Abstract
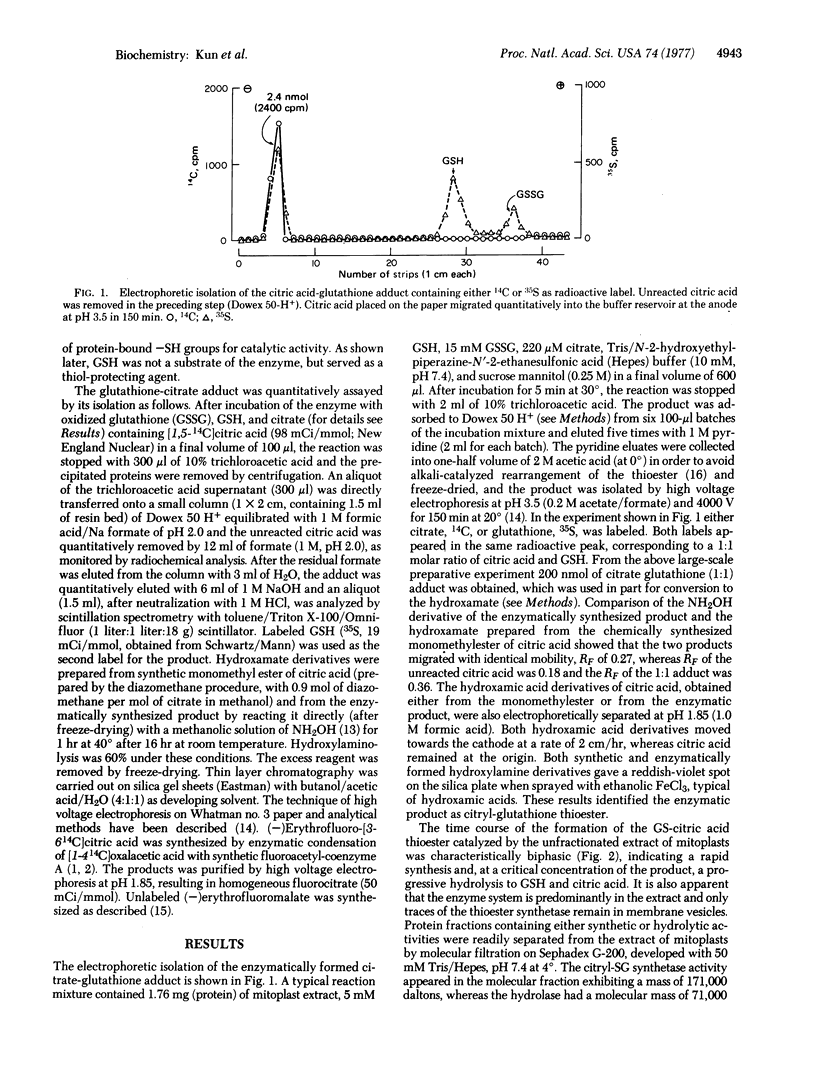
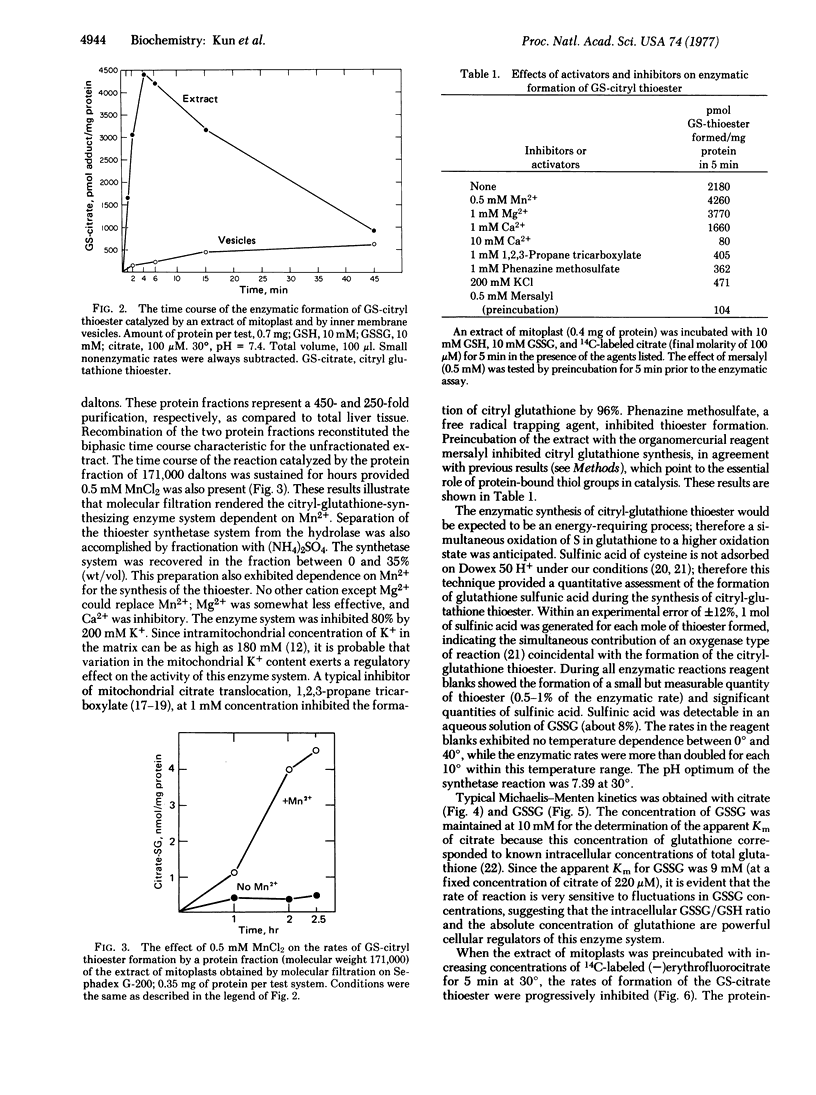
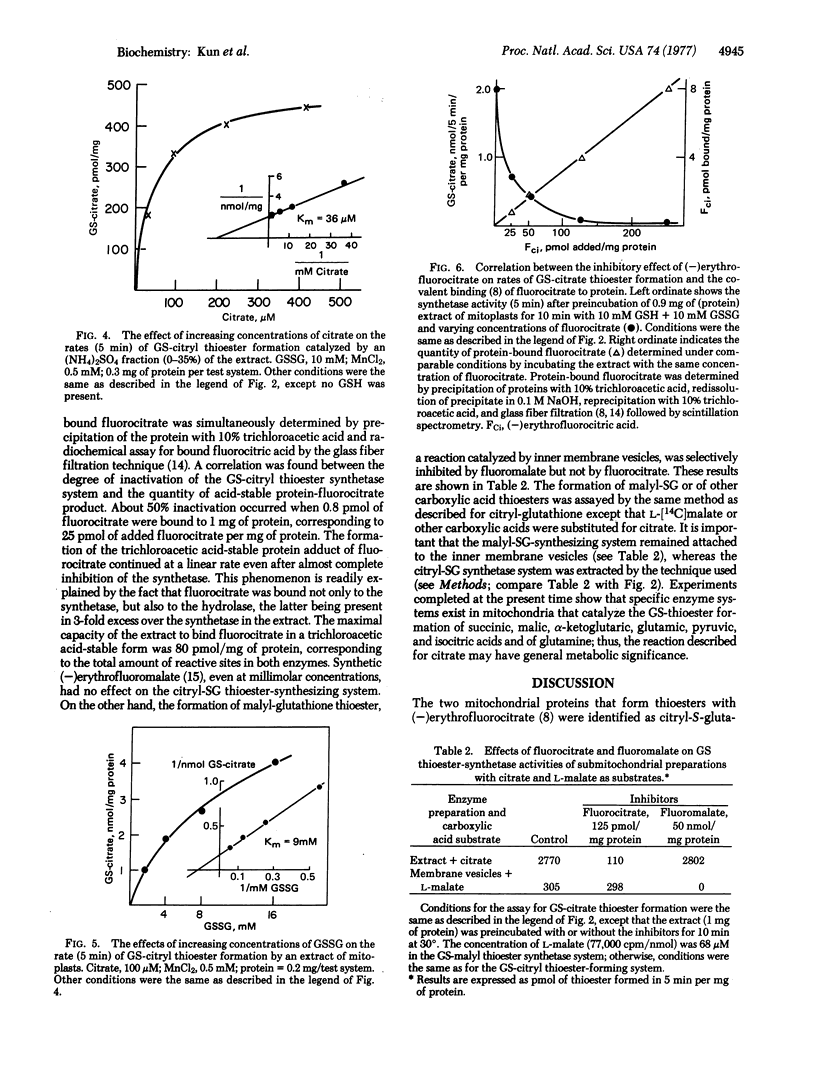
A soluble extract of the mitochondrial compartment composed of the inner membrane and matrix catalyzes the enzymatic synthesis and hydrolysis of the 1:1 adduct of citric acid and glutathione. The adduct was identified as the thioester by isolation with single and double isotope labeling ([14C]citric acid and [35S]glutathione) and by conversion to the monohydroxamate of citric acid and comparison with the synthetic product by thin layer chromatography and high voltage electrophoresis. The enzymatic formation of the thioester (pH optimum 7.39 at 30°) requires oxidized glutathione and citrate; both substrates exhibit a Michaelis-Menten kinetics. During the enzymatic reaction equimolar quantities of thioester and glutathione sulfinic acid are formed. After gel filtration or salt fractionation the enzyme system requires Mn2+ (or Mg2+, which is less effective) for maximal activity. When extracts of mitoplast are tested, the time course of reaction is biphasic due to the rapid synthesis of the product by the thioester-forming system (molecular weight 171,000) followed by its decay by the hydrolase (molecular weight 71,000). The two systems were separated by molecular filtration on Sephadex G-200 and by precipitation with (NH4)2SO4. The thioester-forming system is inhibited by preincubation with 0.5 mM mersalyl. Other inhibitors are 1,2,3-propane tricarboxylic acid, 10 mM Ca2+, 200 mM K+, and the free radical trapping agent, phenazine methosulfate. The citrate-glutathione thioester formation is irreversibly and specifically inhibited by (-)erythrofluorocitrate (50% inhibition at 25 pmol of added fluorocitrate per mg of protein), which forms a trichloroacetic acid-stable adduct with the enzyme protein (at 50% inhibition, 0.8 pmol is bound to 1 mg of protein). Synthesis of malyl-glutathione thioester by inner membrane vesicles is selectively inhibited by (-)erythrofluoromalate.
Keywords: glutathione-S-citryl ester, metalloprotein, inner mitochondrial membrane, fluorocitrate toxic mechanism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand M. D., Evans S. M., Mendes-Mourão J., Chappell J. B. Fluorocitrate inhibition of aconitate hydratase and the tricarboxylate carrier of rat liver mitochondria. Biochem J. 1973 May;134(1):217–224. doi: 10.1042/bj1340217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell H. L., Glusker J. P., Villafranca J. J., Mildvan A. S., Dummel R. J., Kun E. Fluorocitrate inhibition of aconitase: relative configuration of inhibitory isomer by x-ray crystallography. Science. 1970 Dec 25;170(3965):1412–1414. doi: 10.1126/science.170.3965.1412. [DOI] [PubMed] [Google Scholar]
- Eanes R. Z., Kun E. Inhibition of liver aconitase isozymes by (-)-erythro-fluorocitrate. Mol Pharmacol. 1974 Jan;10(1):130–139. [PubMed] [Google Scholar]
- Eanes R. Z., Skilleter D. N., Kun E. Inactivation of the tricarboxylate carrier of liver mitochondria by (-)-erythrofluorocitrate. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1618–1622. doi: 10.1016/0006-291x(72)90794-2. [DOI] [PubMed] [Google Scholar]
- FANSHIER D. W., GOTTWALD L. K., KUN E. STUDIES ON SPECIFIC ENZYME INHIBITORS. VI. CHARACTERIZATION AND MECHANISM OF ACTION OF THE ENZYME-INHIBITORY ISOMER OF MONOFLUOROCITRATE. J Biol Chem. 1964 Feb;239:425–434. [PubMed] [Google Scholar]
- Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1972 Apr 24;26(4):587–594. doi: 10.1111/j.1432-1033.1972.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Kun E., Chang A. C., Sharma M. L., Ferro A. M., Nitecki D. Covalent modification of proteins by metabolites of NAD+. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3131–3135. doi: 10.1073/pnas.73.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun E. Kinetics of ATP-dependent Mg2+ flux in mitochondria. Biochemistry. 1976 Jun 1;15(11):2328–2336. doi: 10.1021/bi00656a013. [DOI] [PubMed] [Google Scholar]
- Lombardini J. B., Singer T. P., Boyer P. D. Cystein oxygenase. II. Studies on the mechanism of the reaction with 18oxygen. J Biol Chem. 1969 Mar 10;244(5):1172–1175. [PubMed] [Google Scholar]
- Meijer A. J., Van Dam K. The metabolic significance of anion transport in mitochondria. Biochim Biophys Acta. 1974 Dec 30;346(3-4):213–244. doi: 10.1016/0304-4173(74)90001-9. [DOI] [PubMed] [Google Scholar]
- Meyer J., Vignais P. M. Kinetic study of glutamate transport in rat liver mitochondria. Biochim Biophys Acta. 1973 Dec 14;325(3):375–384. doi: 10.1016/0005-2728(73)90198-9. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. The effects of 2-ethylcitrate and tricarballylate on citrate transport in rat liver mitochondria and fatty acid synthesis in rat white adipose tissue. Eur J Biochem. 1970 Aug;15(2):263–272. doi: 10.1111/j.1432-1033.1970.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Dummel R. J., Kun E. Studies with specific enzyme inhibitors. XIV. The effects of enzymatically synthesized (-)-erythro-fluoromalic acid on malate dehydrogenase and on anion carriers of liver mitochondria. Mol Pharmacol. 1972 Mar;8(2):139–148. [PubMed] [Google Scholar]
- Stipani I., Prezioso G., Genchi G., Palmieri F. Valutazione quantitativa del trasportatore dell'acido citrico della membrana mitocondriale. - Nota II. Boll Soc Ital Biol Sper. 1976 Aug 30;52(16):1288–1293. [PubMed] [Google Scholar]
- Uotila L. Preparation and assay of glutathione thiol esters. Survey of human liver glutathione thiol esterases. Biochemistry. 1973 Sep 25;12(20):3938–3943. doi: 10.1021/bi00744a024. [DOI] [PubMed] [Google Scholar]
- Uotila L. Purification and characterization of S-2-hydroxyacylglutathione hydrolase (glyoxalase II) from human liver. Biochemistry. 1973 Sep 25;12(20):3944–3951. doi: 10.1021/bi00744a025. [DOI] [PubMed] [Google Scholar]


