Abstract
Neonates have increased susceptibility to infection, which leads to increased mortality. Whether or not this as a result of implicit deficits in neonatal innate immune function or recapitulation of innate immune effector cell populations following infection is unknown. Here, we examine the process of emergency myelopoiesis whereby the host repopulates peripheral myeloid cells lost following the initial infectious insult. As early inflammatory responses are often dependent upon NF-κB and type I IFN signaling, we also examined whether the absence of MyD88, TRIF or MyD88 and TRIF signaling altered the myelopoietic response in neonates to polymicrobial sepsis. Following neonatal polymicrobial septic challenge, hematopoietic stem cell (HSC) expansion in bone marrow and the spleen were both attenuated and delayed in neonates compared with adults. Similar reductions in other precursors were observed in neonates. Similar to adult studies, the expansion of progenitor stem cell populations was also seen in the absence of MyD88 and/or TRIF signaling. Overall, neonates have impaired emergency myelopoiesis in response to sepsis compared with young adults. Despite reports that this expansion may be related to TLR signaling, our data suggest that other factors may be important, as TRIF−/− and MyD88−/− neonatal HSCs are still able to expand in response to polymicrobial neonatal sepsis.
Keywords: Mice, infection, neutrophil, LSK
Introduction
Neonatal sepsis remains a significant global health issue, with over one million deaths per year world-wide.1,2 While numerous efforts to improve critical care have improved outcomes of neonatal sepsis, many facets of the neonatal physiologic and immunologic responses to infection/inflammation require further investigation. Dismal outcomes to sepsis in this vulnerable population are compounded by the fact that few therapeutic modalities have demonstrated any significant clinical benefits.
As our group and others have demonstrated, the distinct functional status of neonatal innate immune response, as well as the reliance of the neonate upon innate immunity, may be one of the reasons why this populations fares so poorly to infection.2 One integral component of the neonatal innate immune response to infection that has not been examined well is the generation of new granulocytes and other myeloid cells following infection or inflammation. This phenomenon, known as emergency myelopoiesis, is the process whereby central stores of innate immune effector cells in the bone marrow and spleen are reconstituted following emigration of neutrophils and other granuloctytes to the periphery in response to inflammation.3–5 Following this exodus of myeloid cells, the hematopoietic stem cells or LSKs (Lin−Sca-1+c-Kit+) expand to form predominantly myeloid progenitor cell populations, such as common myeloid progenitors (CMPs) and granulocyte macrophage progenitors (GMPs).5 These cells subsequently differentiate into mature myeloid populations.
We demonstrate here that neonates have impaired reconstitution of innate immune effector cells, such as neutrophils. Neonates have decreased numbers of LSK cells and other myeloid progenitor cell populations compared with adults in both the bone marrow and spleen. We also show that neonatal LSKs produce significantly fewer CFUs in response to classic myeloid growth factors. Similar to adults, this expansion of LSK and other progenitors does not appear to be dependent upon either MyD88 or TRIF signaling. Together, these data strongly suggest that emergency myelopoiesis in neonates is impaired compared with adults.
Materials and methods
Mice and monitoring
All studies were approved by the Institutional Animal Care and Use Committee at the University of Florida, College of Medicine, prior to their initiation. Six to 8-wk-old male and female C57BL/6J (WT), MyD88−/− [B6.129P2(SJL)-Myd88tm1.1Defr/J] and TRIF−/− (C57Bl/6J-Ticam1Lps2/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and allowed a minimum of 7d to equilibrate to their environment before any experimental use. TRIF−/−MyD88−/− mice were generated from the above MyD88−/− and TRIF−/− strains and bred at our facility. The genotype of adult breeders was verified before experiments were conducted. Adults used in experiments were between 6 and 8 wk of age. To generate neonatal mice, harem mating was established and females were isolated as soon as they were visually identified as pregnant. The females were monitored daily so an accurate date of birth was recorded. Litters aged 5–7 d were used for experimental procedures, and were defined as neonates.
Cecal slurry induced polymicrobial sepsis
Polymicrobial sepsis was induced in mice using the fecal peritonitis method as previously described.6 Briefly, the cecal contents of adult C57BL/6 mice were suspended in 5% dextrose solution (D5W) at a final concentration of 80 mg/ml and injected i.p. into 5–7-d-old neonates or 6–8-wk-old mice. An LD40 (1 mg fecal contents/g body mass) of cecal slurry (CS), as determined from previous experiments, was used.3
Isolation of blood, bone marrow and splenocytes for phenotypic analysis
Blood was obtained via intracardiac puncture of isoflurane-anesthetized mice into a heparinized syringe. Spleens were harvested from euthanized mice and then dissociated through a 70-µm pore size sterile filter. To harvest bone marrow, both femurs and tibias from individual animals were collected and flushed with PBS (Cellgro, Manassas, VA, USA). Splenocyte and bone marrow suspensions were then subjected to erythrocyte lysis using ammonium chloride lysis solution. Cell suspensions were then stained for neutrophil (Ly6G+CD11b+) cell surface markers and analyzed via flow cytometry.7 Single cell suspensions were characterized using anti-Ly6G APC and anti-CD11b-PECy7.
Splenocytes and bone marrow, harvested as described above, were incubated with mouse biotin lineage Ab cocktail (anti-Gr1, anti-B220, anti-Ter119, anti-CD3e and anti-CD11b; Invitrogen, Carlsbad, CA, USA), anti-C-kit, anti-Sca-1, anti-CD34, and either anti-CD135 and anti-CD150 for long- and short-term progenitors, respectively, or anti-CD127 (IL-7Rα) and anti-CD16/32(FcRII/III) for CMPs (Lin−c-Kit+ Sca1−IL-7R−,CD34+,FcγRII/IIIlo), common lymphoid progenitors (CLP; Lin−c-Kitlo,Sca1lo,CD34+,IL-7Rα+) and GMPs (Lin−c-Kit+Sca1−, IL-7α−CD34+,FcγRII/IIIhi).5 All Abs were purchased from Becton Dickinson (Franklin Lakes, NJ, USA) unless otherwise indicated. Cell samples were acquired and analyzed on LSRII flow cytometer with FACSDiva software (Becton Dickinson). At least 1 × 104 non-debris live cells (SYTOX-Blue negative; Invitrogen) were used for analysis.
Cell sorting to isolate LSKs
Bone marrow was harvested as previously described in both adult and neonate mice. Four to five neonates were pooled to acquire enough cells for sorting. Lin− progenitor cells were obtained using biotin–Lin followed by anti-biotin magnetic beads (Miltenyi Biotec, Auburn, CA, USA). To obtain LSK-enriched cells, the Lin− cell fraction was incubated with PerCPcy5.5 Streptavidin for Lin−, FITC c-kit and PECy7 sca-1 Abs, and cells were sorted using a FACSAria sorter (BD Biosciences).
In vitro stimulation of LSKs with growth factors
LSK-enriched progenitors were cultured in mouse methylcellulose base media HSC006 (R&D Systems, Minneapolis, MN, USA) supplemented with murine macrophage colony-stimulation factor (M-CSF; 50 ng/ml), granulocyte macrophage (GM)-CSF (10 ng/ml), granulocyte (G)-CSF(100 ng/ml) or IL-7 (50 ng/ml) (R&D Systems) for 14 d.
Statistical analyses
Continuous variables were tested for normality and equality of variances. All statistical analyses were completed using SigmaStat version 11 (Systat, San Jose, CA, USA). Differences among groups were evaluated by either one-way ANOVA with either Dunn’s or Tukey post hoc analysis, or Student’s t-test. Significance was determined at the 95% confidence level.
Results
Delayed recapitulation of bone marrow and splenic granulocytes in neonates following polymicrobial sepsis
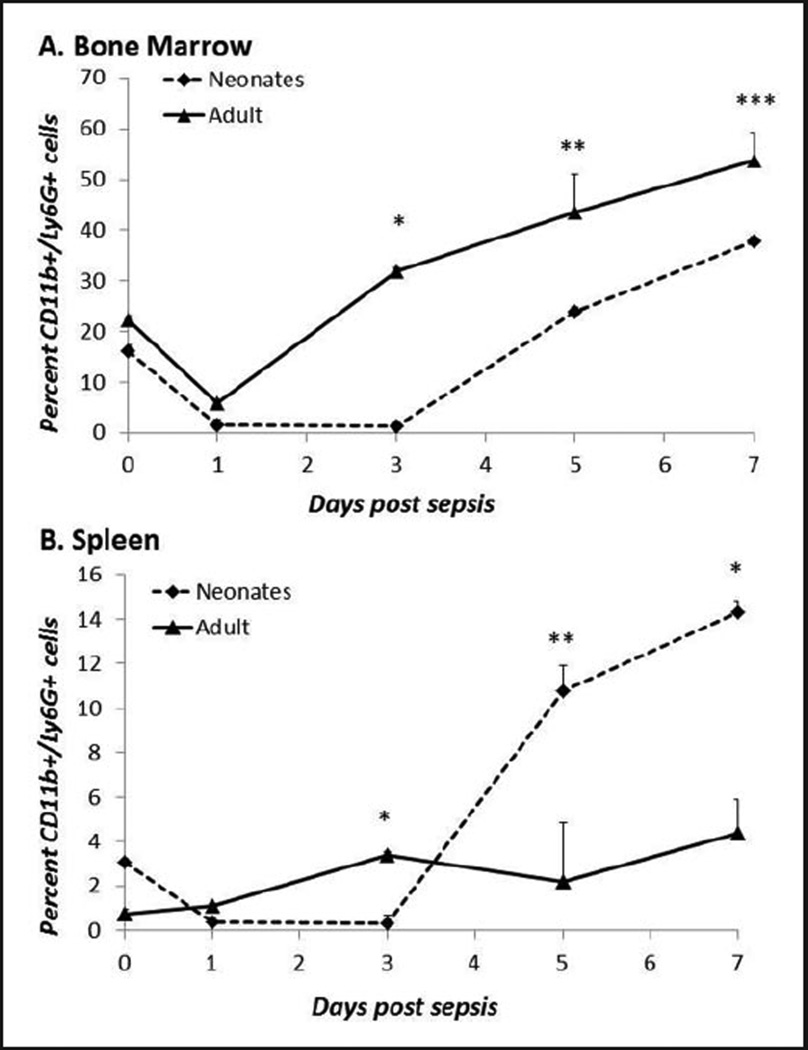
As described above, the process of emergency myelopoiesis is critical for the restoration of peripheral granulocytes following an inflammatory insult. While we and others have previously shown differences in the baseline innate immune function of neonatal granulocytes compared with adult granulocytes,8 emergency myelopoiesis has never been examined in the neonate following sepsis. First, we compared the kinetics of neutrophils in the bone marrow and spleen of both neonates and adults following induction of polymicrobial sepsis. We demonstrate that adults reach a nadir in bone marrow neutrophils at d 1, following CS, but then rebound to baseline levels by d 3 (Figure 1). In contrast, neonatal neutrophils within the bone marrow remained significantly lower than adults for an additional 2 d but rebound to baseline by 5 d in both the spleen and bone marrow following a similar septic insult (Figure 1A, B).
Figure 1.
Delayed restoration of neonatal bone marrow and splenic granulocytes following sepsis. (A) Bone marrow and (B) splenic cell suspensions were harvested at 0, 1, 3, 5 and 7 d post-sepsis and stained for granulocyte cell surface markers (n=4–5/group/time point). In neonates, sepsis is associated with the early and sustained depletion of granulocytes from bone marrow and spleen, as well as delayed reconstitution. (A) *P<0.001, **P=0.019, ***P<0.013. (B) *P<0.001, **P=0.007 by Student’s t-test.
Expansion of hematopoietic stem cells is impaired in neonates compared with adults
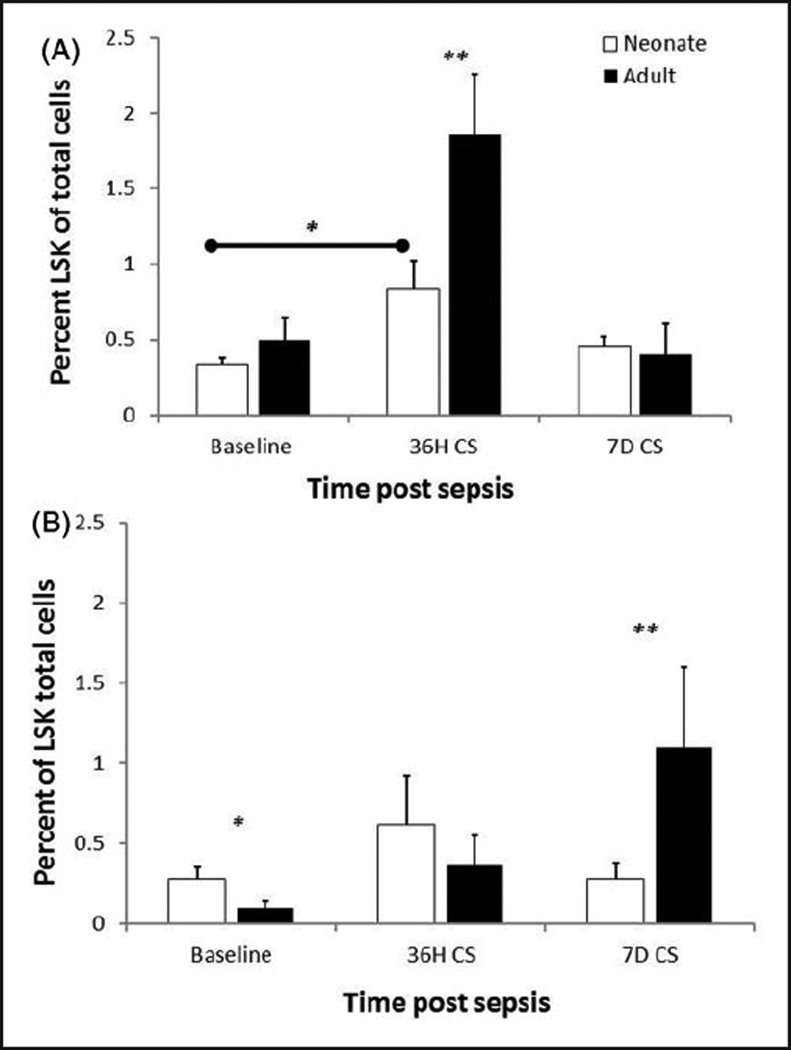
We and others have demonstrated that LSKs expand and that hematopoiesis is skewed towards myelopoiesis following sepsis.5,9,10 To examine if there are any defects in the ability of neonatal LSKs to expand following polymicrobial sepsis, we collected bone marrow and spleens from neonates and adults at several time points following polymicrobial sepsis. While both adult and neonatal populations expand in response to CS, neonatal LSK expansion is only 2.5-fold, whereas adult expansion is greater than 3.5 fold (P<0.05) (Figure 2). Similar findings were observed in the spleen at d 7 in the adult compared with the neonate.
Figure 2.
Decreased expansion of neonatal LSKs following sepsis. (A) Bone marrow and (B) Splenic LSKs were determined at baseline (0 h), 36 h and 7 d after CS. The expansion of bone marrow LSKs was markedly attenuated in neonates compared with young adults. Extramedullary (splenic) expansion of LSKs was also attenuated in the neonates at 7 d post-sepsis (n=4–5/group/time point). *P<0.05, **P<0.001 by Student’s t-test.
Decreased expansion of both neonatal GMP and CMP during sepsis
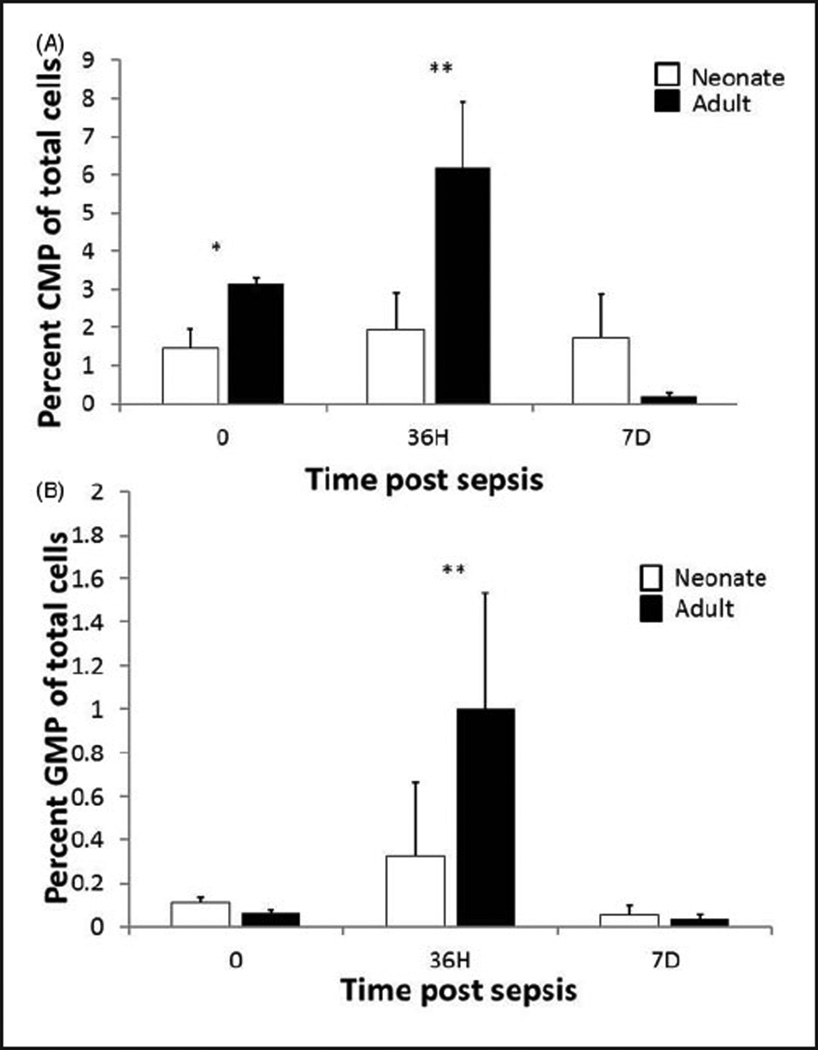
To examine whether or not a similar pattern occurred throughout the myeloid lineage, we stained bone marrow suspensions for CMPs and GMPs, and analyzed them via flow cytometry. As demonstrated in Figure 3, we found that following a septic insult, adults had a significantly greater percentage of CMPs at baseline (0 h) and 36 h, and a greater percentage of GMPs at 36 h. No differences were found between the two groups at 7 d.
Figure 3.
Decreased expansion of neonatal CMPs and GMPs following sepsis. Bone marrow was harvested and stained for CMP and GMP markers at 0, 36 h and 7 d post-CS (n=4–5/group/time point). *P<0.05, **P<0.01 by Student’s t-test.
Decreased formation of neonatal myeloid progenitors following in vitro stimulation
While the expansion of LSKs and myeloid lineage progenitors following polymicrobial sepsis is less than the adult, it is possible that this may either be related to differences in the microenvironment/niche between the neonate and the adult. Alternatively, this difference may be directly attributable to differences in the phenotype of the LSKs. To examine whether or not these factors contributed to the observed differences between the two age groups, we collected bone marrow from neonates and adults at baseline (0 h), 36 h and 7 d post-sepsis, sorted LSKs and then stimulated them in vitro with several myelopoietic growth factors.
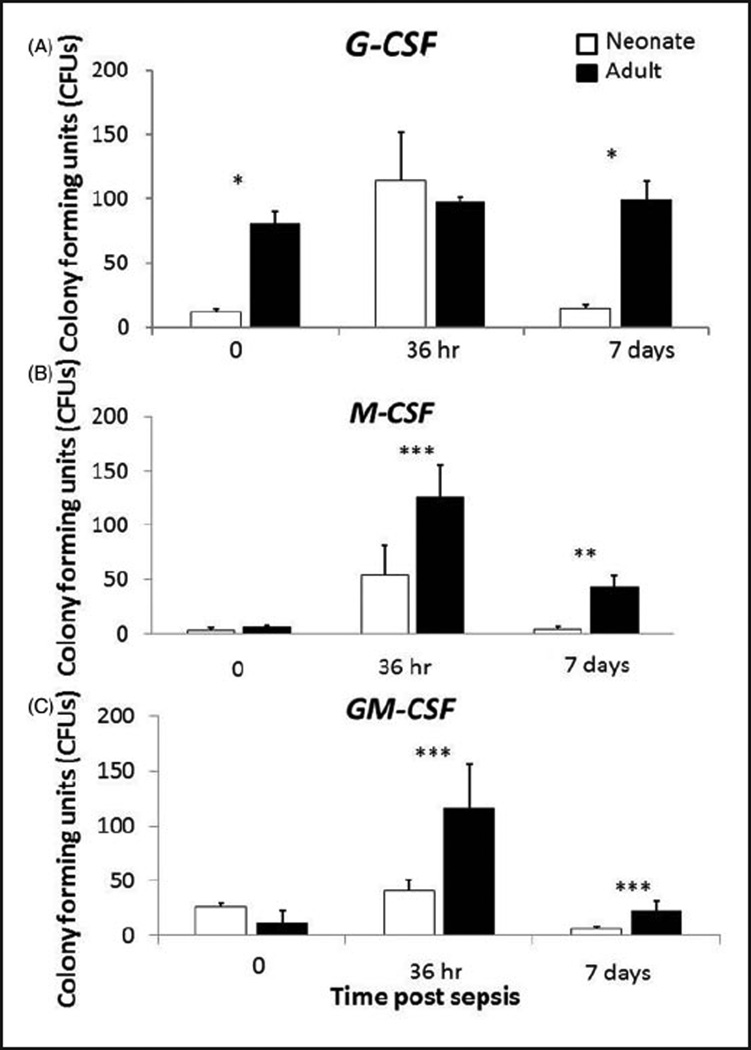
While similar numbers of colonies were produced at 36 h post-sepsis in response to G-CSF, adult LSKs produced significantly more colonies in response to G-CSF at baseline and 7 d post-sepsis (P<0.01). In addition, adult LSKs produced more colonies than neonatal LSKs in response to M-CSF and GM-CSF at 36 h and 7 d post sepsis (all P<0.05) (Figure 4).These data suggest that adult LSKs are better able to respond to growth factors produced following systemic insult to repopulate lost innate immune effector cells.
Figure 4.
Poor in vitro production of CFUs of neonatal LSKs in response to myelopoietic growth factors. Bone marrow was harvested from neonates at baseline (0 h), 36 h and 7 d post-sepsis, and LSKs were sorted via flow cytometry. LSKs were then plated at a density of 500 cells/35-mm plate in the presence of G-CSF (100 ng/ml), M-CSF (50 ng/ml) and GM-CSF (10 ng/ml). Interestingly, the proliferative capacity of neonatal and young adult LSKs to various myeloid growth factors varied dramatically. (A) In response to G-CSF, neonatal LSKs had little baseline proliferative activity that was dramatically increased by sepsis, whereas young adult LSKs were greater at baseline and were unaffected by sepsis. (B, C) In contrast, the proliferative response to GM-CSF and M-CSF was attenuated in neonates after sepsis when compared with young adults (n=4–5/group/time point). *P<0.001, **P<0.01, ***P<0.05 by Student’s t-test.
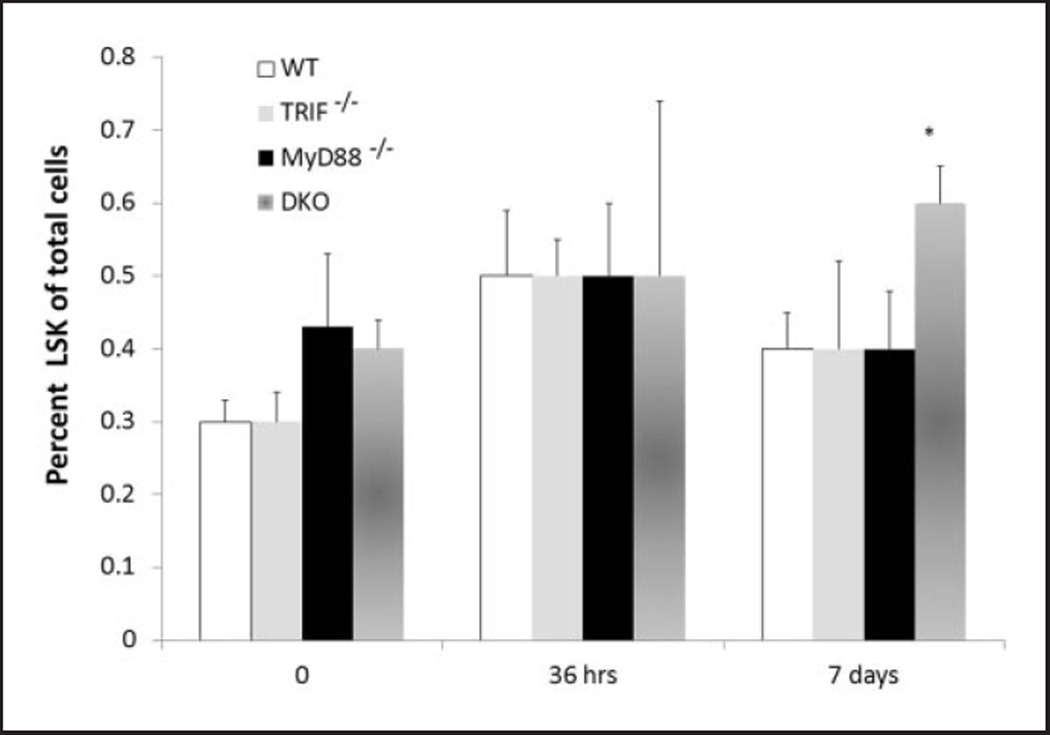
Loss of TLR signaling does not affect the expansion of neonatal LSKs following sepsis
While TLR signaling has been demonstrated to be important for the activation of innate immunity, their role in emergency myelopoiesis is controversial.11 Boettcher et al. have demonstrated that the expansion of LPS-induced emergency myelopoiesis was dependent on the expression of TLR on non-hematopoietic cells.12 We have shown that expansion of LSKs following sepsis is not dependent on TLR signaling.5 While TLR signaling does not play a role in the expansion of LSKs as an adult, many studies have documented differences in TLR signaling between neonates and adults, suggesting that they may control neonatal LSK expansion. To examine this, WT, TRIF−/−, MyD88−/−, or TRIF−/−, MyD88−/− neonatal mice were exposed to CS; bone marrow and splenocytes were harvested at baseline (0 h), 36 h and 7 d post-sepsis. As demonstrated in Figure 5, no differences in the expansion of LSKs were observed between groups. These data suggest that, similar to data obtained in adults, TLR signaling does not play a role in the expansion of LSKs in the neonate in response to a polymicrobial sepsis.
Figure 5.
Deletion of TLR signaling does not affect the expansion of neonatal LSKs following sepsis. LSKs were obtained from bone marrow of WT B6, MyD88−/−, TRIF− and both MyD88−/− TRIF−/−(DKO) at baseline (0 h), 36 h and 7 d after sepsis (n=4–5/group/time point). *P=0.036.
Discussion
Many investigators have demonstrated distinct functional differences in immunological response to sepsis between neonates and adults. We have previously demonstrated that murine neonates have increased susceptibility to polymicrobial sepsis when compared with adults.6,8 One key difference that exists between the two groups is a greater reliance of neonates on the innate immune response.2 In addition, the production of cytokines/chemokines are skewed towards Th2 responses in neonates, while important for humoral responses, may suppress innate immune responses through the production of IL-10 and TNF-α.13 This report adds to this body of knowledge by demonstrating that emergency myelopoiesis in the neonate is quantitatively different than in the young adult in response to sepsis.
Overall, we show that neonates have both delayed and impaired granulopoiesis in response to sepsis compared with adults. Neonatal LSKs and myeloid progenitors are also less able to expand during sepsis. LSKs from neonates produce significantly fewer colonies ex vivo in response to myelopoietic growth factors. In addition, despite reports that this expansion may be activated by TLR signaling, our data suggest that TLR signaling is not obligatory in polymicrobial sepsis as TRIF−/−, MyD88−/− neonatal LSKs are still able to expand. Similar findings in adult polymicrobial sepsis have been described by our group.5
Despite our findings in both adults and neonates that the expansion of LSKs following polymicrobial sepsis can occur in the absence of TLR signaling, several investigators utilizing different models of infection or inflammation have demonstrated a reliance on TLR signaling in emergency granulopoiesis. For example, the expansion of LSKs in both Mycobacterium or candida infection has been suggested to be TLR2-dependent in adults.14,15 Further, adoptively transferred WT LSKs from Ly 5.1+ mice into Ly5.2+ TLR2−/−, TLR4−/− and MyD88−/−, which allowed investigators to track responses in Ly 5.1+ cells, have also been shown to proliferate in response to TLR2, 4 and 9 ligands.16 In addition, Bugl et al. demonstrated that repopulation of neutrophil stores following Gr-1 Ab depletion was reliant on G-CSF–TLR4–TRIF-dependent signaling.17 While these findings suggest that TLR signaling may play a role in the expansion of LSKs or emergency myelopoiesis following infection, the experimental models used are different than the one used in the studies above, and utilize either monomicrobial infections or Ab depletion. In addition, our studies do not necessarily discount the possibility that TLRs could play a role in the expansion of LSKs under certain conditions; however, in the setting of polymicrobial sepsis, the induction of many non-TLR innate immune signaling pathways (i.e. NOD-like receptors) are likely also stimulated and could, therefore, ostensibly participate in the process of emergency granulopoiesis.
Regardless, the implications of these findings are significant. Although we and others have shown a myriad of immunological deficiencies in innate immunity,18 few authors have explored the mechanisms by which cells of the innate immune system are generated and repleted in the septic neonate. The findings are discouraging in the sense that neonates have an attenuated and delayed ability to repopulate their bone marrow, and subsequently their peripheral populations. This defect appears to be an intrinsic property of progenitor cells from neonates, in that when stimulated ex vivo with optimal quantities of growth factors, their proliferation and CFU production are markedly reduced. Such findings suggest that developmental processes of LSKs and early progenitors are still rudimentary in the neonate and not well prepared for systemic inflammation. The findings increase interest in interventional approaches to target hematopoietic stem cell expansion and development.
There are several limitations to this report that deserve mention. Unfortunately, because of the differences in size and cell number between neonates and adults, it is possible that simply comparing the changes in the percentage of cells within a given hematopoietic compartment is not relevant and other metrics may be better suited to demonstrate that there are differences between these two groups. This was tempered by sorting neonatal and adult LSKs and stimulating a fixed number in vitro with various myelopoietic growth factors, which supported our previous finding that neonatal LSKs are, indeed, impaired in their ability to expand. In addition, as hematopoiesis in the fetus shifts from the liver and spleen to the bone marrow, it is possible that what we are observing is simply an artifact of the differences in hematopoietic compartments between these two groups. To account for this, we analyzed the spleen and bone marrow separately in both age groups. Interestingly, we observed impairment in both compartments in the neonate compared with the adult.
In conclusion, these findings highlight profound differences in immunologic response to sepsis between neonates and adults. Importantly, they also suggest a possible avenue for therapeutic design as improving the neonatal emergency myelopoietic response to inflammation would likely not only improve immunological defense against a primary insult, but also against subsequent infection. While beyond the scope of the current study, future efforts will examine whether or not these differences in the generation of myeloid-derived cells are related to the development of the immunological compartment as it matures from fetal to adult life.
Acknowledgments
Funding
Supported, in part, by GM-40586 and GM-81923, awarded by the National Institute of General Medical Sciences (NIGMS). AGC and LFG were supported by the Ruth Kirschstein National Research Service Award (NRSA) training grant (T32 GM-08721) in burns and trauma, awarded by the National Institute of General Medial Sciences (NIGMS). AGC was also supported by an individual NRSA training grant (F32 GM-093665), awarded by NIGMS.
References
- 1.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 2.Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuenca AG, Delano MJ, Kelly-Scumpia KM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2010;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delano MJ, Scumpia PO, Weinstein, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scumpia PO, Kelly-Scumpia KM, Delano MJ, et al. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn JL, Scumpia PO, Delano MJ, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 7.Delano MJ, Kelly-Scumpia KM, Thayer TC, et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol. 2011;187:911–918. doi: 10.4049/jimmunol.1100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca AG, Wynn JL, Kelly-Scumpia KM, et al. Critical role for CXCL10 (IP-10)/CXCR3 signaling in the murine neonatal response to sepsis. Infect Immun. 2011;79:2746–2754. doi: 10.1128/IAI.01291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra R, Villanueva E, Feketova E, et al. Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency. J Leukoc Biol. 2008;83:1541–1550. doi: 10.1189/jlb.1207838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santangelo S, Gamelli RL, Shankar R. Myeloid commitment shifts toward monocytopoiesis after thermal injury and sepsis. Ann Surg. 2001;233:97–106. doi: 10.1097/00000658-200101000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Boettcher S, Ziegler P, Schmid MA, et al. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J Immunol. 2012;188:5824–5828. doi: 10.4049/jimmunol.1103253. [DOI] [PubMed] [Google Scholar]
- 13.Levy O. Innate immunity of the newborn:basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 14.Yanez A, Megias J, O’Connor JE, et al. Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling. PLoS One. 2011;6:e24761. doi: 10.1371/journal.pone.0024761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HH, Kim KK, Kim KD, et al. Effects of mycobacterial infection on proliferation of hematopoietic precursor cells. Microbes Infect. 2011;13:1252–1260. doi: 10.1016/j.micinf.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Megías J, Yáñez A, Moriano S, et al. Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells. 2012;30:1486–1495. doi: 10.1002/stem.1110. [DOI] [PubMed] [Google Scholar]
- 17.Bugl S, Wirths S, Radsak MP, et al. Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood. 2013;121:723–733. doi: 10.1182/blood-2012-05-429589. [DOI] [PubMed] [Google Scholar]
- 18.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37:439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]