Abstract
We show that bimetallic nanoparticles (NPs) of AuPt without any surface modification are potent antibiotic reagents, while pure Au NPs or pure Pt NPs display no antibiotic activities. The most potent antibacterial AuPt NPs happen to be the most effective catalysts for chemical transformations. The mechanism of antibiotic action includes the dissipation of membrane potential and the elevation of adenosine triphosphate (ATP) levels. These bimetallic NPs are unique in that they do not produce reactive oxygen species as most antibiotics do. Being non-toxic to human cells, these bimetallic noble NPs might open an entry to a new class of antibiotics.
Keywords: bacteria, bimetallic nanoparticles, gold, platinum
Some metals such as Ag and Hg are well known to have intrinsic antibacterial properties.1 But these metals also pose serious threat to humans as they are toxic to human cells. Several reports have resorted to using nanoparticles (NPs) made of less toxic metals, such as gold, as safe antibiotics. Even though considered biologically inert in its bulk state, gold in the form of NPs can be activated as antibiotics by modifying with functional organic molecules on their surfaces.2 Because surface modification brings complications in characterization and preparation, we have been seeking NPs of nontoxic metals that do not require surface modifications to use as antibiotics. Our initial screening shows that single-component non-toxic metallic NPs do not have antibiotic capability;2a we thus turn to bimetallic NPs. While bimetallic NPs have found wide uses in chemical catalysis,3 nothing is known about their biological properties. AuPt NPs can catalyze many types of reactions, for example, the oxidation of methanol,4 ethanol,4b glycerol,5 other alcohols,6 formic acid,4b and glucose.3d The catalytic activity is highly dependent on the composition and the atomic arrangement.7 AuPt NPs show the best activity with a Pt content between 15–35 % for methanol oxidation,4b, 7b between 20–40 % for oxygen reduction,8 and between 28–40 % Pt for glucose oxidation.9 Most biological reactions are completed with the aid of enzymes. We hypothesized that the catalytic capability of bimetallic NPs at mild conditions could result in novel biological activities via effects on enzymatic activities (or the catalytic activities in cells). Here, we examined the viability of bacteria and mammalian cells under the influence of AuPt NPs, the morphological change of bacterial cells, and the respiration process to show that AuPt NPs can be potent antibiotics.
We synthesized AuPt NPs by the co-reduction of HAuCl4 and K2PtCl4 with sodium borohydride using Tween 80 as a stabilizer in water in an ice-water bath. We quantified the composition of bimetallic NPs using inductively coupled plasma-optical emission spectroscopy (ICP-OES) and observed their morphology using transmission electron microscopy (TEM). Monometallic NPs were synthesized using only the corresponding salt. These NPs were 2–3 nm in diameter (Figure S1 in the Supporting Information). The characteristic UV/Vis absorption of Au NPs disappeared with the increase of the Pt content (Figure S2). AuPt NPs were negatively charged in water (Table S1), which agrees with reported results.10 When the content of Pt was larger than 50 %, the negative charge of NPs increased with the increase of Pt.
We evaluated the antibacterial activities of AuPt NPs with the minimal inhibitory concentration (MIC) according to the reported method.2b AuPt NPs show significant antibiotic activities when the Pt content is between 10 % and 65 % (Table 1). The tested bacteria include five most important clinically Gram-negative bacteria, Escherichia coli (E. coli), multidrug-resistant E. coli (MDR E. coli), Pseudomonas aeruginosa (P. a), Klebsiella pneumoniae (K. p), and Salmonella choleraesius (S. c). Gram-negative bacteria can induce the infection of almost all organs in body.11 E. coli and K. p can induce the infection of urinary, biliary, gastrointestinal tracts, lung, and blood. P. a and K. p can induce lung infection. E. coli and S. c can cause severe food contamination. MDR E. coli was isolated from a local hospital and carried extended-spectrum β-lactamase (ESBL) resistance genes. In contrast to bimetallic NPs, monometallic Au and Pt NPs showed no antibacterial activity against all tested bacteria. AuPt NPs with the Pt content larger than 65 % showed weak activities and those with the Pt content of 94 % had no activity. AuPt NPs with the Pt content between 10–65 % showed extensive antibacterial activities against both the laboratory standard strains and the clinical MDR strain. AuPt NPs with 20 % Pt showed the best activity. The MIC was 5 μg mL−1 against E. coli, MDR E. coli, P. a, and K. p, and 9 μg mL−1 against S. c. The MIC values thus indicate that the bimetallic AuPt NPs are potent antibiotic reagents.
Table 1.
Antibacterial activities of AuPt NPs and monometallic NPs (minimal inhibitory concentration, MIC, μg mL−1).
| Au100−xPtx[a] | MIC [μg mL−1] | ||||
|---|---|---|---|---|---|
| E. coli[b] | MDR E. coli | P. a | K. p | S. c | |
| Au | >128 | >128 | >128 | >128 | >128 |
| Au95Pt5 | >128 | 16 | >128 | >128 | >128 |
| Au94Pt6 | >128 | 5 | 17 | 9 | 34 |
| Au90Pt10 | 5 | 5 | 9 | 9 | 18 |
| Au80Pt20 | 5 | 5 | 5 | 5 | 9 |
| Au66Pt34 | 6 | 6 | 6 | 12 | 12 |
| Au51Pt49 | 16 | 16 | 16 | 16 | 32 |
| Au35Pt65 | 23 | 23 | 46 | 46 | 46 |
| Au20Pt80 | 41 | >128 | 82 | 82 | 82 |
| Au6Pt94 | >128 | >128 | >128 | >128 | >128 |
| Pt | >128 | >128 | >128 | >128 | >128 |
| gentamicin | 1 | >64 | 2 | 1 | 4 |
| levofloxacin | 0.12 | 32 | 2 | 0.12 | 0.12 |
[a] x is the atomic percentage of Pt in the NP. [b] E. coli=Escherichia coli, P. a=Pseudomonas aeruginosa, K. p=Klebsiella pneumoniae, S. c=Salmonella choleraesius.
We next determined the minimal bactericidal concentrations (MBCs) of E. coli to determine if the bimetallic NPs can be bactericidal. MICs indicate the ability of inhibiting the growth of bacteria but not necessarily killing them, while MBCs indicate the ability of antibiotics in killing bacteria.12 A bactericidal agent is defined as a material with a ratio of MBC to MIC≤4.12b Antibiotics with a ratio of MBC to MIC>4 are defined as bacteriostatic agents. The bactericidal agent kills bacteria rapidly and reduces the development of bacterial resistance, hence as a better choice for clinicians in most cases.12a The MBCs of AuPt NPs with the Pt content between 10–65 % are the same as their MICs, which means that AuPt NPs belong to bactericidal agents against E. coli.
We investigated antibacterial activities of NPs in the presence of biomacromolecules. Biomacromolecules like proteins can adsorb to the surface of NPs and probably change their biological effects.13 When we added 10 % fetal bovine serum in the broth, two representative NPs, Au80Pt20 and Au66Pt34, showed effective activities against bacteria (Table S2). The presence of biomacromolecules thus does not significantly affect the antibacterial activity of NPs.
We note that the most effective antibiotic AuPt NPs are also the best catalysts reported in the literature (Table S3).4b, 7b, 8a Reports in the catalysis of bimetallic NPs state that the synergistic catalysis originates from the change of electron states for both Au and Pt in AuPt NPs.7 The transfer of charge from Pt to Au leads to the increase of d-orbital vacancy in AuPt NPs and the change of the electrocatalytic properties of Au and Pt. When Pt is doped into Au NPs, it increases the adsorption or production of oxygenated species on Au, which is important for catalytic reactions.7 Semiconductor–metal composite Ag2S/Ag NPs killed bacteria presumably by a local circuit loop and reactive oxygen species (ROS) produced under UV irradiation.14 In the following section, we investigate the antibacterial action of AuPt NPs by the observation of bacterial morphology, the detection of membrane permeability, the respiration process (the intracellular redox reaction), and ROS.
We took E. coli as an example to investigate the mechanism of action of AuPt NPs. We visualize the morphological change of E. coli treated with AuPt NPs using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Figure 1). Antibacterial Au90Pt10, Au80Pt20, and Au51Pt49 induced cell lysis (Figure 1 A). TEM images confirmed their structural changes. Au80Pt20 induced blurring of the cytoplasm membrane boundary, loss of interior structures, and the formation of a large-scale light area (Figure 1 B), which suggests that the lysis of bacterial cells took place.15 Hence, AuPt NPs can induce disruption to cell membrane and the lysis of bacterial cells.
Figure 1.
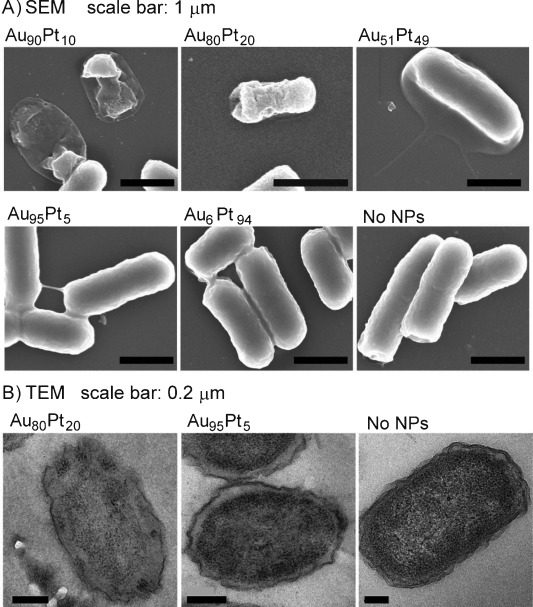
Morphological changes of E. coli treated with AuPt NPs (40 μg mL−1, 2 h) visualized with A) SEM and B) TEM. In (A), antibacterial Au90Pt10, Au80Pt20, and Au51Pt49 induced the lysis of bacterial cells. In (B), Au80Pt20 induced blurring of the cytoplasm membrane, loss of the interior structure, and formation of a large-scale light area (the status of lysis).
We used fluorescent dyes to assess the integrity of the cell membrane in the presence of NPs. The hydrophobic fluorophore 1-N-phenylnaphthylamine (NPN) can bind to the outer membrane and yields increased fluorescence when bacterial outer membrane is disrupted. Hence, the fluorescent dye can indicate the action of NPs on the outer membrane. We treated E. coli with 40 μg mL−1 AuPt NPs for 4 h, collected bacterial cells, and incubated them with NPN for 30 min. All of AuPt NPs can increase the fluorescence to some extent (Figure 2 A). We conclude that the structural change of outer membrane could not be a cause for the antibacterial action of AuPt NPs with 10–65 % of Pt. We used the dye DiSC3(5) to probe the inner membrane potential because the fluorescence of the dye increases when the membrane potential collapses.16 The three best antibacterial AuPt NPs, Au90Pt10, Au80Pt20, and Au66Pt34, can significantly disrupt the inner membrane and decrease the membrane potential (Figure 2 B). We deduced that the collapse of membrane potential probably led to bacterial death, which is in accordance with mechanisms for antibacterial agents.2b, 16, 17
Figure 2.
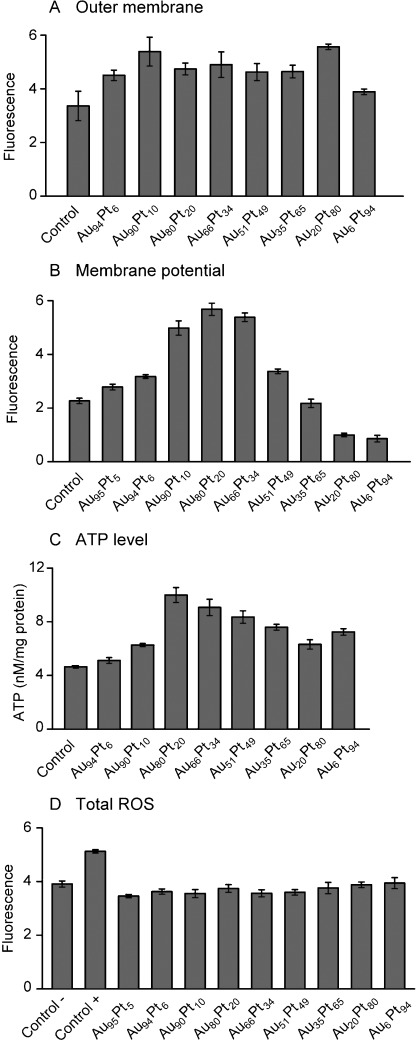
The effects of AuPt NPs on the cell membrane and the respiration chain of E. coli. A) Outer membrane permeability probed with the NPN dye. B) Inner membrane potential probed with the DiSC3(5) dye. C) Intracellular ATP concentrations corrected with the protein content. D) Cellular total ROS probed with 2′,7′-dichlorofluorescein diacetate (DCFH-DA). E. coli without addition of NPs was the control in all assays or the negative control in (D). The positive control in (D) was commercial Rosup producing ROS in the kit.
Because of the vital role ATP plays in bacterial metabolism, we determined the level of intracellular ATP, the activity of F-type ATP synthase, and the NAD+/NADH reaction in the inner membrane. The generation of ATP is an important part in the bacterial respiration chain, which requires the membrane potential, F-type ATP synthase, and protons from the NAD+/NADH reaction. Surprisingly, we found that AuPt NPs with high antibacterial activities significantly increased intracellular ATP levels, among which Au80Pt20 induced a 2-fold increase compared to the control (Figure 2 C). By contrast, AuPt NPs reduced the activity of F-type ATP synthase and did not affect the ratio of NAD+ to NADH (Figure S3). There are two possibilities that can explain the elevation of ATP levels. One is that AuPt as an alternative enzyme could catalyze the generation of ATP. It has been reported that high ATP levels caused by the overexpression of Pck kinase can inhibit the growth of E. coli, and at the same time up-regulate DNA damage-related genes.18 High ATP level can thus be toxic to bacteria. The other possibility is that AuPt could inhibit the synthesis of proteins that consume ATP, thus inducing the accumulation of ATP. In this respect, AuPt NPs resemble the antibiotic chloramphenicol, a kind of protein synthesis inhibitor that is shown to increase the level of ATP.19 The increase of the level of ATP, in addition to being known in some cases of small-molecular antibiotics, is also a strategy that the human immune system employs to kill bacteria.20 ATP-mediated bacterial killing is independent of ROS.21
We examined the production of ROS in NP-treated E. coli to test if bacterial death resulted from oxidative damage, since AuPt NPs can catalyze some redox reactions. Bactericidal antibiotics can induce the generation of ROS to kill bacteria.22 V2O5 NPs as oxidase mimics have been reported to inhibit the bacterial biofilm via ROS.23 Fe3O4-doped silica NPs show composition- and catalysis-related ROS effects on mammalian cells.24 By determining the total ROS with 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and hydroxyl radical (a type of ROS) with hydroxyphenyl fluorescein (HPF), we found that AuPt NPs did not significantly increase the production of either total ROS (Figure 2 D) or hydroxyl radicals (Figure S3). Hence the antibiotic mechanism of bactericidal AuPt NPs does not involve any ROS; this result shows that the mechanisms of action of AuPt NPs are quite different from conventional antibiotics.
We determined the Pt release of AuPt NPs to test if the soluble Pt inside the bacterial cell contributed to bacterial death, since some NPs exert toxicity with their soluble content inside cells.25 In neutralophilic bacteria, cytoplasmic pH values are 7.5–7.7.26 We incubated 50 μg mL−1 of Au95Pt5, Au80Pt20, Au66Pt34, Au6Pt94 NPs in H2O (pH 7.0) and phosphate buffered saline (PBS, pH 7.6) at 37 °C for 96 h, centrifuged with Millipore ultrafilter (MWCO 3000 Da, 1.5 nm or larger NPs cannot pass through the filter), and determined the content of Au and Pt in the filtrate with ICP-MS. The Au release amount is zero for all NPs in H2O and PBS (Table S4). The Pt release percentage is less than 0.2 % for all NPs in H2O and in PBS. The Pt release amount of NPs is far less than MICs. For example, 0.03 μg mL−1 of Pt was released from 50 μg mL−1 of Au80Pt20 while its MIC was 5 μg mL−1 against E. coli. There is no correlation between the Pt release and the antibacterial activity. For example, the Pt amount released from Au6Pt94 was nearly identical to that from Au80Pt20, but Au6Pt94 was totally inactive. Thus, the Pt release could not be a cause for bacterial death.
Our investigation in the antibacterial mechanism of AuPt NPs shows that their excellent antibiotic activities mainly come from: 1) the damage of the inner membrane that compromises the integrity of bacteria; 2) the increase of the intracellular ATP level. A surprise here is that the antibiotic mechanism does not involve ROS at all. This mechanism is therefore unique, because most commercially available bactericidal antibiotics or metal-based agents (e.g., Ag, Cu, ZnO, TiO2) invariably involve elevated levels of ROS to kill bacteria.
For a preliminary evaluation of the potential toxicity of AuPt NPs, we tested their effect on the viability of human umbilical vein endothelial cells (HUVECs) with a CCK-8 kit, which quantifies the number of viable cells. Antibiotic AuPt NPs did not affect the cell viability (>95 %) at the concentration as high as 80 μg mL−1 after 24 h of incubation (Figure 3). After the longer-term incubation such as 48 h and 72 h, NPs at 40 μg mL−1 did not affect the cell viability (>95 %) (Figure S4). Hence, AuPt NPs showed selective toxicity to bacteria but not to mammalian cells. This selectivity could result from the difficulty for NPs to act on the respiration chain in the mammalian mitochondria, which need to overcome several barriers including the escape from lysosomes, targeting mitochondria, and entry into of mitochondria through the membrane.27
Figure 3.
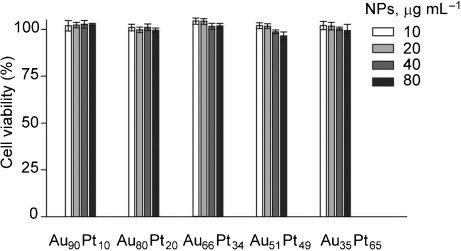
Cell viability of HUVECs after incubation for 24 h with antibiotic AuPt NPs at different concentrations.
In conclusion, our work reports that AuPt NPs are potent antibacterial agents and harmless to human cells. By contrast, pure Au NPs or pure Pt NPs are not antibiotic at all. The antibiotic mechanisms of AuPt NPs include the rupture in the bacterial inner membrane and the increase of intracellular ATP levels, but do not involve the generation of ROS. Further work is required to elucidate the identity of the substrate in the intracellular catalytic reactions related to this process. Nevertheless, we believe that our findings in this work not only extend the application of bimetallic NPs (made of inert noble metals) as new classes of antibacterial agents, but may also provide new perspectives in biological reactions to broaden the application of NPs in medicine.
Dedicated to Professor George M. Whitesides on the occasion of his 75th birthday
Supporting Information
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201401035.
References
- [1a].Lemire JA, Harrison JJ, Turner RJ. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- [1b].Chernousova S, Epple M. Angew. Chem. 2013;125 doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52 [Google Scholar]
- [2a].Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X. J. Am. Chem. Soc. 2010;132:12349–12356. doi: 10.1021/ja1028843. [DOI] [PubMed] [Google Scholar]
- [2b].Zhao Y, Chen Z, Chen Y, Xu J, Li J, Jiang X. J. Am. Chem. Soc. 2013;135 doi: 10.1021/ja4058635. [DOI] [PubMed] [Google Scholar]
- [2c].Zhao Y, Jiang X. Nanoscale. 2013;5 doi: 10.1039/c3nr01990j. [DOI] [PubMed] [Google Scholar]
- [2d].Gu HW, Ho PL, Tong E, Wang L, Xu B. Nano Lett. 2003;3 [Google Scholar]
- [3a].Lim B, Jiang M, Camargo PH, Cho EC, Tao J, Lu X, Zhu Y, Xia Y. Science. 2009;324:1302–1305. doi: 10.1126/science.1170377. [DOI] [PubMed] [Google Scholar]
- [3b].Peng Z, Yang H. Nano Today. 2009;4 [Google Scholar]
- [3c].Debe MK. Nature. 2012;486 doi: 10.1038/nature11115. [DOI] [PubMed] [Google Scholar]
- [3d].Zhang Y, Cui X, Shi F, Deng Y. Chem. Rev. 2012;112 doi: 10.1021/cr200260m. [DOI] [PubMed] [Google Scholar]
- [4a].Suntivich J, Xu Z, Carlton CE, Kim J, Han B, Lee SW, Bonnet N, Marzari N, Allard LF, Gasteiger HA, Hamad-Schifferli K, Shao-Horn Y. J. Am. Chem. Soc. 2013;135:7985–7991. doi: 10.1021/ja402072r. [DOI] [PubMed] [Google Scholar]
- [4b].Ilayaraja N, Prabu N, Lakshminarasimhan N, Murugan P, Jeyakumar D. J. Mater. Chem. A. 2013;1 [Google Scholar]
- [5a].Villa A, Veith GM, Prati L. Angew. Chem. 122 doi: 10.1002/anie.201000762. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:4601–4604. [Google Scholar]
- [5b].Brett GL, He Q, Hammond C, Miedziak PJ, Dimitratos N, Sankar M, Herzing AA, Conte M, Lopez-Sanchez JA, Kiely CJ, Knight DW, Taylor SH, Hutchings GJ. Angew. Chem. 2011;123 doi: 10.1002/anie.201101772. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50 [Google Scholar]
- [6].Schrinner M, Proch S, Mei Y, Kempe R, Miyajima N, Ballauff M. Adv. Mater. 2008;20:1928–1933. [Google Scholar]
- [7a].Mokkath JH, Schwingenschlogl U. RSC Adv. 2013;3:15350–15353. [Google Scholar]
- [7b].Mott D, Luo J, Njoki PN, Lin Y, Wang LY, Zhong CJ. Catal. Today. 2007;122 [Google Scholar]
- [8a].Luo J, Njoki PN, Lin Y, Wang LY, Zhong CJ. Electrochem. Commun. 2006;8 [Google Scholar]
- [8b].Ferrando R, Jellinek J, Johnston RL. Chem. Rev. 2008;108 doi: 10.1021/cr040090g. [DOI] [PubMed] [Google Scholar]
- [9].Comotti M, Della Pina C, Rossi M. J. Mol. Catal. A. 2006;251:89–92. [Google Scholar]
- [10].Wang D, Li Y. Adv. Mater. 2011;23:1044–1060. doi: 10.1002/adma.201003695. [DOI] [PubMed] [Google Scholar]
- [11].Alekshun MN, Levy SB. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [12a].Finberg RW, Moellering RC, Tally FP, Craig WA, Pankey GA, Dellinger EP, West MA, Joshi M, Linden PK, Rolston KV, Rotschafer JC, Rybak MJ. Clin. Infect. Dis. 2004;39:1314–1320. doi: 10.1086/425009. [DOI] [PubMed] [Google Scholar]
- [12b].Pankey GA, Sabath LD. Clin. Infect. Dis. 2004;38 doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- [13a].Zhao YY, Wang Z, Zhang W, Jiang XY. Nanoscale. 2011;2:2114–2119. doi: 10.1039/c0nr00309c. [DOI] [PubMed] [Google Scholar]
- [13b].Stark WJ. Angew. Chem. 2010;123 [Google Scholar]; Angew. Chem. Int. Ed. 2011;50 [Google Scholar]
- [13c].Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ. Environ. Sci. Technol. 2005;39 doi: 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- [14].Pang M, Hu J, Zeng HC. J. Am. Chem. Soc. 2010;132:10771–10785. doi: 10.1021/ja102105q. [DOI] [PubMed] [Google Scholar]
- [15].Witte A, Wanner G, Sulzner M, Lubitz W. Arch. Microbiol. 1992;157:381–388. doi: 10.1007/BF00248685. [DOI] [PubMed] [Google Scholar]
- [16].Cui Y, Zhao Y, Tian Y, Zhang W, Lu X, Jiang X. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- [17a].Lu P, Haagsma AC, Pham H, Maaskant JJ, Mol S, Lill H, Bald D. Antimicrob. Agents Chemother. 2011;55:5354–5357. doi: 10.1128/AAC.00507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17b].Hurdle JG, O’Neill AJ, Chopra I, Lee RE. Nat. Rev. Microbiol. 2011;9 doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kwon YD, Lee SY, Kim P. Biosci. Biotechnol. Biochem. 2008;72:1138–1141. doi: 10.1271/bbb.70831. [DOI] [PubMed] [Google Scholar]
- [19].Schneider DA, Gourse RL. J. Biol. Chem. 2004;279:8262–8268. doi: 10.1074/jbc.M311996200. [DOI] [PubMed] [Google Scholar]
- [20a].Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. Proc. Natl. Acad. Sci. USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20b].Coutinho-Silva R, Ojcius DM. Microbes Infect. 2012;14 doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20c].Xiang Y, Wang X, Yan C, Gao Q, Li SA, Liu J, Zhou K, Guo X, Lee W, Zhang Y. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063759. , e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- [22].Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- [23].Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R, Tremel W. Nat. Nanotechnol. 2012;7:530–535. doi: 10.1038/nnano.2012.91. [DOI] [PubMed] [Google Scholar]
- [24].Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Environ. Sci. Technol. 2007;41:4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- [25].Studer AM, Limbach LK, Van Duc L, Krumeich F, Athanassiou EK, Gerber LC, Moch H, Stark WJ. Toxicol. Lett. 2010;197:169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- [26].Krulwich TA, Sachs G, Padan E. Nat. Rev. Microbiol. 2011;9:330–343. doi: 10.1038/nrmicro2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marrache S, Dhar S. Proc. Natl. Acad. Sci. USA. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


