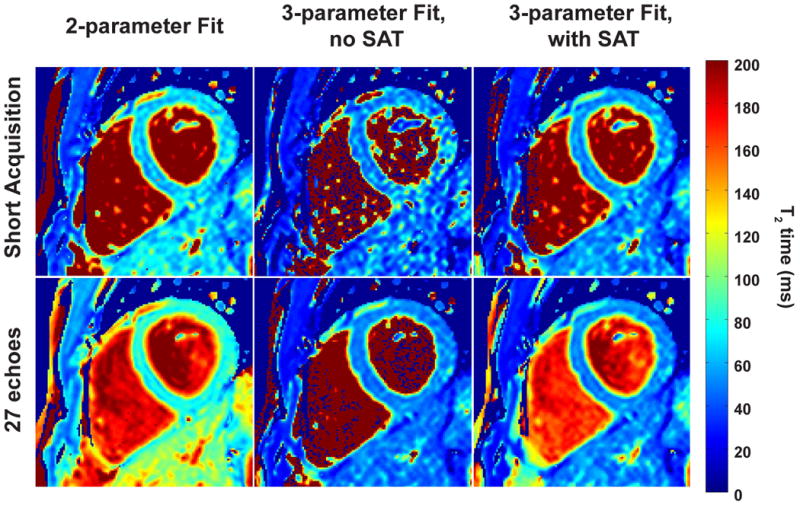
Example T2 maps from a healthy adult subject (No. 3), generated using 2-parameter fitting (left column), 3-parameter fitting without SAT image (middle column) and the proposed 3-parameter with SAT image (right column) with the short acquisition and using all 27 (top and bottom row respectively) T2prep echoes. The myocardial T2 value increases when going from 3 to 27 echoes using the 2-parameter fit. For the short acquisition, the T2 map generated using the 3-parameter fit without SAT image has 1.8-fold more variation in the septum compared to that generated using the proposed 3-parameter fit with SAT image. When using all 27 T2prep echoes, the 3-parameter fits with and without SAT image leads to similar quality in the myocardium.
Figure 8 shows the estimated T2 values (averaged over an ROI in the septum) from the same subject in Figure 7 using the three fitting methods (2-parameter, 3-parameter without SAT image and the proposed 3-parameter with SAT image) on various subsets of images corresponding to different T2prep echo times. Similar to phantom imaging, the T2 value estimated with the 2-parameter fitting method increased with increasing number of T2prep echo times. The T2 value estimated using the 3-parameter fit without SAT image showed a convergence trend with increased number of T2prep echo times. The proposed 3-parameter fitting with SAT image yielded T2 values which are independent of number of T2prep echoes. The standard deviation of T2 values in the ROI decreased with higher number of echoes for all fitting methods.
Figure 8.
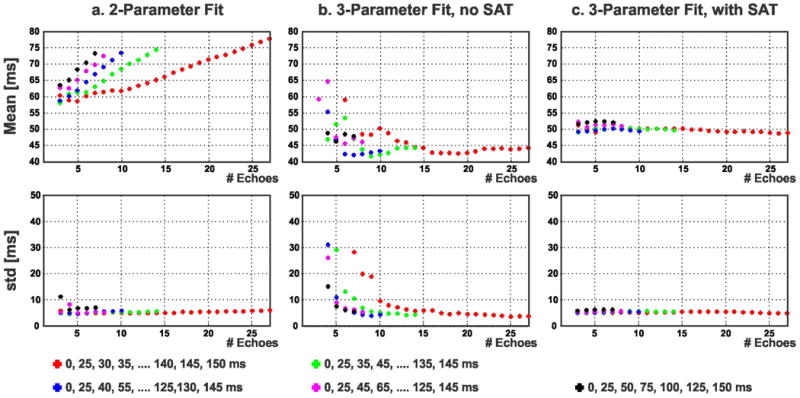
Myocardial T2 values from the same subject in Figure 7 (averaged over an ROI in the septum) using 2-parameter fit, 3-parameter fit without SAT image and the proposed 3-parameter fit with SAT image, on various subsets of image corresponding to different T2prep echo times. The 2-parameter model (a) shows dependence on the choice and number of T2prep echo times. The 3-parameter fit without SAT image (b) converges to the T2 value with a large number of T2prep echoes, but shows deviations otherwise. The proposed 3-parameter fit with the SAT image (c) results in T2 values that are almost constant (variation: 3.6 ms) over different subsets of T2prep echo times.
Table 1 summarizes the ventricular septum T2 values for all of the healthy adult subjects using the three fitting methods, and the short acquisition or 27 T2prep echoes. The maximum variation (among all subjects) of the myocardial T2 values between using 3 or 27 T2prep echoes, was 3.7 ms with the proposed 3-parameter fitting with SAT image. The range of increase in the T2 values, among all subjects, was 4.6 - 20.0 ms when the 2-parameter fit is used with 27 T2prep echoes instead of 3 T2prep echoes. T2 measurements could not be performed on two maps generated using the 3-parameter fitting without SAT image and the short acquisition due to the high levels of inhomogeneity in the myocardium. Furthermore, for the T2 maps from the short acquisitions where measurements could be performed, the precision of the 3-parameter fit with the SAT image was significantly better than that of the 3-parameter fit without the SAT image (8.5 ± 2.1 ms vs. 15.5 ± 5.1 ms, P = 0.009).
Table 1.
Quantitative myocardial T2 values in the ventricular septum using 2-parameter fitting, 3-parameter fitting with and without SAT image for the short acquisition and 27 T2prep echoes. The short acquisition consists of T2prep echoes (0 25, 50 ms) for the 2-parameter fit; (0, 25, 50, 90 ms) 3-parameter fit without SAT image; and (0 25, 50, ∞ ms) for the 3-parameter fit with SAT image.
| 2-parameter Fit | 3-parameter Fit, no SAT | 3-parameter Fit, with SAT | ||||
|---|---|---|---|---|---|---|
| Subject | Short Acq (ms) | 27-echoes (ms) | Short Acq (ms) | 27-echoes (ms) | Short Acq (ms) | 27-echoes (ms) |
| 1 | 68.2 ± 8.0 | 88.2 ± 10.3 | 56.2 ± 12.0 | 49.1 ± 4.3 | 54.5 ± 7.0 | 52.4 ± 6.1 |
| 2 | 78.4 ± 14.3 | 87.3 ± 8.4 | N/A | 61.7 ± 8.7 | 62.9 ± 11.5 | 61.1 ± 7.2 |
| 3 | 63.3 ± 11.3 | 77.5 ± 6.1 | 50.3 ± 8.8 | 46.9 ± 4.0 | 51.7 ± 5.0 | 49.0 ± 4.3 |
| 4 | 56.3 ± 10.5 | 74.3 ± 15.8 | 36.6 ± 13.0 | 44.0 ± 7.9 | 46.5 ± 10.7 | 46.3 ± 9.9 |
| 5 | 71.0 ± 9.4 | 75.6 ± 8.4 | 75.9 ± 22.4 | 58.8 ± 8.5 | 58.3 ± 9.0 | 56.2 ± 7.5 |
| 6 | 71.0 ± 14.2 | 79.4 ± 9.7 | N/A | 54.2 ± 7.7 | 57.7 ± 11.3 | 54.0 ± 7.1 |
| 7 | 67.7 ± 11.0 | 75.4 ± 14.1 | 68.3 ± 19.5 | 57.1 ± 9.0 | 56.8 ± 10.1 | 56.2 ± 9.9 |
| 8 | 62.4 ± 10.6 | 73.5 ± 12.2 | 55.0 ± 17.1 | 48.0 ± 5.7 | 51.7 ± 9.4 | 49.2 ± 7.7 |
| average | 67.3 | 78.9 | 57.1 | 52.5 | 55.0 | 53.1 |
Table 2 depicts the results of the segment-based analysis for the proposed 3-parameter fit with 27 echoes. The range variation for the average T2 values across the six segments is 4.2 ms (between 52.6 and 56.8 ms), showing less than 10% variation. The range of variation for the B/A values is 0.01, with a mean value of 0.14 or 0.15 across all segments. The B/A value predicted by Equation [6] for these sequence parameters is 0.13 (with T1 = 1200 ms and T2 = 55 ms), which is consistent with the experimental findings.
Table 2.
Segment-based analysis for the proposed 3-parameter fit with 27 echoes. The results show that the range of variation for average T2 values across the six mid-ventricular myocardial segments is 4.2 ms. The range of variation for the B/A parameter is 0.01. The value predicted for B/A by Equation [6] for the given sequence parameters is 0.13 (subj = subject, seg = segment).
| T2 (ms) | B/A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subj\Seg | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 70 | 67 | 60 | 56 | 51 | 55 | 0.13 | 0.18 | 0.20 | 0.19 | 0.21 | 0.18 |
| 2 | 60 | 56 | 58 | 60 | 55 | 56 | 0.17 | 0.16 | 0.18 | 0.17 | 0.17 | 0.17 |
| 3 | 53 | 53 | 49 | 51 | 52 | 52 | 0.17 | 0.16 | 0.15 | 0.14 | 0.16 | 0.16 |
| 4 | 43 | 39 | 43 | 47 | 46 | 41 | 0.18 | 0.20 | 0.18 | 0.14 | 0.13 | 0.14 |
| 5 | 59 | 58 | 59 | 59 | 53 | 56 | 0.09 | 0.10 | 0.13 | 0.12 | 0.12 | 0.13 |
| 6 | 53 | 56 | 55 | 53 | 52 | 50 | 0.16 | 0.13 | 0.13 | 0.15 | 0.15 | 0.15 |
| 7 | 58 | 53 | 54 | 59 | 55 | 61 | 0.12 | 0.10 | 0.12 | 0.12 | 0.12 | 0.14 |
| 8 | 58 | 53 | 54 | 59 | 55 | 61 | 0.12 | 0.10 | 0.12 | 0.12 | 0.12 | 0.14 |
| average | 56.8 ± 7.6 | 54.3 ± 7.6 | 53.8 ± 5.6 | 55.5 ± 4.7 | 52.6 ± 3.2 | 54.2 ± 6.7 | 0.14 ± 0.03 | 0.14 ± 0.04 | 0.15 ± 0.03 | 0.14 ± 0.03 | 0.15 ± 0.03 | 0.15 ± 0.02 |
Discussion
In this study, we proposed a 3-parameter model for T2 relaxation to characterize T2-prepared bSSFP acquisitions. For efficient estimation of these three parameters, we also proposed a novel sequence that incorporates saturation-prepared images in addition to T2-prepared images, as well as an efficient navigator-gating scheme for free-breathing acquisitions. This new sequence and the 3-parameter model improve the accuracy of myocardial T2 mapping.
The 3-parameter model for curve-fitting was found to be independent of the choice of T2prep echo times, whereas the estimated T2 values changed with T2prep echo times using the 2-parameter model. Since the 2-parameter model does not take into account the disturbance in magnetization due to the startup and imaging pulses until the acquisition of central k-space, this leads to a model mismatch between the curve-fitting and the underlying acquisition, which makes the estimated T2 value a function of the T2prep echo times. This model mismatch is resolved using the 3-parameter model, and the dependence of the estimated T2 value on where the T2 relaxation curve is sampled is eliminated.
Apart from its independence from the sequence parameters, the 3-parameter model with the SAT image is accurate with respect to the spin echo sequence, after the proposed modifications to account for RF pulse imperfection. The inaccuracy of T2 mapping procedure with the 2-parameter curve-fitting with respect to the spin echo sequence, as well as its dependence on k-space profile ordering, has been noted previously (11). However, this discrepancy was not examined further in (11).
The reference T2 maps with CPMG spin echo sequence were generated with a 2-parameter fit. The issue of magnetization disturbance due to imaging pulses in single shot sequences is not present for this acquisition, thus a 2-parameter fit is appropriate. The 3-parameter fit for the spin echo acquisition (not shown) also yields the same values.
We chose to acquire 27 images with different T2prep echo times in each scan for this study. This was done to study the effect of different choices of T2prep times on the overall estimation procedure using the 3-parameter and 2-parameter models. This number of echoes is not required for attaining accuracy and precision for in-vivo imaging using the proposed 3-parameter curve fitting with the additional SAT image. Furthermore, the precision gain going from 3 echoes to 27 echoes is at most 4.3 ms for the myocardium in this technique.
In (28), it was concluded that the 3-parameter fit cannot be robustly used with 4 finite T2prep echoes. However, our experience indicates that 3 T2prep echoes of 0, 25, 50 ms, and an additional SAT image are sufficient to provide accurate and precise T2 maps. Using 6 second rest cycles, this exam can be completed in 16 seconds at 60 bpm heart-rate, which is attainable with a breath-hold acquisition. The improvement in our sequence in terms of robustness with a small number of T2prep echoes comes from the use of the SAT image instead of a large T2prep echo time, which significantly improves the precision in-vivo. Compared to the sampling of a large T2prep echo time, such as 90 ms, the SAT image (equivalently T2prep echo time ∞) enables direct estimation of the B parameter in Equation [2], and higher quality estimates of T2 values. Furthermore, in Appendix A, we analytically show that from an estimation theoretic perspective, sampling the SAT image is more beneficial in terms of precision of T2 maps in the presence of noise compared to sampling a large but finite T2prep echo time. Another benefit of using the SAT image is that it can be acquired without any preceding rest periods, whereas a 6 second rest period would be necessary to acquire a large T2prep echo time.
The segment-based analysis of the B/A parameter from the fitting procedure leads to values which are consistent with the theoretical predictions. The minor pixel-dependent differences may be due to the least squares fitting procedure, which approximates the Rician noise in the images as Gaussian noise with a non-zero mean (34), which would be reflected in the B parameter. Due to this good correspondence, between theory as predicted in Equation [6] and the experimental results, the utility of the B term is expected to extend to different phase encode schemes. Small regional variations of the T2 values were also observed.
For both the SAT image and for images acquired with large T2prep echo times, the underlying SNR may be too low to approximate the Rician noise as Gaussian noise, which is implicitly done in the least squares estimation process. This may cause a bias in the estimation procedure. However, this was not observed in our phantom experiments. Nonetheless, it might be beneficial to acquire multiple SAT images, since no rest cycles are required between them, and average them prior to fitting to further mitigate any bias.
Apart from the use of SAT images instead of large T2prep echo times, optimal selection of T2prep echo times to further improve robustness was not explored experimentally. In Appendix A, an estimation theoretic analysis to maximize the precision of the T2 maps shows that it would be beneficial to choose a tri-modal distribution of T2prep echo times, with the points concentrating at 0 ms, at an echo time near the T2 value of interest and at ∞, with the multiplicity changing based on the total number of echoes. However, in our experiments, we used a more standard distribution of T2prep echo times based on the existing literature. Further experiments are warranted to systematically optimize the T2prep echo time distribution, but this is not the focus of the current work.
A new navigator-gating scheme was proposed to improve the efficiency for free-breathing acquisitions. In the conventional scheme proposed in (35), the T2prep follows the NAV signal, however it is performed regardless of the position of the NAV signal, necessitating additional rest periods if the NAV signal is outside the gating window. In our proposed approach, the T2prep is conditionally applied based on the position of the NAV signal. Thus, if the NAV signal is outside the gating window, no preparation or imaging pulses are applied, and the magnetization remains undisturbed. This also eliminates the necessity for rest periods if the NAV signal is outside the gating window. Thus, the overall efficiency of the acquisition is improved.
Since the NAV signal is placed before the T2 preparation, there is a longer separation between the image acquisition and the NAV signal. This may lead to residual motion in the images, necessitating image registration to mitigate residual motion artifacts. The efficacy of the particular image registration algorithm in T2 mapping was not systematically studied in this study, and is beyond the scope and focus of this work.
A 6 second rest period was used to allow for a full magnetization recovery between subsequent T2prep modules. This choice was based on a 5×T1 approximation, using reported myocardial T1 values in the literature. While a 6 second rest period was used in this study to ensure sufficient recovery, phantom results indicated that 4 seconds may be sufficient, reducing the breath-hold duration for an acquisition with 3 T2prep echoes and a SAT image. Although shorter rest periods are desirable, phantom results showed arbitrary, T1-dependent biases in the estimated T2 values when shorter durations were used.
This study has several limitations. Only a small number of healthy subjects were recruited. Further clinical evaluations on larger cohorts are warranted to quantify changes in T2 relaxation times in different populations. No validation of the T2 values has been performed in vivo, since a reference T2 time cannot be assessed in the myocardium in a reasonable scan time. The intra-patient reproducibility of the T2 values was also not studied. We have only considered single-shot sequences with linear ordering. The effect of including the third parameter on accuracy may be less for centric ordering or multi-shot sequences. Only a single mid-ventricular short-axis slice was imaged in this study. The low in-plane resolution used in this study may lead to partial imaging artifacts if more apical slices are acquired.
Conclusion
We propose a 3-parameter model for T2 relaxation accurately models myocardial T2 mapping using T2-prepared bSSFP acquisitions. This model exhibits no dependency on the choice of T2prep echo times, whereas such dependence is observed if a conventional 2-parameter model is used for curve-fitting. The proposed sequence incorporates SAT images in addition to T2-prepared images, and the improved navigator-gating technique augments the efficiency of the myocardial T2 mapping acquisition, allowing for accurate and precise T2 maps.
Acknowledgments
The project described was partially supported by NIH R01EB008743-01A2, NIH K99HL111410-01 and Samsung Electronics, Suwon, South Korea.
The authors thank Warren J. Manning for his editorial comments.
Appendix A
We use the Cramér-Rao Bound (CRB) to provide a lower bound on the precision of an unbiased T2 estimator, and subsequently minimize this bound numerically to find the optimal selection of T2prep echo times, similar to the approaches in (36,37). For the model in Equation [7] with least squares estimation of T2, and for K T2prep echo times, {x1, x2, …, xk}, the Fisher information matrix is given by
| [A1] |
The CRB on the variance of the T2 estimate is given by
| [A2] |
where Iij denotes the (i,j)th entry of I. To find the selection of T2prep echo times that minimizes the variance of the error, we propose to solve
| [A3] |
for a given range of T2 values of interest. We also note that J(A, T2, {xk}) scales with 1/A2, and thus J(A, T2, {xk}) = J(1, T2, {xk})/A2, and hence the selection of T2prep echo times does not depend on A or B, but only on the T2 values of interest.
J(1, T2, {xk}) was numerically minimized for T2 values of interest from 45 ms to 60 ms, for K = 4 and K = 28. For K = 4, this yielded a tri-modal distribution with T2prep echo times of 0, 49 ms (sampled twice) and ∞. For K = 28, the distribution of T2prep echo times of 0 (sampled six times), 53 ms (sampled fourteen times) and ∞ (sampled 8 times). This kind of tri-modal distribution is consistent with the bi-modal distribution in (36) for the 2-parameter T2 model, and the tri-modal one in (37) for the 3-parameter T1 model.
Furthermore, if instead of the ∞ T2prep echo time, one could only sample a maximum finite value of 90 ms, the distributions changed to 0, 32 ms (sampled twice) and 90 ms for K = 4; and 0 (sampled six times), 33 ms (sampled fourteen times) and 90 (sampled 8 times) for K = 28. In this case, the variance of the T2 estimate increased by 5.4-fold and 5.5-fold for K = 4 and 28, respectively. A direct comparison for K = 4 also showed that T2prep echo times {0, 25, 50, 90} had 5.6-fold higher variance compared to {0, 25, 50, ∞}. These results indicate that sampling the ∞ T2prep echo time improves the precision of the fit compared to sampling a large but finite T2prep echo time.
We note that this derivation is based on the least squares estimation, which has a one-to-one correspondence with a Gaussian noise model in the images. However, the noise in the magnitude images is Rician, which can be well-approximated by Gaussian noise for images with sufficient SNR (34), and assumption that may not hold for ISAT images. This may lead to a model mismatch and an apparent bias in T2 estimates, although this was not observed in our study.
Footnotes
The first two authors contributed equally to this work.
References
- 1.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109(20):2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 2.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118(8):837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 3.Raman SV, Simonetti OP, Winner MW, 3rd, Dickerson JA, He X, Mazzaferri EL, Jr, Ambrosio G. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55(22):2480–2488. doi: 10.1016/j.jacc.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45(11):1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 6.Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J Cardiovasc Magn Reson. 2009;11:7. doi: 10.1186/1532-429X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Aty H, Cocker M, Friedrich MG. Myocardial edema is a feature of Tako-Tsubo cardiomyopathy and is related to the severity of systolic dysfunction: insights from T2-weighted cardiovascular magnetic resonance. Int J Cardiol. 2009;132(2):291–293. doi: 10.1016/j.ijcard.2007.08.102. [DOI] [PubMed] [Google Scholar]
- 8.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199(1):49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging. 2007;26(3):452–459. doi: 10.1002/jmri.21028. [DOI] [PubMed] [Google Scholar]
- 10.Arai AE. Using magnetic resonance imaging to characterize recent myocardial injury: utility in acute coronary syndrome and other clinical scenarios. Circulation. 2008;118(8):795–796. doi: 10.1161/CIRCULATIONAHA.108.797373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara MT, Higgins CB, Schechtmann N, Botvinick E, Lipton MJ, Chatterjee K, Amparo EG. Detection and characterization of acute myocardial infarction in man with use of gated magnetic resonance. Circulation. 1985;71(4):717–724. doi: 10.1161/01.cir.71.4.717. [DOI] [PubMed] [Google Scholar]
- 13.Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys. 1984;11(4):425–448. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- 14.He T, Gatehouse PD, Anderson LJ, Tanner M, Keegan J, Pennell DJ, Firmin DN. Development of a novel optimized breathhold technique for myocardial T2 measurement in thalassemia. J Magn Reson Imaging. 2006;24(3):580–585. doi: 10.1002/jmri.20681. [DOI] [PubMed] [Google Scholar]
- 15.Foltz WD, Stainsby JA, Wright GA. T2 accuracy on a whole-body imager. Magn Reson Med. 1997;38(5):759–768. doi: 10.1002/mrm.1910380512. [DOI] [PubMed] [Google Scholar]
- 16.Blume U, Lockie T, Stehning C, Sinclair S, Uribe S, Razavi R, Schaeffter T. Interleaved T(1) and T(2) relaxation time mapping for cardiac applications. J Magn Reson Imaging. 2009;29(2):480–487. doi: 10.1002/jmri.21652. [DOI] [PubMed] [Google Scholar]
- 17.van Heeswijk RB, Feliciano H, Bongard C, Bonanno G, Coppo S, Lauriers N, Locca D, Schwitter J, Stuber M. Free-breathing 3 T magnetic resonance T2-mapping of the heart. JACC Cardiovasc Imaging. 2012;5(12):1231–1239. doi: 10.1016/j.jcmg.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4(3):269–278. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zia MI, Ghugre NR, Connelly KA, Strauss BH, Sparkes JD, Dick AJ, Wright GA. Characterizing myocardial edema and hemorrhage using quantitative T2 and T2* mapping at multiple time intervals post ST-segment elevation myocardial infarction. Circ Cardiovasc Imaging. 2012;5(5):566–572. doi: 10.1161/CIRCIMAGING.112.973222. [DOI] [PubMed] [Google Scholar]
- 20.Foltz WD, Al-Kwifi O, Sussman MS, Stainsby JA, Wright GA. Optimized spiral imaging for measurement of myocardial T2 relaxation. Magn Reson Med. 2003;49(6):1089–1097. doi: 10.1002/mrm.10467. [DOI] [PubMed] [Google Scholar]
- 21.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 22.Huang TY, Liu YJ, Stemmer A, Poncelet BP. T2 measurement of the human myocardium using a T2-prepared transient-state TrueFISP sequence. Magn Reson Med. 2007;57(5):960–966. doi: 10.1002/mrm.21208. [DOI] [PubMed] [Google Scholar]
- 23.Foltz WD, Yang Y, Graham JJ, Detsky JS, Dick AJ, Wright GA. T2 fluctuations in ischemic and post-ischemic viable porcine myocardium in vivo. J Cardiovasc Magn Reson. 2006;8(3):469–474. doi: 10.1080/10976640600572897. [DOI] [PubMed] [Google Scholar]
- 24.Park CH, Choi EY, Kwon HM, Hong BK, Lee BK, Yoon YW, Min PK, Greiser A, Paek MY, Yu W, Sung YM, Hwang SH, Hong YJ, Kim TH. Quantitative T2 mapping for detecting myocardial edema after reperfusion of myocardial infarction: validation and comparison with T2-weighted images. Int J Cardiovasc Imaging. 2013;29(Suppl 1):65–72. doi: 10.1007/s10554-013-0256-0. [DOI] [PubMed] [Google Scholar]
- 25.Usman AA, Taimen K, Wasielewski M, McDonald J, Shah S, Giri S, Cotts W, McGee E, Gordon R, Collins JD, Markl M, Carr JC. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circ Cardiovasc Imaging. 2012;5(6):782–790. doi: 10.1161/CIRCIMAGING.111.971101. [DOI] [PubMed] [Google Scholar]
- 26.Wassmuth R, Prothmann M, Utz W, Dieringer M, von Knobelsdorff-Brenkenhoff F, Greiser A, Schulz-Menger J. Variability and homogeneity of cardiovascular magnetic resonance myocardial T2-mapping in volunteers compared to patients with edema. J Cardiovasc Magn Reson. 2013;15:27. doi: 10.1186/1532-429X-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker PM, Marie PY, Mezeray C, Bessieres M, Escanye JM, Karcher G, Danchin N, Mattei S, Villemot JP, Bertrand A. Synchronized inversion recovery-spin echo sequences for precise in vivo T1 measurement of human myocardium: a pilot study on 22 healthy subjects. Magn Reson Med. 1993;29(5):637–641. doi: 10.1002/mrm.1910290509. [DOI] [PubMed] [Google Scholar]
- 28.Giri S, Chung Y, Shah S, Xue H, Guehring J, Zuehlsdorff S, Simonetti OP. T2 mapping using T2prepared-SSFP: optimizing echo time, flip angle and parameter fitting. 2010 May; Stockholm. Proceedings of the 18th Scientific Meeting of ISMRM; p. 2960. [Google Scholar]
- 29.Levitt M, Freeman R, Frenkiel T. Broadband heteronuclear decoupling. J Magn Reson. 1982;47:328–330. [Google Scholar]
- 30.Scheffler K. On the transient phase of balanced SSFP sequences. Magn Reson Med. 2003;49(4):781–783. doi: 10.1002/mrm.10421. [DOI] [PubMed] [Google Scholar]
- 31.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1 mapping. Magn Reson Med. 2013 doi: 10.1002/mrm.24878. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Roujol S, Foppa M, Weingartner S, Manning WJ, Nezafat R. Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): Application to T1 mapping. Magn Reson Med. 2014 doi: 10.1002/mrm.25270. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 34.Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995;34(6):910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giri S, Shah S, Xue H, Chung YC, Pennell ML, Guehring J, Zuehlsdorff S, Raman SV, Simonetti OP. Myocardial T(2) mapping with respiratory navigator and automatic nonrigid motion correction. Magn Reson Med. 2012;68(5):1570–1578. doi: 10.1002/mrm.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JA, Hodgkinson P, Barker AL, Hore PJ. Optimal sampling strategies for the measurement of spin–spin relaxation times. J Magn Res - Series B. 1996;113:25–34. [Google Scholar]
- 37.Akcakaya M, Weingartner S, Roujol S, Nezafat R. On the selection of sampling points for myocardial T1 mapping. Magn Reson Med. 2014 doi: 10.1002/mrm.25285. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


