Abstract
Known for centuries, the geographical pattern of increasing biodiversity from the poles to the equator is one of the most pervasive features of life on Earth. A longstanding goal of biogeographers has been to understand the primary factors that generate and maintain high diversity in the tropics. Many ‘historical’ and ‘ecological’ hypotheses have been proposed and debated, but there is still little consensus. Recent discussions have centred around two main phenomena: phylogenetic niche conservatism and ecological productivity. These two factors play important roles, but accumulating theoretical and empirical studies suggest that the single most important factor is kinetics: the temperature dependence of ecological and evolutionary rates. The relatively high temperatures in the tropics generate and maintain high diversity because ‘the Red Queen runs faster when she is hot’.
Keywords: Ecological interactions, evolutionary rates, Janzen–Connell dynamics, latitudinal diversity gradient, metabolic theory, Red Queen, species diversity, tropics
There is, however, one natural feature of this country, the interest and grandeur of which may be fully appreciated in a single walk: it is the ‘virgin forest’. Here no one who has any feeling of the magnificent and the sublime can be disappointed; the sombre shade, scarce illumined by a single direct ray even of the tropical sun, the enormous size and height of the trees, most of which rise like huge columns a hundred feet or more without throwing out a single branch, the strange buttresses around the base of some, the spiny or furrowed stems of others, the curious and even extraordinary creepers and climbers which wind around them, hanging in long festoons from branch to branch, sometimes curling and twisting on the ground like great serpents, then mounting to the very tops of the trees, thence throwing down roots and fibres which hang waving in the air, or twisting round each other form ropes and cables of every variety of size and often of the most perfect regularity. These, and many other novel features – the parasitic plants growing on the trunks and branches, the wonderful variety of the foliage, the strange fruits and seeds that lie rotting on the ground – taken altogether surpass description, and produce feelings in the beholder of admiration and awe. It is here, too, that the rarest birds, the most lovely insects, and the most interesting mammals and reptiles are to be found. Here lurk the jaguar and the boa-constrictor, and here amid the densest shade the bell-bird tolls his peal.
Alfred Russel Wallace on tropical forest in Brazil in his 1849 letter to the members of the Mechanics' Institution, published in Wallace, 1905 (p. 270).
Introduction
For more than three centuries Western science has known that biodiversity is greatest in the tropics. European explorers and traders returned from Africa, Asia and the Americas with thousands of specimens of previously unknown kinds of animals and plants. Many were sent to Carl Linnaeus, who by his last edition of Systema Naturae in 1758, had catalogued 7700 species of plants and 4400 species of animals, including 17 species of hummingbirds from South America and the Caribbean (Linnaeus, 1758). Explorer-naturalists who accompanied voyages to the tropics were awed by the variety of species, form and function, and wrote accounts comparable to Wallace's letter from Brazil (above). Many giants of 19th century natural science, including Joseph Banks, Thomas Belt, Alexander von Humboldt, Joseph Dalton Hooker, Charles Darwin, Henry Walter Bates and Alfred Russel Wallace, were indelibly influenced by their exposure to tropical biodiversity.
In the mid-20th century, most of the great synthesizers who laid the foundations of modern evolution, systematics, biogeography and ecology commented on the pattern of increasing biodiversity from the poles to the equator (e.g. Theodosius Dobzhansky, Ernst Mayr, Charles Elton, Evelyn Hutchinson, Philip Darlington, Alfred Fischer, Geoge Gaylord Simpson, Robert MacArthur and Edward O. Wilson). But the data were still sketchy and there was little consensus as to the causal processes. Within the last few decades, biogeographers have taken advantage of new biological inventories of previously poorly studied taxa and regions and of technological advances in computers, electronic databases and geographical information systems to quantify and clarify the empirical patterns. It is now clear that the tropics harbour not only the most species of plants and animals, but also the most diverse genomes, clades of higher taxa (e.g. Willig et al., 2003; Lomolino et al., 2010), and even languages and cultures of subsistence human societies (Collard & Foley, 2002; Pagel & Mace, 2004; Gavin et al., 2013). And the pattern is ancient, apparent in the fossil record dating back hundreds of millions of years (e.g. Stehli et al., 1969; Crane & Lidgard, 1989; Crame, 2001). Even as the patterns have become clearer, however, the explanations have remained elusive and controversial.
So why is life most diverse in the tropics? The number of hypotheses to explain the latitudinal diversity gradient (LDG) has only increased in recent decades. Pianka (1966) listed 6, Brown (1988) 8, Rohde (1992) 23, Willig et al. (2003) 27, and Lomolino et al. (2010) 32. These proliferating hypotheses are a mixed bag, ranging from specific ideas for restricted taxonomic or functional groups to general phenomena potentially applicable to all organisms. They invoke different kinds and levels of explanation from proximate to ultimate, random to deterministic, historical to ecological, abiotic to biotic. Most importantly, many are not alternatives in the sense that they offer mutually exclusive explanations. There are too many for me to review and evaluate all of them here.
Instead, I offer a personal overview: highlighting some theoretical and empirical advances, evaluating the present state of the art, and offering a unifying, but admittedly incomplete, synthesis. I focus my treatment on the LDG of species richness across spatial scales from local communities to regional biotas.
Background
Patterns: diversity of species, clades and cultures
The LDG is pervasive. It occurs in nearly all kinds of organisms – plants, animals and microbes – and environments – terrestrial, freshwater and marine. It occurs at all levels of evolutionary differentiation, not only at the species level, but also for intraspecific genetic and phenotypic differentiation and for lineages and higher taxa of multiple species. Many examples are shown in Lomolino et al. (2010; see also Willig et al., 2003; Hawkins et al., 2012).
Although the LDG is pervasive, it is definitely not universal. There are many clear exceptions. For example, species of conifers, amphipods, crayfish, ichneumonid wasps, voles and penguins are most diverse at mid- or high latitudes and greatly reduced or absent in the tropics (e.g. Willig et al., 2003; Lomolino et al., 2010). These exceptions are among the ‘natural experiments’ that offer potentially powerful insights into the mechanisms that generate the more general pattern.
Processes: historical and ecological
Efforts to explain both the cases that exhibit LDGs and the cases that are exceptions have typically focused on effects of either ‘history’ or ‘ecology’. The historical hypotheses suggest that the LDGs are legacies of past geological, climatic and evolutionary events, most of which occurred thousands to millions of years ago. Some historical hypotheses are non-equilibrial in the sense that they propose that diversity is still changing in a lagged response to past perturbations. An example is the idea that the LDG in at least some groups in North America and Eurasia is a legacy of past glaciations: the high latitudes were uninhabitable or inhospitable during the glacial epochs, and there has not been sufficient time for animals and plants to disperse and adapt to the habitats that became available during the interglacial periods, including the current one (e.g. Fischer, 1960; Hortal et al., 2011). Other historical hypotheses suggest that many LDGs reflect a longstanding, approximately steady-state relationship between the abiotic template of the Earth and the evolutionary processes that have shaped biodiversity. An example is the out of the tropics hypothesis which is discussed in more detail below.
In contrast to historical hypotheses, ecological hypotheses assume that, regardless of their evolutionary origin, most LDGs were originally caused and are now maintained by biological responses to the Earth's abiotic template, especially variables that are due to solar radiation. The poles-to-equator gradients of temperature and seasonality and the corresponding gradient of biodiversity have existed for hundreds of millions of years, although the details of the patterns have fluctuated over time in response to tectonic events, Milankovich orbital cycles and other factors. One class of ecological hypotheses invokes productivity: there is a latitudinal gradient of primary production, and the more productive tropics support more individuals apportioned among more species. A second class invokes niche relationships: adaptations to some combination of abiotic conditions and biotic interactions allow tropical species to be more specialized, dividing resources more finely among more species.
The Role of History
Out of the tropics
Both fossil and phylogenetic reconstructions provide compelling evidence that most lineages originated in the tropics. The relevant dynamics are perhaps most simply and cogently presented in the ‘out of the tropics’ model (Jablonski et al., 2006; Roy et al., 2009; Cavender-Bares et al., 2011; Bowen et al., 2013) and supporting empirical evidence, mostly from the fossil record. Here is my depiction: (1) rates of origination of new species are highest in the tropics; (2) higher rates of speciation than extinction generate high diversity of species and clades within the tropics; (3) most species and clades of tropical origin remain confined to low latitudes, because abiotic environmental constraints inhibit colonization and range expansion out of the tropics; (4) a minority of tropical species overcome these constraints and expand their ranges to colonize and sometimes diversify secondarily at higher latitudes; and (5) at these latitudes high rates of extinction result in lower standing stocks of species and clades. According to this model the tropics are both a ‘cradle’, because most lineages originate there, and a ‘museum’, because some of these lineages survive for long periods. Progressively higher latitudes tend to contain progressively younger species and clades, because of the higher extinction rates. The predicted patterns appear to be well supported by the fossil record, especially when sampling effort and other complications are taken into account (Jablonski et al., 2006).
Niche conservatism
A complementary historical hypothesis invokes ‘phylogenetic niche conservatism’ (Wiens & Graham, 2005; Hawkins et al., 2007, 2012; Donoghue, 2008; Wiens et al., 2010; Buckley et al., 2010; Cavender-Bares et al., 2011). My interpretation is as follows: (1) closely related species tend to share similar traits inherited from their common ancestors; (2) among these phylogenetically conservative traits are niche attributes, requirements and tolerances for environmental conditions; (3) tropical environments with relatively warm, aseasonal climatic regimes have been present throughout most of Earth's history, whereas more extreme conditions, including periods of continental glaciation, have been more intermittent; (4) because of a long evolutionary history in relatively equable environments, most tropical species and lineages cannot tolerate the abiotic stresses at higher latitudes – especially cold temperature, low water (in terrestrial environments), and extreme seasonality – and so they are restricted to the tropics; (5) novel adaptive traits are required to tolerate stressful abiotic conditions and expand ranges to higher latitudes; and (6) the increasing severity of stress acts as a filter, resulting in a decreasing number of species and lineages with increasing latitude. The predicted patterns appear to be generally supported by phylogenetic analyses of trait evolution, climatic niche models, palaeoclimatic conditions and geographical range limits of naturalized exotic species. Tropical species experience and are adapted to only a narrow and equable range of abiotic conditions (e.g. Janzen, 1967; Terborgh, 1973; Gaston & Chown, 1999; Colwell, 2011). So, niche conservatism offers historical, ecological and evolutionary mechanisms to explain why only a minority of species and lineages have expanded out of the tropics to colonize and sometimes diversify in the more variable and stressful environments at higher latitudes.
Together, the out of the tropics and niche conservatism hypotheses provide a compelling account of the historical dynamics of the LDG. I do not question most parts of this explanation. In my judgement, however, several issues still need to be addressed. Why are rates of speciation highest in the tropics? How does variation in speciation and extinction rates and the severity of abiotic conditions across latitudes generate and maintain the standing stocks of species richness seen in the LDGs? What are the implications of the exceptions, such as the diversification in tropical South America of lineages of placental mammals and cultures of aboriginal humans that colonized the New World relatively recently via the cold, seasonal environment of the Bering land bridge?
The Role of Ecology
It is apparent that the above ‘historical’ hypotheses ultimately rely, implicitly and explicitly, on ‘ecology’. G.E. Hutchinson (1959, p. 347) perceptively addressed these issues in his famous Homage to Santa Rosalia:
If we can have one or two species of a large family adapted to the rigors of Arctic existence, why can we not have more? It is reasonable to suppose that the total biomass may be involved. If the fundamental productivity of an area is limited by a short growing season to such a degree that the total biomass is less than under more favorable conditions, then the rarer species in a community may be so rare that they do not exist. It is also probable that certain absolute limitations on growth-forms of plants, such as those that make the development of forest impossible above a certain latitude, may in so acting, severely limit the number of niches.
Here Hutchinson, in just a few elegantly worded sentences, addresses the role of ecology in generating and maintaining the LDG: (1) how productivity ultimately limits the total biomass of living matter; (2) how that biomass is apportioned among the ‘number of niches’ and hence among species; and (3) how rare species with specialized niches persist in the face of extinction.
Productivity
For the last few decades, the main ecological explanation for the LDG has been that regions of high productivity have higher biodiversity because more species can obtain sufficient resources to maintain viable populations. This explanation is based on the well-documented pattern that terrestrial net primary production, which is controlled largely by temperature and seasonality, increases from effectively zero at the poles to a maximum in the lowland wet tropics. Consequently, resources to support organisms of all trophic levels and most lifestyles are most abundant in the tropics, so these resources can be divided among more species with each getting ‘a large-enough piece of the pie’ to persist in the face of stochastic extinction. The productivity hypothesis has been advanced in several forms by multiple investigators (e.g. Hutchinson, 1959; Connell & Orias, 1964; MacArthur, 1965, 1972; Pianka, 1966; Brown, 1981; Wright, 1983; Currie, 1991; O'Brien et al., 1998).
There can be no doubt that the LDG is ultimately due, at least in part, to productivity. The poles and driest deserts are nearly devoid of life because they are simply too cold or too dry for organisms to survive and reproduce there. Fundamental physical, chemical and biological constraints limit the capacities of organisms to convert energy and nutrients into biomass (e.g. O'Brien et al., 1998). In contrast, the tropics teem with life because the warm moist environment offers relatively benign abiotic conditions and abundant resources. Recently, the metabolic theory of ecology (MTE; Brown et al., 2004; Sibly et al., 2012) and empirical research in ecosystem ecology have made progress in synthesizing our understanding of the linkages between physiochemical limits on biological metabolism and the major environmental variables that affect photosynthesis and respiration. Nearly all of the energy that supports life comes from the sun, and the rate of photosynthesis or net primary production (NPP) is limited primarily by temperature and water in terrestrial environments and by nutrients and solar radiation in marine environments. NPP sets absolute limits on total resource use, biomass and number of individuals in an ecosystem, although there are tradeoffs, largely due to body size, in how energy and biomass are apportioned among individuals.
Strong correlations between species richness and NPP in terrestrial environments led many ecologists, myself included, to infer that productivity affects biodiversity in the way that Hutchinson suggested, by limiting the number of individuals per species that could persist in the face of extinction (e.g. Brown, 1981; Wright, 1983; Currie, 1991; Wright et al., 1993; Fraser & Currie, 1996; Francis & Currie, 1998; Kaspari et al., 2000; Kilpatrick et al., 2006). Now, however, several lines of evidence suggest that this mechanism, by itself, is inadequate to explain the ubiquity and magnitude of LDGs in different organisms and habitats.
Kinetics
In 1992 Klaus Rohde (see also Fischer, 1960) reviewed the hypotheses and supporting evidence for the LDG, and proposed a relatively novel mechanism: ‘It is concluded that greater species diversity is due to greater “effective” evolutionary time (evolutionary speed) in the tropics, probably as the result of shorter generation times, faster mutation rates, and faster selection at greater temperatures’ (Rohde, 1992, p. 514). Rohde suggested that the effect of temperature on physiological processes causes faster rates of evolution and more rapid responses to selection in the tropics. Empirical studies have provided strong support for the idea that biotic interactions are ‘more important’ in the tropics, and that the density-dependent relationships play a major role in generating and maintaining the LDG (e.g. Janzen, 1970; Connell, 1971; MacArthur, 1972; Sax, 2001; Mittelbach et al., 2007; Schemske et al., 2009; Comita et al., 2010; Ricklefs, 2010; Swamy & Terborgh, 2010; Johnson et al., 2012; Terborgh, 2012). But it is not clear how temperature or other abiotic factors that vary with latitude affect the rates and outcomes of interactions so that ‘diversity begets diversity’.
The MTE has modelled explicitly and quantitatively the linkages between temperature, metabolic biochemistry, and physiological, ecological and evolutionary rates (e.g. Allen et al., 2002, 2006; Brown et al., 2004; Savage et al., 2004; Gillooly et al., 2005; Gillooly & Allen, 2007; Sibly et al., 2012). The theory assumes that over a biologically realistic range of temperatures, biological rates increase exponentially with temperature: 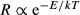 , where R is the rate of some process such as metabolism, population growth or speciation, e is the root of the natural logarithm, E is an ‘activation energy’ that gives the temperature dependence, k is Boltzmann's constant (8.62 × 10−5 eVK−1), and T is temperature in Kelvin (Gillooly et al., 2001; Brown et al., 2004; Sibly et al., 2012). The theory is still incomplete, but it provides a framework for analysing temperature dependence of phenomena related to biodiversity. I show an example, using preliminary data on forests from our National Science Foundation (NSF)-supported Macrosystems research project (Table1). The upper panel of Fig.1 shows productivity as a function of average annual temperature in an ‘Arrhenius plot’, where the logarithm of NPP is plotted as a function of inverse temperature, 1/kT. The relationship is highly significant, with E = 0.46, which is intermediate between empirical values for rates of photosynthesis and secondary succession (E ≈ 0.33) and respiration (E ≈ 0.65; Allen et al., 2005; Anderson et al., 2006). The lower panel of Fig.1 shows the temperature dependence of tree abundance and species richness. Both relationships are significant, but the relationship is much stronger for species than for individuals (E = 0.80 and 0.16, respectively). Similar relationships for species richness have been published elsewhere (e.g. Allen et al., 2002, 2006; Allen & Gillooly, 2006; Wang et al., 2009).
, where R is the rate of some process such as metabolism, population growth or speciation, e is the root of the natural logarithm, E is an ‘activation energy’ that gives the temperature dependence, k is Boltzmann's constant (8.62 × 10−5 eVK−1), and T is temperature in Kelvin (Gillooly et al., 2001; Brown et al., 2004; Sibly et al., 2012). The theory is still incomplete, but it provides a framework for analysing temperature dependence of phenomena related to biodiversity. I show an example, using preliminary data on forests from our National Science Foundation (NSF)-supported Macrosystems research project (Table1). The upper panel of Fig.1 shows productivity as a function of average annual temperature in an ‘Arrhenius plot’, where the logarithm of NPP is plotted as a function of inverse temperature, 1/kT. The relationship is highly significant, with E = 0.46, which is intermediate between empirical values for rates of photosynthesis and secondary succession (E ≈ 0.33) and respiration (E ≈ 0.65; Allen et al., 2005; Anderson et al., 2006). The lower panel of Fig.1 shows the temperature dependence of tree abundance and species richness. Both relationships are significant, but the relationship is much stronger for species than for individuals (E = 0.80 and 0.16, respectively). Similar relationships for species richness have been published elsewhere (e.g. Allen et al., 2002, 2006; Allen & Gillooly, 2006; Wang et al., 2009).
Table 1.
Preliminary data for mean annual temperature, annual net primary productivity (NPP), number of individual trees and number of tree species at six forest sites of our Macrosystems project (five Long-Term Ecological Research sites and the Smithsonian's Barro Colorado Island). NPP data are from Kaspari et al. (2000) except for Luquillo, which is from http://daac.ornl.gov/NPP/guides/npp_doc.html. Data for number of individual trees > 0.25 cm d.b.h. and number of tree species in standardized modified ‘Gentry plots’ totalling 5000 m2 in area were collected by V. Buzzard, C. Sides, A. Henderson and B.J. Enquist.
| Site | Latitude | Longitude | Temperature (°C) | NPP (g C/m2/yr) | Number of individuals | Number of species |
|---|---|---|---|---|---|---|
| Niwot Ridge (Colorado, USA) | 40° 3′ 34″ N | 105° 37′ 1″ W | −4 | 180 | 2031 | 6 |
| H.J. Andrews Forest (Oregon, USA) | 44° 13′ 59″ N | 122° 10′ 34″ W | 9 | 200 | 2322 | 17 |
| Harvard Forest (Massachusetts, USA) | 42° 31′ 53″ N | 72° 11′ 23″ W | 7 | 450 | 1384 | 25 |
| Coweeta Forest (Georgia, USA) | 35° 3′ 38″ N | 83° 24′ 59″ W | 13 | 550 | 1730 | 52 |
| Luquillo Forest (Puerto Rico) | 18° 16′ 52″ N | 65° 47′ 58″ W | 23 | 1033 | 2128 | 96 |
| Barro Colorado Island (Panama) | 9° 9′ 7″ N | 79° 50′ 47″ W | 27 | 1310 | 4971 | 263 |
Figure 1.
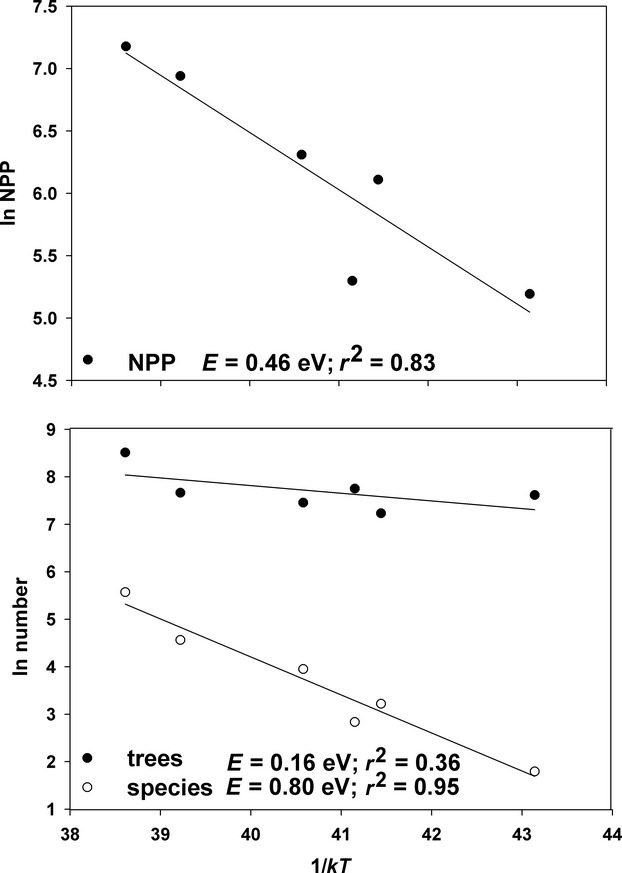
Temperature dependence of productivity, number of trees and number of tree species on the six forest study sites of our Macrosystems project (Table1). Data are presented as Arrhenius plots, with the natural logarithm of rate as a function of inverse temperature, 1/kT (so colder temperatures are to the right). Data were fitted by ordinary least squares regression: values of E give the slopes, the measure of temperature dependence, and values of r2 give the proportion of variation explained. Above: rate of net primary production (NPP); below: number of individual trees and number of tree species: data from V. Buzzard, C. Sides, A. Henderson and B.J. Enquist. Note that the temperature dependence for species richness was substantially higher than for NPP, which was substantially higher than for the number of individuals.
The important point to be made here, however, is that species diversity increases with increasing temperature much more rapidly than the number of individuals and NPP. This implies that productivity alone is not a sufficient explanation for the LDG. Instead, it suggests that the LDG is due in large part to relatively direct effects of kinetics. The higher temperatures in the tropics cause higher rates of metabolism, ecological dynamics and coevolutionary processes, which generate and maintain higher biodiversity.
Synthesis
I offer the synthetic framework outlined in Fig.2 and fleshed out below.
Figure 2.
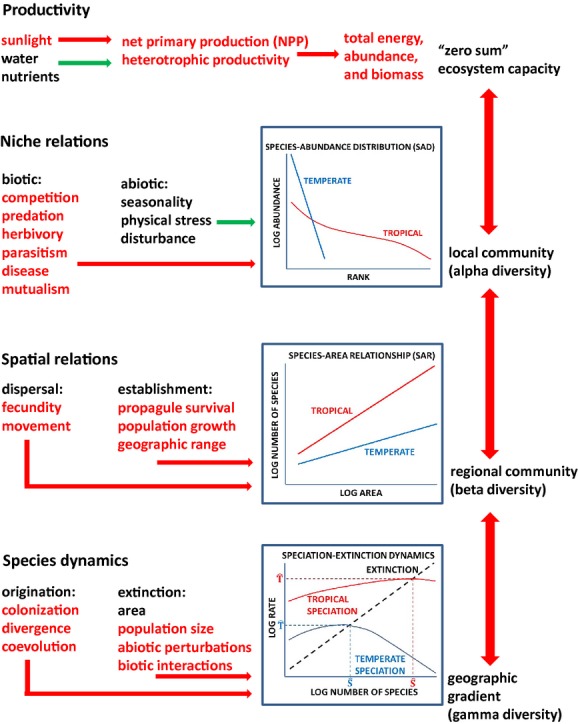
A tentative synthetic framework for the causal mechanisms that generate and maintain the latitudinal gradient of species diversity. Cause–effect relationships are indicated by arrows. Mechanisms that are at least in part temperature dependent, and hence consistent with the kinetics of metabolic theory, are in red. Other mechanisms are in green. Some emergent outcomes are shown in the graphs. The presentation is arranged in approximate order of increasing spatial scale and evolutionary time, starting with local ecological processes at the top and ending with regional species dynamics at the bottom. But the mechanisms operate both bottom-up and top-down, as indicated by the double-headed vertical arrows on the right, which indicate important feedbacks among processes and across scales. See text for additional explanation. Ŝ, carrying capacity for species; 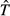 , turnover rate of species.
, turnover rate of species.
Productivity
The flow of energy through an ecosystem determines its capacity to support life. Net primary production (NPP) gives the whole-ecosystem rate of energy supply. NPP sets powerful constraints on total abundance and biomass of all organisms. Given a fixed NPP, an ecosystem can support either a high biomass composed of a relatively few large individuals or a low biomass composed of many small individuals (e.g. trees in a forest compared to planktonic algae in the ocean). The rate of photosynthesis increases with increasing temperature in both terrestrial plants and aquatic algae (Allen et al., 2005; Yvon-Durocher et al., 2010; Anderson-Teixeira & Vitousek, 2012). However, geographical variation in NPP is complicated by water limitation in terrestrial ecosystems (e.g. Lieth, 1975; O'Brien et al., 1998) and nutrient limitation in marine and freshwater ecosystems (e.g. Bunt, 1975; Smith, 1979; Moore et al., 2013).
The effect of productivity on diversity is positive, but modest: too small to account for the magnitude of the LDG. The number of species increases much more rapidly with decreasing latitude and increasing temperature than the increase in total ecosystem energy supply (NPP), biomass or abundance (Fig.1; see also Enquist & Niklas, 2001; Currie et al., 2004). The effect of productivity on biodiversity is addressed by species–energy theory and its extensions and empirical tests (Wright, 1983; Wright et al., 1993; Currie et al., 2004; Hurlbert, 2006). Species–energy relationships are conceptually similar to species–area relationships in island biogeography. The empirical patterns are comparable to other species–energy and species–area relationships (MacArthur & Wilson, 1967; Wright, 1983; Wright et al., 1993; Currie et al., 2004; Hurlbert, 2006), where a typical z-value of 0.25 would require four orders of magnitude increase in NPP to increase species richness by one order of magnitude (i.e. a 10,000-fold increase in productivity for a 10-fold increase in diversity). I conclude that there is a latitudinal gradient of productivity due to the temperature dependence of NPP (see below), and it plays a small but significant role in the LDG.
Niches
The LDG in alpha diversity depends, therefore, not so much on the rate of resource supply as on how these resources are apportioned among individuals and species. Indeed, it has long been known that tropical communities typically contain disproportionately more specialized and rare species than assemblages at higher latitudes (Klopfer & MacArthur, 1961; Hubbell, 1979, 2008). This is perhaps best depicted by comparing the shapes of ranked species-abundance distributions (SADs): when the number of individuals is scaled logarithmically, high latitude communities are typically steep and approximately linear, whereas tropical communities are much flatter and strongly curvilinear (Fig.2, top).
The temperature dependence of alpha diversity and the shape of SADs imply that local species richness is regulated by temperature-dependent ecological and evolutionary processes, mediated through the kinetics of metabolism as Rohde suggested in 1992. The kinetics of ecological interactions and coevolutionary adaptation result in species occupying more specialized niches in the tropics. Local species richness is indeed strongly temperature-dependent. Arrhenius plots of diversity as a function of inverse temperature typically have negative slopes, with E = 0.6–0.8 (e.g. Allen et al., 2002, 2006; Wang et al., 2009; Fig.1).
Metabolic theory has the potential to model and account for these relationships. There have been attempts to do so (e.g. Allen et al., 2002; : Brown et al., 2004; Allen & Gillooly, 2006; Wang et al., 2009), but I agree with David Storch (2012) that ‘a metabolic theory of biodiversity is a work in progress’. The obvious questions are: what are the temperature-dependent processes, and how do they generate and maintain standing stocks of local species richness? I believe that the answer lies primarily in the effect of temperature on interspecific ecological interactions and Red Queen coevolution. ‘Diversity begets diversity’ to generate the LDG, because ecological and evolutionary rates increase exponentially with temperature and are highest in the tropics. But we need theoretical and simulation models and relevant empirical studies to show how this occurs.
To go from typical log–linear temperate to curvilinear tropical SADs requires species-specific density-dependent processes that (1) suppress broad-niched dominant species, freeing up resources, and (2) facilitate the persistence of specialized species, allowing them to increase when rare, colonize from the metacommunity, and speciate. One phenomenon that has this effect is the Janzen–Connell process (Janzen, 1970; Connell, 1971). The idea is that ‘enemies’, some combination of predators, herbivores, parasites and diseases, have negative density-dependent impacts on their prey or hosts through both short-term ecological interactions and longer-term coevolutionary adaptations. The Janzen–Connell process has increasingly gained empirical support (e.g. MacArthur, 1972; Clark & Clark, 1984; Sax, 2001; Mittelbach et al., 2007; Schemske et al., 2009; Comita et al., 2010; Ricklefs, 2010; Swamy & Terborgh, 2010; Johnson et al., 2012; Terborgh, 2012).
It is far from clear, however, how temperature, by increasing the rate of interactions and coevolution, generates and maintains higher species richness. Recent meta-analyses have collected and analysed published data on temperature dependence of rates of ecological interactions, including competition, predation, herbivory and parasitism (Brown et al., 2004; Dell et al., 2011; Englund et al., 2011). The results are variable, sample sizes are generally small, and statistical resolution is correspondingly limited. A more comprehensive compilation and analysis reveals a clear central tendency, with values clustering around the value of E ≈ 0.65 as predicted by metabolic theory (W.R. Burnside, S.T. Hammond & J.H. Brown, unpublished). Additional theoretical and empirical studies are needed to show how warmer temperatures, by speeding up rates of interaction and coevolution, cause higher species diversity in the tropics.
Spatial relationships
The vast majority of empirical studies of the LDG have focused on alpha diversity or local species richness. Much less attention has been devoted to the spatial context of diversity, explicitly to beta diversity or spatial turnover in species composition. Higher beta diversity in the tropics, however, appears to be another pervasive feature of the LDG (Condit et al., 2002; Qian et al., 2004, 2009; Rodríguez & Arita, 2004; Qian & Ricklefs, 2007; Wang et al., 2009; Kraft et al., 2011). This phenomenon is expressed on a range of spatial scales. With decreasing latitude and increasing temperature: (1) similarity in species composition of local communities decreases more rapidly with increasing distance between samples (distance-decay relationships: e.g. Nekola & White, 1999); (2) the number of species increases more rapidly with increasing sample area (species–area relationships: Fig.2, middle; e.g. Wang et al., 2009); and (3) species occupy smaller geographical ranges and a narrower range of abiotic environmental conditions (Rapoport's rule: e.g. Rapoport, 1982; Stevens, 1989; Brown et al., 1996).
These patterns of beta diversity are consistent with the effect of temperature on ecological interactions and coevolutionary processes as outlined above. In a spatial context, ‘diversity begets diversity’ because biotic resistance from other species restricts species to their specialized niches and limits their capacities to disperse and invade other environments, which also have a diverse complement of well-adapted species. The patterns of beta diversity are uniquely explained by biotic limiting factors; otherwise the relatively benign conditions in the tropics should allow species to be widespread (e.g. Terborgh, 1973; Gaston & Chown, 1999; Colwell, 2011).
Again, it remains to be explained how temperature generates and maintains these patterns of beta diversity. Critical here is the effect of temperature on dispersal. I suggest that in general, temperature tends to increase the rates but decrease the distances of dispersal. On the one hand, the large number of very rare species in tropical communities suggests that relatively high rates of colonization of species from some larger, more extensive metacommunity are essential to replenish local richness after stochastic extinctions. On the other hand, the small ranges and difficulty of invading distant sites due to biotic resistance suggest that long-distance dispersal is rare and probably maladaptive for most tropical species. Metabolic theory addresses the temperature dependence of metabolic rate, life history traits and dispersal distance in ectothermic organisms. The effect of temperature on development and lifespan predicts shorter dispersal distances in at least some tropical organisms. One example is planktonic larvae, the primary dispersal stage of benthic marine algae, invertebrates and fish. In warmer environments larvae develop more rapidly and consequently disperse over shorter distances (O'Connor et al., 2007). And finally, higher rates of ecological interactions with enemies and the resulting mortality should additionally limit dispersal distances and establishment of propagules in the tropics.
Species dynamics
Ultimately the generation and maintenance of diversity depends on species dynamics, on how rates of colonization, speciation and extinction vary with the number of species. When speciation rate exceeds extinction rate, the number of species will tend to increase exponentially; when extinction rate exceeds speciation rate, the number of species will tend to decline exponentially towards zero. Because such exponential trajectories cannot be continued for very long, species diversity must come to an approximate equilibrium or steady state between rates of origination and extinction. So, it is really this ‘carrying capacity for species’, rather than speciation and extinction rates per se, that maintains species diversity over both ecological and evolutionary time.
It is constructive to develop a simple graphical model, similar to MacArthur & Wilson's (1967) equilibrium model of island biogeography (Fig.2, bottom). Here, both speciation and extinction rates are depicted as increasing functions of species richness, but the extinction curve is steeper than the speciation curve so that they cross to give a stable equilibrium. I have drawn the graph with a single extinction curve for both temperate and tropical environments, but with a higher rate of speciation for any given richness, S, in the tropical environment, reflecting how ‘diversity begets diversity’ due to the rates of biotic interactions and coevolutionary processes (see also Emerson & Kolm, 2005). This model predicts a higher ‘carrying capacity’ for species, Ŝ, in the tropics, maintained by a higher turnover rate of species,  , i.e. higher rates of both speciation and extinction at this equilibrium. There is much room to elaborate and improve on this model: e.g. changing the shapes and positions of the curves, making the extinction rates different in the tropic and temperate environments, and adding a colonization rate curve to depict species migrating out of the tropics into the temperate zone, and so on. Nevertheless, this simple model can be useful in guiding our thinking about the fundamentals of species dynamics and the temperature dependence of the underlying rate processes.
, i.e. higher rates of both speciation and extinction at this equilibrium. There is much room to elaborate and improve on this model: e.g. changing the shapes and positions of the curves, making the extinction rates different in the tropic and temperate environments, and adding a colonization rate curve to depict species migrating out of the tropics into the temperate zone, and so on. Nevertheless, this simple model can be useful in guiding our thinking about the fundamentals of species dynamics and the temperature dependence of the underlying rate processes.
The model in Fig.2 is at least qualitatively consistent with metabolic theory and empirical observations. There is increasing evidence that rates of evolution, from nucleotide and nucleic acid substitution to speciation and phyletic diversification, are higher in warmer environments and, when examined, exhibit a latitudinal gradient (Gillooly et al., 2005; Allen et al., 2006; Estabrook et al., 2007; Gillooly & Allen, 2007; Mittelbach et al., 2007; Gillman et al., 2010; Wright et al., 2010, 2011; Machac et al., 2012). In particular, the model in the bottom panel of Fig.2 predicts that at equilibrium both speciation and extinction rates are temperature dependent. This is supported by turnover of fossil morphospecies of planktonic organisms as a function of palaeolatitude and sea-surface temperatures (Allen et al., 2006; Allen & Gillooly, 2006).
It remains to be explained just how higher rates of evolution and diversification generate and maintain higher species diversity in warmer environments and at tropical latitudes. The model of Allen et al. (2002) that predicted a universal temperature dependence of biodiversity has not been supported. A single value of E is unlikely on theoretical grounds (Storch, 2012), and empirical studies have shown that E varies with spatial scale (beta diversity: see above), taxon and geographical region (Wang et al., 2009; Hawkins et al., 2007). It is tempting to suggest that the temperature dependence of biodiversity and the LDG are not simply due to higher rates of evolution in warmer environments, but to higher rates of Red Queen coevolution due to more and faster biotic interactions. Given the evidence presented above, I am confident that ‘the Red Queen runs faster when she is hot’, but additional theoretical and empirical work will be required to elucidate the mechanisms.
Feedbacks
I have narrated the synthesis above from the bottom-up, starting with how productivity and niche relationships affect local (alpha) diversity and working up to spatial turnover (beta diversity) and finally to geographical scale species dynamics (gamma diversity). I do not mean to imply, however, that the causal relationships all flow in this direction. In fact, as depicted by the arrows on the right side of Fig.2, there are top-down feedbacks at all these levels. The large-scale patterns of colonization, speciation and extinction feed down to influence regional diversity and the composition of metacommunities, and these feed down to affect the diversity and composition of local communities. Some of these cross-scale feedbacks are implicit in the presentation above and Fig.2, but a few warrant some elaboration.
One, described by Darwin (1859), has been called the Dobzhansky–MacArthur phenomenon (DMP): the equatorial limits of species geographical ranges are usually due to biotic interactions, whereas the polar limits are due to stressful abiotic conditions. There is considerable empirical support for the DMP (e.g. MacArthur, 1972; Root, 1988; Stephens & Wiens, 2003; Lomolino et al., 2010; Sunday et al., 2011, 2012; and other cases summarized in Lomolino et al., 2010). Perhaps the most convincing evidence comes from the latitudinal limits of geographical ranges of exotic species on continents and islands where they are native and where they have been introduced (e.g. Sax, 2001; Wiens & Graham, 2005). The DMP offers a way that the historical ‘out of the tropics’ dynamics and phylogenetic niche conservatism of lineages can be reconciled with the fact that the LDG is an ancient pattern, reflecting a steady-state relationship to climate dating back hundreds of millions of years (e.g. Stehli et al., 1969; Crane & Lidgard, 1989; Crame, 2001). Needless to say, the DMP is consistent with the above suggestions that the LDG is due in large part to the temperature dependence of biotic interactions and Red Queen coevolution.
Another interesting cross-scale feedback is the influence of the geography of speciation on local and regional diversity. It has long been recognized that diversity is highest not just in the tropics, but in topographically diverse regions: mountain terrain on land, such as the slopes of the Andes and Himalayas, and island archipelagos in the sea, such as the Indo-West Pacific and Caribbean. Janzen (1967) called attention to the former in a wonderful paper entitled ‘Why mountain passes are higher in the tropics’. He pointed out that because of seasonal variation in climate, a given change in elevation poses a greater barrier to dispersal in the tropics than at higher latitudes (Fig.3, top). By dispersing in a particular season (winter or summer) an elevationally restricted temperate species can potentially cross mountain passes at lower or higher elevations without encountering temperatures outside the range that it normally experiences during an annual cycle. An elevationally restricted tropical species, by contrast, cannot cross over passes at substantially higher or lower elevations without being exposed to more extreme temperatures than it normally experiences. Janzen suggested that adaptations to such limited seasonal climatic variation result in tropical organisms with narrow thermal niches and restricted distributions. McCain (2009) has recently shown that elevational ranges are narrower in the tropics than at higher latitudes, a pattern consistent with both Janzen's hypothesis and Rapoport's rule (see above). Although Janzen was cautious about extrapolating his idea to explain higher speciation rates and species diversity in the tropics, it is easy to see how temperature-limited dispersal would have this effect.
Figure 3.
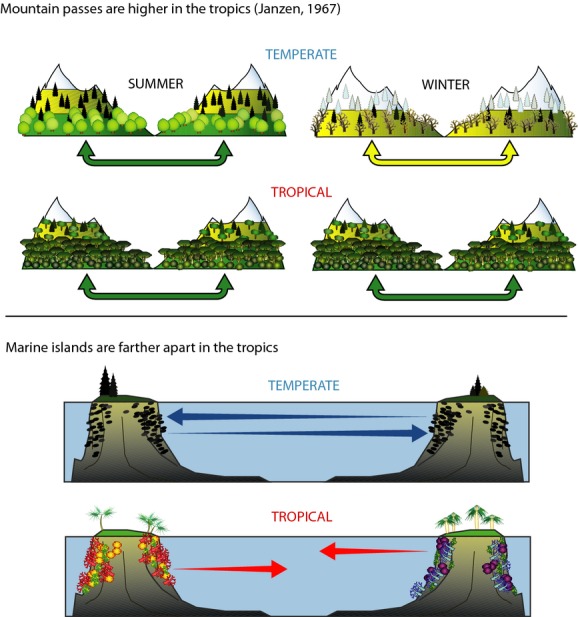
Schematic diagram illustrating a feedback between topography, temperature regime and speciation rates that plays a major role in the latitudinal diversity gradient. Above: Janzen's (1967) explanation for ‘Why mountain passes are higher in the tropics’. Organisms confined to elevational zones can more easily disperse across mountain passes at either higher or lower elevations in temperate regions (yellow and green arrows) than in the tropics (green arrows only), because temperate organisms living at any elevation experience wider seasonal temperature fluctuations and have broader thermal tolerances than tropical organisms. Below: my explanation for ‘Why marine islands are farther apart in the tropics’. Organisms that are confined to benthic habitats and disperse as planktonic larvae can travel longer distances in the temperate zones (blue arrows) than in the tropics (red arrows), because they have lower metabolic and developmental rates and longer survival in colder waters (O'Connor et al., 2007). In both cases, shorter-distance dispersal in the tropics results in reduced gene flow and allows higher rates of diversification and speciation. In both cases, in addition to the direct effect of temperature on physiology, the higher diversity and activity of enemies in warm tropical environments create more severe barriers to dispersal.
I suggest that a somewhat similar phenomenon may facilitate speciation in marine organisms in tropical archipelagos (Fig.3, bottom). Species richness of benthic marine organisms is highest in the islands of the Indo-West Pacific and Caribbean (Tittensor et al., 2010; Bowen et al., 2013). Benthic fish, invertebrates and algae disperse between suitable substrates as planktonic larvae. Metabolic theory predicts and empirical studies confirm that survival of larvae in the plankton is temperature dependent, because the rate of development depends on metabolic rate (Gillooly et al., 2002; O'Connor et al., 2007; O'Connor & Bruno, 2012). In warmer waters larvae develop to the settling stage more rapidly, so they spend less time in the plankton and disperse shorter distances. So, from the standpoint of barriers to dispersal and gene flow, ‘islands are farther apart in the tropics’. Janzen emphasized the direct effect of environmental temperature as a barrier to dispersal in tropical mountains. Warm waters present somewhat similar physiological barriers to dispersal of planktonic marine larvae in archipelagos. In both cases, however, the effect is potentially exacerbated by biotic resistance from enemies, which are more diverse and active where the temperatures are warmer.
These effects of topography on speciation appear to feed down to smaller scales to augment regional and local diversity. High origination rates would facilitate maintenance of higher equilibrium species richness by counteracting high extinction rates of rare species, (Hubbell, 2008). Additionally, the generation of new species with novel combinations of traits that more completely fill available niche space would tend to augment diversity at all spatial scales. So while temperature-dependent biotic interactions act from the bottom-up to promote local diversity by promoting the coexistence of many specialized rare species, temperature-dependent speciation rates act from the top-down to pump in new species and traits to fill the niches with specialized species.
Future Prospects
It should be clear from the above that we do not yet have a widely accepted, general, synthetic explanation for the LDG. Even if all or parts of the above synthesis are substantially correct, many parts are incomplete and many holes need to be filled. I am optimistic that substantial progress can be made. Several areas of research should be especially promising.
Comparative biogeography and macroecology
There has been great progress in documenting empirical patterns of biodiversity: across scales of space and time, levels of biological organization, taxa and habitats. There has been less progress in comparing and synthesizing the results, in part because systematists, ecologists and biogeographers tend to be specialists. This is unfortunate. On the one hand, the LDG is very general, suggesting that similar processes operate in similar ways on the different organisms in different habitats. On the other hand, the roles of historical events and contemporary environments, and of evolutionary and ecological processes, are not independent or mutually exclusive. Moreover, the LDG is just the most well known of several pervasive geographical patterns of biodiversity. Others are the gradients of species richness with elevation on land, depth in the ocean, and aridity in terrestrial environments (e.g. Lomolino et al., 2010; Colwell, 2011).
There is much to be learned by taking advantage of ‘natural experiments’ and making comparisons among the different gradients, scales, levels of organization, taxa and habitats. The variables that potentially affect diversity – tectonic and glacial history, seasonality, temperature, productivity, abiotic stress, biotic interactions, abundance and biomass – sort out in different ways in these different systems, offering potentially valuable insights. For example, the correlations among seasonality, NPP, temperature and latitude, which potentially confound analyses of the LDG in the terrestrial realm are effectively absent in the marine realm. In the oceans NPP is controlled largely by nutrient supply and is highest in areas of upwelling and outflows of large rivers. So productivity is largely independent of temperature and latitude, but biodiversity is highest in tropical waters (e.g. Tittensor et al., 2010). The historical effects of Pleistocene glaciations on pelagic marine organisms, although not inconsequential, were likely to have been quite different from those on plants and animals on the northern continents. Similarly, comparisons of elevational and latitudinal diversity patterns have the potential to separate the effects of glacial history and seasonality from those of productivity and temperature. Also relevant are mechanisms responsible for the frequently observed peak of diversity at intermediate elevations, a phenomenon seldom seen across latitude (e.g. McCain, 2004; Colwell, 2011).
Comparisons of diversity at different levels of biological organization will also be relevant. The LDG is not restricted to species; it also holds at higher and lower levels of organization. Recent studies have documented LDGs of clades of multiple species (e.g. Hawkins et al., 2012; Romdal et al., 2012). Supplemented with information from the fossil record and molecular phylogenies on the timing and location of dispersal, speciation, divergence and extinction, such studies are elucidating historical patterns of biodiversity and providing insights into the dynamical processes. At the other extreme, there is increasing evidence of LDGs within species, including human cultures and languages (Collard & Foley, 2002; Pagel & Mace, 2004). Rapoport's rule was originally based on within-species variation as expressed in range sizes of recognized subspecies. Finally, there appears to be a LDG of genetic diversity within populations, as evidenced by numbers of mitochondrial genotypes within local populations of several kinds of terrestrial vertebrates (Adams & Hadly, 2012).
Comparisons of species richness, genetic diversity, geographical range limits, local abundance and spatial distributions between alien and native species offer additional insights. Exotics introduced by humans within the last few hundred years provide invaluable ‘unintentional experiments’ in ecology and evolution (Sax, 2001; Sax et al., 2002; Wiens & Graham, 2005). For example, the pattern that polar but not equatorial limits of geographical ranges of introduced terrestrial vertebrates are closely correlated, and that tropical continents but not islands, appear to be resistant to colonization by introduced species are consistent with the DMP, limitation by abiotic factors at high latitudes, and biotic interactions in the tropics (Sax, 2001). There is much to learn by expanding macroecological studies of exotics to other systems, such as terrestrial insects and marine organisms.
The most invasive organism is our own species. In only about 50,000 years anatomically modern humans have spread out of tropical Africa to colonize the entire Earth and become the most dominant species. Recent studies of subsistence cultures have documented a Rapoport's rule of tribal ranges and a LDG of languages and cultures (Collard & Foley, 2002; Pagel & Mace, 2004; Burnside et al., 2012; Gavin et al., 2013). Because these patterns have been established rapidly and independently on different continents, they presumably reflect convergent responses to similar ecological conditions. They are consistent with effects of biotic interactions, especially with diseases and plant and animal food resources.
Theory and models
There is also a need for more and better theory to articulate promising questions, guide the design and analysis of empirical studies, and evaluate mechanistic hypotheses. To produce a widely accepted general synthetic theory of biodiversity will be a real challenge. Just to provide a relatively satisfying explanation of the LDG would be a major accomplishment, let alone to develop a more comprehensive theory that places the LDG in the context of other pervasive patterns of diversity across geographical space and evolutionary time.
A major challenge in developing biodiversity theory is the inherent complexity of the problem. Most efforts have used either qualitative verbal or graphical frameworks (Connell & Orias, 1964; Janzen, 1970; Connell, 1971; Rosenzweig, 1995), simple analytical treatments that incorporate only a few coexisting species (e.g. MacArthur, 1972; Tilman, 2004), or whole-system models that focus on emergent patterns rather than underlying mechanisms (e.g. Hubbell, 2008; Harte et al., 2008; Harte, 2011; Storch, 2012). This is understandable, but for ideal interplay between theory and data we need models that can incorporate explicit mechanistic processes and make predictions for assemblages of tens to hundreds of species. This will necessarily entail computer simulation models.
I am fairly optimistic that considerable progress can be made using models that incorporate a relatively small number of assumptions and parameters and are firmly grounded in basic biophysical principles such as mass and energy balance, biomechanics and physiology, and metabolic scaling (e.g. Hammond & Niklas, 2009, 2011a,b).
Manipulative experiments
I also see considerable promise in using real experiments to address general questions about biodiversity. Such manipulations can be viewed as empirical models: deliberately oversimplified systems designed to make progress by using controlled conditions and standardized treatments to focus on the effects of critical variables and processes. Experiments with microbes in microcosms have been used to great effect to address questions in population ecology, interspecific interactions and evolution. So far, however, inferences about biodiversity have generally been limited, because most experiments have used a small number of genotypes or species. But the general approach could be extended by inoculating microcosms with high-diversity cultures from natural sources and using metagenomic molecular tools to quantify the outcomes, while manipulating and controlling environmental variables.
Concluding Remarks
More than three centuries after the spectacularly high biodiversity in the tropics became known, accounting for this phenomenon remains one of the greatest challenges for Western science. New databases and analytical techniques have increasingly documented the empirical manifestations of the LDG, both the pervasiveness of the gradient across different habitats, taxa, and levels of biological organization, and the variations and exceptions to the general pattern. Still missing, however, is a unified theory of biodiversity to explain these relationships.
In particular, I see a need for frameworks that show how the historical events and environmental conditions affect the dynamics of fundamental ecological and evolutionary processes to generate and maintain variation in standing stocks of biodiversity. In other words, we need to understand the linkages between the rates of mechanistic processes and the resulting states of diversity at different levels of organization. We can look to the fossil record, phylogenetic reconstructions, analyses of variation in genomes and niche traits, and metabolic theory to guide the search for these linkages. I suspect that the latitudinal gradient of biodiversity is so ancient and pervasive because the relationship of the Earth to the sun and the variation in solar energy input creates a gradient of environmental temperature. Temperature affects the rate of metabolism and all biological activity, including the rates of ecological interactions and coevolution. ‘Diversity begets diversity’ in the tropics, because ‘the Red Queen runs faster when she is hot’.
Acknowledgments
This paper really should have at least 100 co-authors, because so many colleagues have so generously and spiritedly shared their ideas and data about the LDG with me. I cannot thank them all individually, but I am most grateful. Special thanks to: (1) NIH grant T32EB009414 and NSF grant DEB-0083422 for financial support; (2) personnel of our Macrosystems project for helpful discussions; (3) V. Buzzard, C. Sides, A. Henderson and B.J. Enquist for providing the data in Table1 and Fig.1; (4) R. Colwell, J.-C. Svenning, M. Kaspari, A. Kodric-Brown, I. Simova, R.J. Whittaker and an anonymous referee for valuable comments on the manuscript; and (5) T. Fristoe, who prepared Fig.3.
Biosketch
James H. Brown grew up in upstate New York, attended Cornell University, and received his PhD from the University of Michigan. He held faculty appointments at the University of California at Los Angeles, University of Utah, University of Arizona, Santa Fe Institute, and University of New Mexico, where he has been Professor Emeritus since 2012. He is known for his empirical research in desert ecosystems and biogeography and his theoretical research in macroecology, biological scaling and metabolic ecology. He has trained many undergraduates, graduate students and postdocs. He has received several honors and awards, including election to the National Academy of Sciences and the American Academy of Arts and Sciences, the Wallace Award from the International Biogeography Society, the Odum and MacArthur Awards from the Ecological Society of America, the Merriam and Grinnell Awards from the American Society of Mammalogists, the Marsh Award from the British Ecological Society, and the Grinnell Medal from the University of California at Berkeley. In retirement he is trying to spend a bit less time doing science and a bit more time travelling and with family. This paper derives from Brown's Alfred Russel Wallace Award Address at the 2013 annual meeting of the International Biogeography Society.
References
- Adams RI. Hadly EA. Genetic diversity within vertebrate species is greater at lower latitudes. Evolutionary Ecology. 2012;27:133–143. &. [Google Scholar]
- Allen AP. Gillooly JF. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecology Letters. 2006;9:947–954. doi: 10.1111/j.1461-0248.2006.00946.x. &. [DOI] [PubMed] [Google Scholar]
- Allen AP, Brown JH. Gillooly JF. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. &. [DOI] [PubMed] [Google Scholar]
- Allen AP, Gillooly JF. Brown JH. Linking the global carbon cycle to individual metabolism. Functional Ecology. 2005;19:202–213. &. [Google Scholar]
- Allen AP, Gillooly JF, Savage VM. Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proceedings of the National Academy of Sciences USA. 2006;103:9130–9135. doi: 10.1073/pnas.0603587103. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KJ, Allen AP, Gillooly JF. Brown JH. Temperature-dependence of biomass accumulation rates during secondary succession. Ecology Letters. 2006;9:673–682. doi: 10.1111/j.1461-0248.2006.00914.x. &. [DOI] [PubMed] [Google Scholar]
- Anderson-Teixeira KJ. Vitousek PM. Ecosystems. In: Sibly RM, Brown JH, Kodric-Brown A, editors; Metabolic ecology: a scaling approach. Chichester, UK: Wiley-Blackwell; 2012. pp. 99–111. (ed. by ) &. [Google Scholar]
- Bowen BW, Rocha LA, Toonen RJ. Karl SA. The origins of tropical marine biodiversity. Trends in Ecology and Evolution. 2013;20:1–8. doi: 10.1016/j.tree.2013.01.018. &. [DOI] [PubMed] [Google Scholar]
- Brown JH. Two decades of homage to Santa Rosalia: toward a general theory of diversity. American Zoologist. 1981;21:877–888. [Google Scholar]
- Brown JH. Species diversity. In: Myers AA, Giller PS, editors. Analytical biogeography. London: Chapman and Hall; 1988. pp. 57–89. (ed. by ) [Google Scholar]
- Brown JH, Stevens GC. Kaufman DM. The geographic range: size, shape, boundaries, and internal structure. Annual Review of Ecology and Systematics. 1996;27:597–623. &. [Google Scholar]
- Brown JH, Gillooly JF, Allen AP, Savage VM. West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. &. [Google Scholar]
- Buckley LB, Davies TJ, Ackerly DD, Kraft NJ, Harrison SP, Anacker BL, Cornell HV, Damschen EI, Grytnes J-A, Hawkins BA, McCain CM, Stephens PR. Wiens JJ. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2131–2138. doi: 10.1098/rspb.2010.0179. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt JS. Primary productivity of marine ecosystems. Berlin: Springer; 1975. [Google Scholar]
- Burnside WR, Brown JH, Burger O, Hamilton MJ, Moses M. Bettencourt L. Human macroecology: linking pattern and process in big-picture human ecology. Biological Reviews. 2012;87:194–208. doi: 10.1111/j.1469-185X.2011.00192.x. &. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Gonzalez-Rodriguez A, Pahlich A, Koehler K. Deacon N. Phylogeography and climatic niche evolution in live oaks (Quercus series Virentes) from the tropics to the temperate zone. Journal of Biogeography. 2011;38:962–981. &. [Google Scholar]
- Clark DA. Clark DB. Spacing dynamics of a tropical rain forest tree: evaluation of the Janzen-Connell model. The American Naturalist. 1984;124:769–788. &. [Google Scholar]
- Collard IF. Foley RA. Latitudinal patterns and environmental determinants of recent human cultural diversity: do humans follow biogeographical rules? Evolutionary Ecology Research. 2002;4:371–383. &. [Google Scholar]
- Colwell RK. Biogeographical gradient theory. In: Scheiner SM, Willig MR, editors. The theory of ecology. Chicago: University of Chicago Press; 2011. pp. 309–330. (ed. by ) [Google Scholar]
- Comita LS, Muller-Landau HC, Aguilar S. Hubbell SP. Asymmetric density dependence shapes species abundances in a tropical tree community. Science. 2010;329:330–332. doi: 10.1126/science.1190772. &. [DOI] [PubMed] [Google Scholar]
- Condit R, Pitman N, Leigh EG, Jr, Chave J, Terborgh J, Foster RB, Núñez P, Aguilar S, Valencia R, Villa G, Muller-Landau HC, Losos E. Hubbell SP. Beta-diversity in tropical forest trees. Science. 2002;295:666–669. doi: 10.1126/science.1066854. &. [DOI] [PubMed] [Google Scholar]
- Connell J. On the role of the natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR, editors. Dynamics of populations. Wageningen: Centre for Agricultural Publishing and Documentation; 1971. pp. 298–310. (ed. by ) . Proceedings of the Advanced Study Institute, [Google Scholar]
- Connell JH. Orias E. The ecological regulation of species diversity. The American Naturalist. 1964;98:399–414. &. [Google Scholar]
- Crame JA. Taxonomic diversity gradients through geological time. Diversity and Distributions. 2001;7:175–189. [Google Scholar]
- Crane PR. Lidgard S. Angiosperm diversification and paleolatitudinal gradients in Cretaceous floristic diversity. Science. 1989;246:675–678. doi: 10.1126/science.246.4930.675. &. [DOI] [PubMed] [Google Scholar]
- Currie DJ. Energy and large-scale patterns of animal- and plant-species richness. The American Naturalist. 1991;137:27–49. [Google Scholar]
- Currie DJ, Mittelbach GG, Cornell HV, Field R, Guégan JF, Hawkins BA, Kaufman DM, Kerr JT, Oberdorff T, O'Brien E. Turner JRG. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecology Letters. 2004;7:1121–1134. &. [Google Scholar]
- Darwin C. On the origin of species by means of natural selection. London: J. Murray; 1859. [Google Scholar]
- Dell AI, Pawar S. Savage VM. Systematic variation in the temperature dependence of physiological and ecological traits. Proceedings of the National Academy of Sciences USA. 2011;108:10591–10596. doi: 10.1073/pnas.1015178108. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ. A phylogenetic perspective on the distribution of plant diversity. Proceedings of the National Academy of Sciences USA. 2008;105:11549–11555. doi: 10.1073/pnas.0801962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson BC. Kolm N. Species diversity can drive speciation. Nature. 2005;434:1015–1017. doi: 10.1038/nature03450. &. [DOI] [PubMed] [Google Scholar]
- Englund G, Öhlund G, Hein CL. Diehl S. Temperature dependence of the functional response. Ecology Letters. 2011;14:914–921. doi: 10.1111/j.1461-0248.2011.01661.x. &. [DOI] [PubMed] [Google Scholar]
- Enquist BJ. Niklas KJ. Invariant scaling relations across tree-dominated communities. Nature. 2001;410:655–660. doi: 10.1038/35070500. &. [DOI] [PubMed] [Google Scholar]
- Estabrook GF, Smith GR. Dowling TE. Body mass and temperature influence rates of mitochondrial DNA evolution in North American cyprinid fish. Evolution. 2007;61:1176–1187. doi: 10.1111/j.1558-5646.2007.00089.x. &. [DOI] [PubMed] [Google Scholar]
- Fischer AG. Latitudinal variations in organic diversity. Evolution. 1960;14:64–81. [Google Scholar]
- Francis AP. Currie DJ. Global patterns of tree species richness in moist forests: another look. Oikos. 1998;81:598–602. &. [Google Scholar]
- Fraser RH. Currie DJ. The species richness–energy hypothesis in a system where historical factors are thought to prevail: coral reefs. The American Naturalist. 1996;148:138–159. &. [Google Scholar]
- Gaston KJ. Chown SL. Why Rapoport's rule does not generalise. Oikos. 1999;84:309–312. &. [Google Scholar]
- Gavin MC, Botero CA, Bowern C, Colwell RK, Dunn M, Dunn RR, Gray R, Kirby KR, McCarter J, Powell A, Rangel TF, Stepp JR, Trautwein M, Verdolin JL. Yanega G. Towards a mechanistic understanding of linguistic diversity. BioScience. 2013;63:524–535. &. [Google Scholar]
- Gillman LN, Keeling DJ, Gardner RC. Wright SD. Faster evolution of highly conserved DNA in tropical plants. Journal of Evolutionary Biology. 2010;23:1327–1330. doi: 10.1111/j.1420-9101.2010.01992.x. &. [DOI] [PubMed] [Google Scholar]
- Gillooly JF. Allen AP. Linking global patterns in biodiversity to evolutionary dynamics using metabolic theory. Ecology. 2007;88:1890–1894. doi: 10.1890/06-1935.1. &. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM. Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. &. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Charnov EL, West GB, Savage VM. Brown JH. Effects of size and temperature on developmental time. Nature. 2002;417:70–73. doi: 10.1038/417070a. &. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Allen AP, West GB. Brown JH. The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proceedings of the National Academy of Sciences USA. 2005;102:140–145. doi: 10.1073/pnas.0407735101. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond ST. Niklas KJ. Emergent properties of plants competing in silico for space and light: seeing the tree from the forest. American Journal of Botany. 2009;96:1430–1444. doi: 10.3732/ajb.0900063. &. [DOI] [PubMed] [Google Scholar]
- Hammond ST. Niklas KJ. Modeling forest self-assembly dynamics using allometric and physical first principles. BioScience. 2011a;61:663–676. &. [Google Scholar]
- Hammond ST. Niklas KJ. Computer simulations of plant biodiversity in stable and unstable environments: a test of the neutral biodiversity theory. Journal of Biological Systems. 2011b;19:1–17. &. [Google Scholar]
- Harte J. Maximum entropy and ecology: a theory of abundance, distribution, and energetics. Oxford: Oxford University Press; 2011. [Google Scholar]
- Harte J, Zillio T, Conlisk E. Smith AB. Maximum entropy and the state-variable approach to macroecology. Ecology. 2008;89:2700–2711. doi: 10.1890/07-1369.1. &. [DOI] [PubMed] [Google Scholar]
- Hawkins BA, Diniz-Filho JAF, Jaramillo CA. Soeller SA. Climate, niche conservatism, and the global bird diversity gradient. The American Naturalist. 2007;170:S16–S27. doi: 10.1086/519009. &. [DOI] [PubMed] [Google Scholar]
- Hawkins BA, McCain CM, Davies TJ, Buckley LB, Anacker BL, Cornell HV, Damschen EI, Grytnes J-A, Harrison SP, Holt RD, Kraf NJB. Stephens PR. Different evolutionary histories underlie congruent species richness gradients of birds and mammals. Journal of Biogeography. 2012;39:825–841. &. [Google Scholar]
- Hortal J, Diniz-Filho JAF, Bini LM, Rodríguez MÁ, Baselga A, Nogués-Bravo D, Rangel TF, Hawkins BA. Lobo JM. Ice age climate, evolutionary constraints and diversity patterns of European dung beetles. Ecology Letters. 2011;14:741–748. doi: 10.1111/j.1461-0248.2011.01634.x. &. [DOI] [PubMed] [Google Scholar]
- Hubbell SP. Tree dispersion, abundance, and diversity in a tropical dry forest. Science. 1979;203:1299–1309. doi: 10.1126/science.203.4387.1299. [DOI] [PubMed] [Google Scholar]
- Hubbell SP. The unified neutral theory of biodiversity and biogeography. Princeton NJ: Princeton University Press; 2008. [Google Scholar]
- Hurlbert AH. Linking species–area and species–energy relationships in Drosophila microcosms. Ecology Letters. 2006;9:287–294. doi: 10.1111/j.1461-0248.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist. 1959;93:145–159. [Google Scholar]
- Jablonski D, Roy K. Valentine JW. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. &. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Why mountain passes are higher in the tropics. The American Naturalist. 1967;101:233–249. [Google Scholar]
- Janzen DH. Herbivores and the number of tree species in tropical forests. The American Naturalist. 1970;104:501–528. [Google Scholar]
- Johnson DJ, Beaulieu WT, Bever JD. Clay K. Conspecific negative density dependence and forest diversity. Science. 2012;336:904–907. doi: 10.1126/science.1220269. &. [DOI] [PubMed] [Google Scholar]
- Kaspari M, Alonso L, Alonso L. O'Donnell S. Three energy variables predict ant abundance at a geographical scale. Proceedings of the Royal Society B: Biological Sciences. 2000;267:485–489. doi: 10.1098/rspb.2000.1026. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Mitchell WA, Porter WP. Currie DJ. Testing a mechanistic explanation for the latitudinal gradient in mammalian species richness across North America. Evolutionary Ecology Research. 2006;8:333–344. &. [Google Scholar]
- Klopfer PH. MacArthur RH. On the causes of tropical species diversity: niche overlap. The American Naturalist. 1961;94:223–226. &. [Google Scholar]
- Kraft NJ, Comita LS, Chase JM, Sanders NJ, Swenson NG, Crist TO, Stegen JC, Vellend M, Boyle B, Anderson MJ, Cornell HV, Davies KF, Freestone AL, Inouye BD, Harrison SP. Myers JA. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science. 2011;333:1755–1758. doi: 10.1126/science.1208584. &. [DOI] [PubMed] [Google Scholar]
- Lieth H. Primary production of the major vegetation units of the world. In: Leith H, Whittaker RH, editors. Primary productivity of the biosphere. Berlin: Springer; 1975. pp. 203–215. Ecological Studies Vol. 14. [Google Scholar]
- Linnaeus C. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Holmiae: Laurentius Salvius; 1758. . Editio decima, reformata. [Google Scholar]
- Lomolino MV, Riddle BR, Whittaker RJ. Brown JH. Biogeography. 4th edn. Sunderland MA: Sinauer; 2010. &. [Google Scholar]
- MacArthur RH. Patterns of species diversity. Biological Reviews. 1965;40:510–533. [Google Scholar]
- MacArthur RH. Geographical ecology: patterns in the distribution of species. New York: Harper & Row; 1972. [Google Scholar]
- MacArthur RH. Wilson EO. The theory of island biogeography. Princeton, NJ: Princeton University Press; 1967. &. [Google Scholar]
- Machac A, Zrzavý J, Smrckova J. Storch D. Temperature dependence of evolutionary diversification: differences between two contrasting model taxa support the metabolic theory of ecology. Journal of Evolutionary Biology. 2012;25:2449–2456. doi: 10.1111/jeb.12019. &. [DOI] [PubMed] [Google Scholar]
- McCain CM. The mid-domain effect applied to elevational gradients: species richness of small mammals in Costa Rica. Journal of Biogeography. 2004;31:19–31. [Google Scholar]
- McCain CM. Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecology Letters. 2009;12:550–560. doi: 10.1111/j.1461-0248.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- Mittelbach GG, Schemske DW, Cornell HV, et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecology Letters. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- Moore CM, Mills MM, Arrigo KR, et al. Processes and patterns of oceanic nutrient limitation. Nature Geoscience. 2013 , doi: 10.1038/ngeo1765. [Google Scholar]
- Nekola JC. White PS. The distance decay of similarity in biogeography and ecology. Journal of Biogeography. 1999;26:867–878. &. [Google Scholar]
- O'Brien EM, Whittaker RJ. Field R. Climate and woody plant diversity in southern Africa: relationships at species, genus and family levels. Ecography. 1998;21:495–509. &. [Google Scholar]
- O'Connor MI. Bruno JF. Marine invertebrates. In: Sibly RM, Brown JH, Kodric-Brown A, editors; Metabolic ecology: a scaling approach. Chichester, UK: Wiley-Blackwell; 2012. pp. 188–197. ) &. [Google Scholar]
- O'Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP. Weiss JM. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proceedings of the National Academy of Sciences USA. 2007;104:1266–1271. doi: 10.1073/pnas.0603422104. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. Mace R. The cultural wealth of nations. Nature. 2004;428:275–278. doi: 10.1038/428275a. &. [DOI] [PubMed] [Google Scholar]
- Pianka ER. Latitudinal gradients in species diversity: a review of concepts. The American Naturalist. 1966;100:33–46. [Google Scholar]
- Qian H. Ricklefs RE. A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecology Letters. 2007;10:737–744. doi: 10.1111/j.1461-0248.2007.01066.x. &. [DOI] [PubMed] [Google Scholar]
- Qian H, Ricklefs RE. White PS. Beta diversity of angiosperms in temperate floras of eastern Asia and eastern North America. Ecology Letters. 2004;8:15–22. &. [Google Scholar]
- Qian H, Badgley C. Fox D. The latitudinal gradient of beta diversity in relation to climate and topography for mammals in North America. Global Ecology and Biogeography. 2009;18:111–122. &. [Google Scholar]
- Rapoport EH. Aerography: geographical strategies of species. Oxford: Pergamon; 1982. [Google Scholar]
- Ricklefs RE. Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proceedings of the National Academy of Sciences USA. 2010;107:1265–1272. doi: 10.1073/pnas.0913626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez P. Arita HT. Beta diversity and latitude in North American mammals: testing the hypothesis of covariation. Ecography. 2004;27:547–556. &. [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- Romdal TS, Araújo MB. Rahbek C. Life on a tropical planet: niche conservatism and the global diversity gradient. Global Ecology and Biogeography. 2012;22:344–350. &. [Google Scholar]
- Root T. Energy constraints on avian distributions and abundances. Ecology. 1988;69:330–339. [Google Scholar]
- Rosenzweig ML. Species diversity in space and time. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- Roy K, Hunt G, Jablonski D, Krug AZ. Valentine JW. A macroevolutionary perspective on species range limits. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1485–1493. doi: 10.1098/rspb.2008.1232. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage VM, Gillooly JF, Brown JH, West GB. Charnov EL. Effects of body size and temperature on population growth. The American Naturalist. 2004;163:429–441. doi: 10.1086/381872. &. [DOI] [PubMed] [Google Scholar]
- Sax DF. Latitudinal gradients and geographic ranges of exotic species: implications for biogeography. Journal of Biogeography. 2001;28:139–150. [Google Scholar]
- Sax DF, Gaines SD. Brown JH. Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. The American Naturalist. 2002;160:766–783. doi: 10.1086/343877. &. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM. Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics. 2009;40:245–269. &. [Google Scholar]
- Sibly RM, Brown JH. Kodric-Brown A. Metabolic ecology: a scaling approach. Chichester, UK: Wiley-Blackwell; 2012. & (eds) ( [Google Scholar]
- Smith VH. Nutrient dependence of primary productivity in lakes. Limnology and Oceanography. 1979;24:1051–1064. [Google Scholar]
- Stehli FG, Douglas RG. Newell ND. Generation and maintenance of gradients in taxonomic diversity. Science. 1969;164:947–949. doi: 10.1126/science.164.3882.947. &. [DOI] [PubMed] [Google Scholar]
- Stephens PR. Wiens JJ. Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. The American Naturalist. 2003;161:112–128. doi: 10.1086/345091. &. [DOI] [PubMed] [Google Scholar]
- Stevens GC. The latitudinal gradient in geographical range: how so many species coexist in the tropics. The American Naturalist. 1989;133:240–256. [Google Scholar]
- Storch D. Biodiversity and its energetic and thermal controls. In: Sibly RM, Brown JH, Kodric-Brown A, editors. Metabolic ecology: a scaling approach. Chichester, UK: Wiley-Blackwell; 2012. pp. 120–131. (ed. by ) [Google Scholar]
- Sunday JM, Bates AE. Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1823–1830. doi: 10.1098/rspb.2010.1295. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday JM, Bates AE. Dulvy NK. Thermal tolerance and the global redistribution of animals. Nature Climate Change. 2012;2:686–690. &. [Google Scholar]
- Swamy V. Terborgh JW. Distance-responsive natural enemies strongly influence seedling establishment patterns of multiple species in an Amazonian rain forest. Journal of Ecology. 2010;98:1096–1107. &. [Google Scholar]
- Terborgh J. On the notion of favorableness in plant ecology. The American Naturalist. 1973;107:481–501. [Google Scholar]
- Terborgh J. Enemies maintain hyperdiverse tropical forests. The American Naturalist. 2012;179:303–314. doi: 10.1086/664183. [DOI] [PubMed] [Google Scholar]
- Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proceedings of the National Academy of Sciences USA. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittensor DP, Mora C, Jetz W, Lotze HK, Ricard D, Berghe EV. Worm B. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466:1098–1101. doi: 10.1038/nature09329. &. [DOI] [PubMed] [Google Scholar]
- Wallace AR. My life: a record of events and opinions. London: G. Bell & Sons; 1905. [Google Scholar]
- Wang Z, Brown JH, Tang Z. Fang J. Temperature dependence, spatial scale, and tree species diversity in eastern Asia and North America. Proceedings of the National Academy of Sciences USA. 2009;106:13388–13392. doi: 10.1073/pnas.0905030106. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ. Graham CH. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics. 2005;36:519–539. &. [Google Scholar]
- Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Davies J, Grytnes J-A, Harrison SP, Hawkins BA, Holt RD, McCain CM. Stephens PR. Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. &. [DOI] [PubMed] [Google Scholar]
- Willig MR, Kaufman DM. Stevens RD. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annual Review of Ecology, Evolution, and Systematics. 2003;34:273–309. &. [Google Scholar]
- Wright DH. Species-energy theory: an extension of species-area theory. Oikos. 1983;47:496–506. [Google Scholar]
- Wright DH, Currie DJ. Maurer BA. Energy supply and patterns of species richness on local and regional scales. In: Ricklefs RE, Schluter D, editors; Species diversity in ecological communities: historical and geographical perspectives. Chicago: University of Chicago Press; 1993. pp. 66–74. (ed. by ) & . . [Google Scholar]
- Wright SD, Gillman LN, Ross HA. Keeling DJ. Energy and the tempo of evolution in amphibians. Global Ecology and Biogeography. 2010;19:733–740. &. [Google Scholar]
- Wright SD, Ross HA, Keeling DJ, McBride P. Gillman LN. Thermal energy and the rate of genetic evolution in marine fishes. Evolutionary Ecology. 2011;25:525–530. &. [Google Scholar]
- Yvon-Durocher G, Allen AP, Montoya JM, Trimmer M. Woodward G. The temperature dependence of the carbon cycle in aquatic ecosystems. Advances in Ecological Research. 2010;43:267–313. &. [Google Scholar]


