Abstract
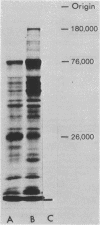
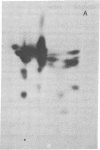
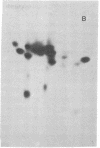
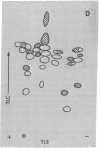
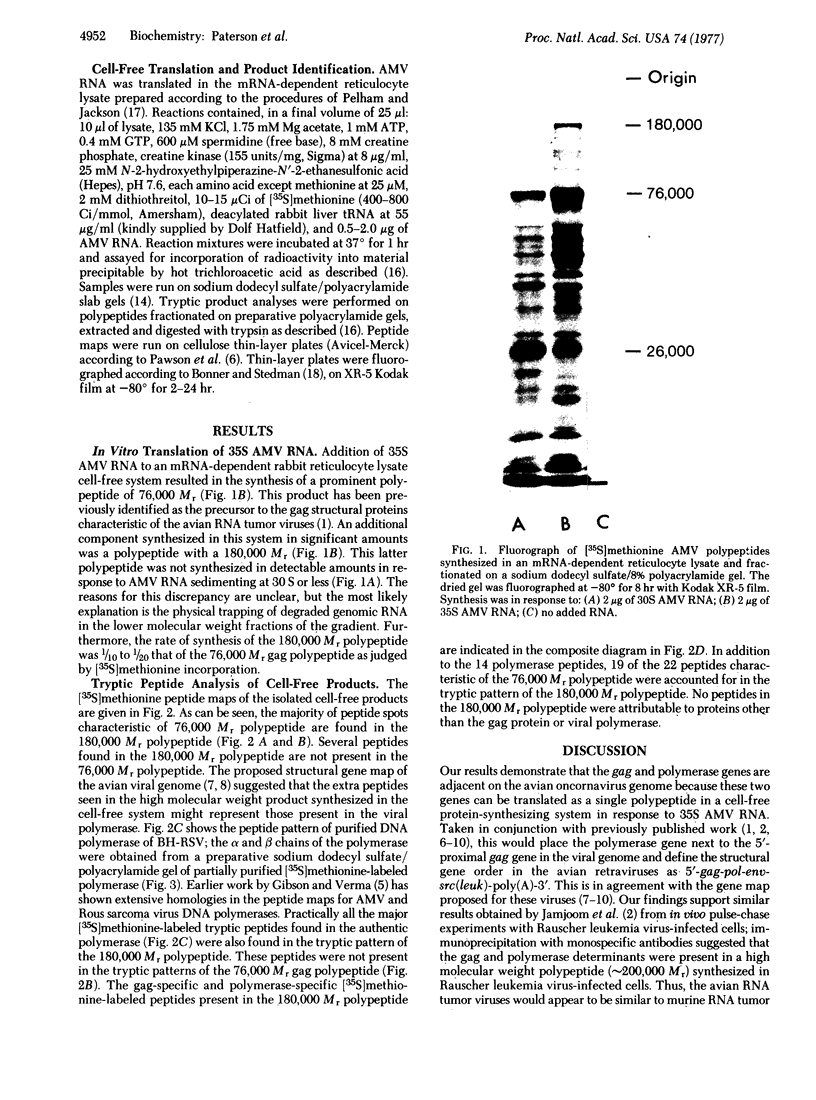
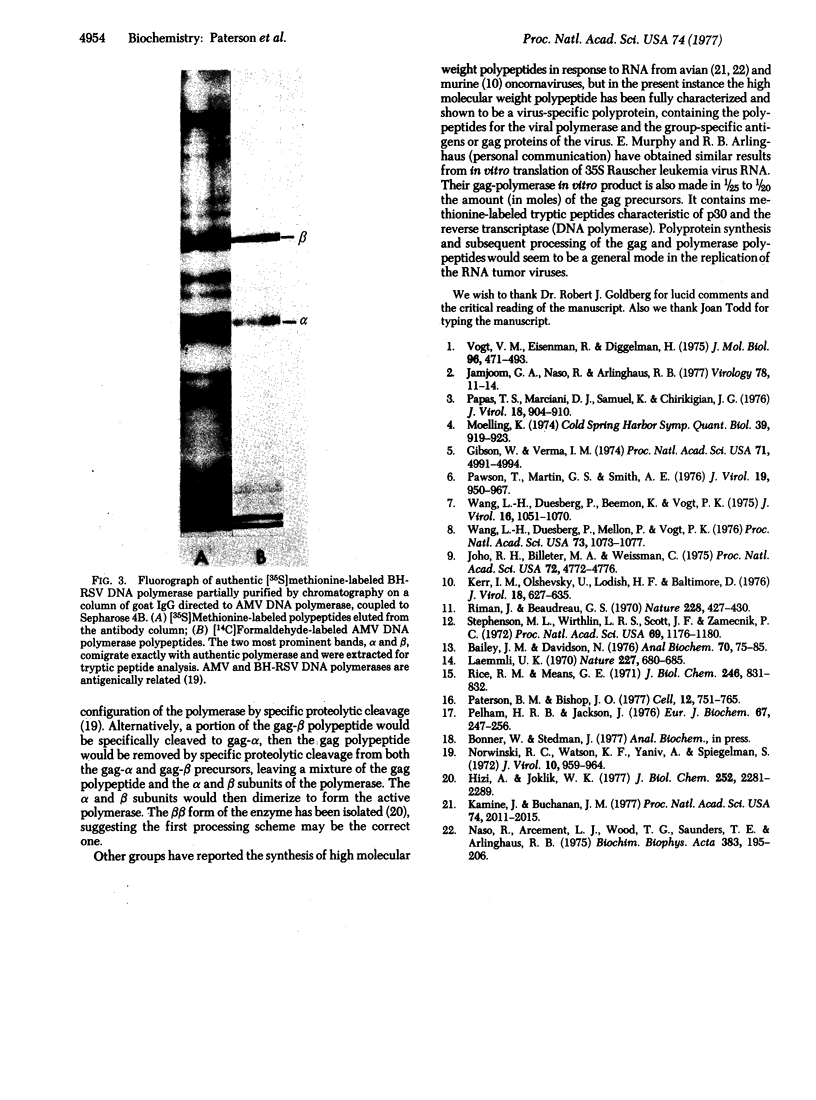
High molecular weight RNA (35S) isolated from avian myeloblastosis virus directs the cell-free synthesis of two prominent polypeptides of 180,000 and 76,000 molecular weight. The latter polypeptide has previously been identified as the precursor to the group-specific antigens of the virus ("gag" proteins) [Vogt, V. M., Eisenman, R. & Diggelmann, H. (1975) J. Mol. Biol. 96, 471-493]. Two-dimensional tryptic peptide analyses of the [35S]methionine-labeled peptides demonstrate that the 180,000-dalton product is a polyprotein that can account for all the peptides of the avian myeloblastosis virus DNA polymerase (DNA nucleotidyltransferase, EC 2.7.7.7) and those of the gag viral proteins. This is direct confirmation of the genomic order of the viral structural genes, placing the polymerase gene adjacent to the 5'-proximal gag gene of the virus. Furthermore, our findings suggest that the primary polymerase gene product is the beta subunit of the enzyme. These results are discussed in relation to the proposed structural gene map for the avian retraviruses and suggest a model for the in vivo processing of the viral polymerase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Cell-free synthesis of two proteins unique to RNA of transforming virions of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1977 May;74(5):2011–2015. doi: 10.1073/pnas.74.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Olshevsky U., Lodish H. F., Baltimore D. Translation of murine leukemia virus RNA in cell-free systems from animal cells. J Virol. 1976 May;18(2):627–635. doi: 10.1128/jvi.18.2.627-635.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Wood G., Saunders T. E., Arlinghaus R. B. The cell-free translation of Rauscher leukemia virus RNA into high molecular weight polypeptides. Biochim Biophys Acta. 1975 Mar 10;383(2):195–206. doi: 10.1016/0005-2787(75)90261-0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Watson K. F., Yaniv A., Spiegelman S. Serological analysis of the deoxyribonucleic acid polymerase of avian oncornaviruses. II. Comparison of avian deoxyribonucleic acid polymerases. J Virol. 1972 Nov;10(5):959–964. doi: 10.1128/jvi.10.5.959-964.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas T. S., Marciani D. J., Samuel K., Chirikjian J. G. Mechanism of release of active alpha subunit from dimeric alpha beta avian myeloblastosis virus DNA polymerase. J Virol. 1976 Jun;18(3):904–910. doi: 10.1128/jvi.18.3.904-910.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Ríman J., Beaudreau G. S. Viral DNA-dependent DNA polymerase and the properties of thymidine labelled material in virions of an oncogenic RNA virus. Nature. 1970 Oct 31;228(5270):427–430. doi: 10.1038/228427a0. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Mellon P., Vogt P. K. Distribution of envelope-specific and sarcoma-specific nucleotide sequences from different parents in the RNAs of avian tumor virus recombinants. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1073–1077. doi: 10.1073/pnas.73.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]