Abstract
Sleep and circadian rhythms are intrinsically linked, with several sleep traits, including sleep timing and duration, influenced by both sleep homeostasis and the circadian phase. Genetic variation in several circadian genes has been associated with diurnal preference (preference in timing of sleep), although there has been limited research on whether they are associated with other sleep measurements. We investigated whether these genetic variations were associated with diurnal preference (Morningness–Eveningness Questionnaire) and various sleep measures, including: the global Pittsburgh Sleep Quality index score; sleep duration; and sleep latency and sleep quality. We genotyped 10 polymorphisms in genes with circadian expression in participants from the G1219 sample (n = 966), a British longitudinal population sample of young adults. We conducted linear regressions using dominant, additive and recessive models of inheritance to test for associations between these polymorphisms and the sleep measures. We found a significant association between diurnal preference and a polymorphism in period homologue 3 (PER3) (P < 0.005, recessive model) and a novel nominally significant association between diurnal preference and a polymorphism in aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) (P < 0.05, additive model). We found that a polymorphism in guanine nucleotide binding protein beta 3 (GNβ3) was associated significantly with global sleep quality (P < 0.005, recessive model), and that a rare polymorphism in period homologue 2 (PER2) was associated significantly with both sleep duration and quality (P < 0.0005, recessive model). These findings suggest that genes with circadian expression may play a role in regulating both the circadian clock and sleep homeostasis, and highlight the importance of further studies aimed at dissecting the specific roles that circadian genes play in these two interrelated but unique behaviours.
Keywords: circadian expressed genes, genetic association, single nucleotide polymorphisms, sleep duration, sleep quality
Introduction
Circadian rhythms are daily cycles controlled by a central hypothalamic pacemaker and affect a wide range of physiological and behavioural, outputs including the sleep–wake cycle (Franken and Dijk, 2009). This central pacemaker is known to be governed by a complex molecular clock, which consists of various transcriptional feedback loops and output systems (reviewed in Zhang and Kay, 2010). Previous research has determined a number of the genes involved in these transcriptional feedback loops that drive the central pacemaker (the clock genes) and numerous other genes whose expression are rhythmically regulated by these core clock genes (reviewed in Zhang and Kay, 2010).
Diurnal preference, an individual's preference of timing of the sleep–wake cycle, is influenced by both the circadian clock and by sleep homeostatis (Kerkhof, 1991; Mongrain et al., 2004, 2006; Schmidt et al., 2009; Taillard et al., 2003). Diurnal preference and certain sleep measures, including sleep quality and duration, vary across individuals. Twin studies suggest that genetic influences partially underlie the variation seen for diurnal preference, sleep quality and sleep duration (Barclay et al., 2010a,b; Partinen et al., 1983). Previously, genetic variation in several clock genes [circadian locomotor output cycles kaput (CLOCK), period homologue (PER)1, PER2, PER3] has been shown to be associated with diurnal preference in the general population (Archer et al., 2010; Carpen et al., 2006; Katzenberg et al., 1998; Lee et al., 2007, 2011). Genetic variation in guanine nucleotide binding protein beta 3 (GNβ3), which has circadian expression in the mouse pituitary (Hughes et al., 2009), has also been associated with diurnal preference (Johansson et al., 2004). Similarly, other studies have suggested that variation in other circadian genes, aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) and D site of albumin promoter (albumin D-box) binding protein (DBP), may affect specific circadian outputs, such as daily changes in mood (Shi et al., 2008).
Sleep and circadian rhythms are intrinsically linked, with a number of sleep traits, including sleep timing and duration, influenced by both sleep homeostasis and the circadian phase (Borbely, 1982). Given the influences of the circadian clock on sleep behaviours, we hypothesized that the same single nucleotide polymorphisms (SNPs) that had been associated with diurnal preference could also be associated with additional subjective sleep measures. We thus attempted to both replicate the associations of these SNPs in genes with circadian expressionwith diurnal preference and to further investigate whether they were associated with specific subjective sleep measures in the G1219 sample. We limited our investigations to those SNPs that were associated previously with diurnal preference and circadian outputs, e.g. worse evening mood (summarized in Table 1), including a rare, unpublished F-box and leucine-rich repeat protein 3(FBXL3) SNP that was found previously to be associated with extreme values of diurnal preference (S. N. Archer, unpublished data).
Table 1.
Summary of Investigated single nucleotide polymorphisms (SNPs)
| Gene | SNP ID | Allele | Genotypic frequency | Gene region | Related phenotype | Citation | ||
|---|---|---|---|---|---|---|---|---|
| M/M | M/m | m/m | ||||||
| ARNTL2 | rs922270 | T/C | 690 (72.9) | 240 (25.3) | 17 (1.8) | Intronic region | Worse evening mood | Shi et al. (2008) |
| CLOCK | rs2070062 | T/C | 530 (56.1) | 341 (36.1) | 73 (7.7) | 3′ UTR region | Diurnal preference | Pedrazzoli et al. (2007) |
| DBP | rs3848543 | C/T | 673 (73.3) | 231 (25.2) | 14 (1.5) | Intronic region | Worse evening mood | Shi et al. (2008) |
| FBXL3 | 825679097 | G/T | 939 (99.8) | 2 (0.2) | 0 (0) | Coding (Gly/Val) | Diurnal preference | S. N. Archer (unpublished data) |
| GNβ3 | rs5443 | C/T | 466 (49.4) | 387 (41) | 91 (9.6) | Affects splicing | Diurnal preference | Johansson et al. (2004) |
| PER1 | rs2735611 | T/C | 688 (73.7) | 224 (24) | 22 (2.4) | Coding (silent) | Diurnal preference | Carpen et al. (2006) |
| PER2 | rs934945 | G/A | 604 (64.2) | 295 (31.4) | 42 (4.5) | Coding (Gly/Glu) | Diurnal preference | Lee et al. (2007) and Carpen et al. (2006) |
| PER2 | rs2304672 | C/G | 796 (84.4) | 144 (15.3) | 3 (0.3) | Promoter region | Diurnal preference | Carpen et al. (2006) and Lee et al. (2011) |
| PER3 | rs2797687 | G/T | 611 (67.1) | 266 (29.2) | 33 (3.6) | Promoter region | DSPD | Archer et al. (2010) |
| PER3 | rs10462020 | G/T | 606 (64.3) | 274 (29.1) | 63 (6.7) | Coding (Val/Gly) | Diurnal preference and DSPD | Johansson et al. (2003); Ebisawa et al. (2001) |
ARNTL2, aryl hydrocarbon receptor nuclear translocator-like 2; CLOCK, circadian locomotor output cycles kaput; DBP, D site of albumin promoter (albumin D-box) binding protein; PER, period homologue (PER1, PER2, PER3); FBXL3, F-box and leucine-rich repeat protein 3; GNβ3, guanine nucleotide binding protein beta 3; DSPD, delayed sleep phase disorder.
The genotypic frequencies for the 10 SNPs that varied in the G1219 sample are listed in this table. The allele lists the major allele first (M) and minor allele second (m). The genotypic frequencies relate to the major and minor alleles. Additional information is listed for each SNP, including its position relative to the gene and the related phenotype.
Materials and Methods
Participants
The participants in this study come from the G1219 and G1219 Twins longitudinal studies (McAdams et al., 2013). The G1219 sample comprises the adolescent offspring of adults from a large-scale population-based study (Eley et al., 2004), while the G1219 Twins sample comprises randomly selected twin sets born between 1985 and 1988 (McAdams et al., 2013). Informed consent was obtained from parents/guardians of all adolescents aged <16 years, and from the adolescents themselves when ≥16 years old. Ethical approval for different stages of this study has been provided by the Research Ethics Committees of the Institute of Psychiatry, South London and Maudsley NHS Trust, and Goldsmiths, University of London.
This study focuses exclusively on wave 4, comprising 1556 individuals (collected in 2007) as, at the time of analyses, subjective sleep data were available at this wave only. Individuals were selected for inclusion if sleep measures were available and DNA had been collected. The total sample was 1130 (73% of those participating at wave 4). For monozygotic (MZ) twin pairs, data from only one individual, selected at random, was included in the analyses. The analyses are based on sleep data and DNA from 952 wave four participants [mean age = 20.3 years, standard deviation (SD) = 1.77, age range 18–27 years; 61.0% female] consisting of 562 dizygotic (DZ) individuals, 180 MZ individuals and 210 individuals from sibling pairs.
Measures
Diurnal preference
The Morningness–Eveningness Questionnaire (MEQ, Horne and Oostburg, 1976) measured diurnal preference. The MEQ is a 19-item self-report questionnaire which assesses individual preference for the timing of a number of diurnal activities, sleeping habits, hours of peak performance and times of ‘feeling best’ and maximum alertness. Individual items are rated on a 4- or 5-point scale and the total score ranges from 16 to 86. Higher scores indicate a greater ‘morningness’ preference. For further details of the validity of the MEQ in the G1219 sample, see elsewhere (Barclay et al., 2010a). The MEQ score had a mean of 48.6 (SD = 8.2).
Sleep quality
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989), which is a widely used questionnaire measure containing 18 items assessing sleep over the previous month. Global scores range from 0 to 21, with higher scores indicating poorer sleep quality. For further details of the validity of the MEQ in the G1219 sample, see elsewhere (Barclay et al., 2011). The global PSQI mean score was 5.9 (SD = 3.1).
In addition to the global score, three measures of sleep (sleep duration, sleep latency and sleep quality) were assessed using the PSQI. Sleep duration was measured using question 4 of this questionnaire: ‘During the past month, how many hours of actual sleep did you get at night?’. Sleep duration ranged from 2 to 12 h with a mean of 7.5 h (SD = 1.2). Sleep latency was measured using question 2 of this questionnaire: ‘During the past month, how long (in min) has it usually taken you to fall asleep each night?’. Sleep latency ranged from 0 to 240 min and had a mean of 27.8 (SD = 23.3). Sleep quality was measured using question 6 of this questionnaire: ‘During the past month, how would you rate your sleep quality overall?’, and was scored: very good = 0; fairly good = 1; fairly bad = 2; and very bad = 3. Sleep quality had a mean score of 1.13 (SD = 0.71).
DNA extraction and genotyping
Cheek swab kits were posted to participants in order to collect DNA, primarily during wave 4. The DNA extractions are described elsewhere (Barclay et al., 2011). Ten single nucleotide polymorphisms (SNPs) were genotyped from a number of genes with circadian expression (see Table 1). The FBXL3_1362G/T SNP that was found previously to be associated with extreme values of diurnal preference (S. N. Archer, unpublished data) was submitted to dbSNP and has the SNP submission number: 825679097. All the genotyping assays were performed by KBioscience (http://www.lgcgenomics.com/) using KASPar chemistry (for more details see: http://www.lgcgenomics.com/genotyping/kasp-genotyping-reagents/kasp-overview/). Blind duplicates and Hardy–Weinberg equilibrium (HWE) tests were used as quality control tests. Linkage disequilibrium and HWE were calculated using the Haploview program (Barrett et al., 2005). The PER3 SNP rs10462020 failed to reach HWE in the total sample (χ2 = 16.1, P = 0.0001), but was in HWE in the male subset (P < 0.05). All other SNPs were in HWE (P < 0.05).
Statistical analyses
All analyses were performed in stata (StataCorp., 2002). Linear regressions were conducted to model the main effect of the SNPs on diurnal preference, global PSQI sleep quality, sleep duration, sleep latency and sleep quality. Age and sex were first entered into the regression models, followed by main effects of genotype. We investigated the three non-independent models of inheritance: additive, dominant and recessive (Lewis and Knight, 2012). To investigate the combined effects of SNPs on specific sleep measures, we also conducted regression analyses for each sleep phenotype adding all 10 of the SNPs we investigated into the model simultaneously. So that we could determine additionally if the significant individual associations were independent of each other, we varied the model of inheritance included in the combined analysis for each measure investigated. For each measure we included the model of inheritance that was most significant for each SNP from the individual analysis; for those SNPs with no significant model of inheritance for that measure we used the additive model of inheritance. As our sample included related individuals, all analyses were corrected conservatively for the non-independence of the twin/sibling observations using the ‘robust’ cluster command in stata, as is standard in analyses of this type (see Rogers, 1993). This analysis should correct for any failures to reach the assumptions that are required for using linear models, such as linearity, independence, normality and homoscedasticity. If these assumptions are met, then a standard linear regression model should account for differences in group size across the genotype groups. We applied a Bonferroni correction to control for multiple testing independently for each of the sleep measures. We adjusted the P-value by using the total number of SNPs investigated for each trait, thus the corrected P-values for diurnal preference, global PSQI sleep quality, sleep duration, sleep latency and sleep quality are: corrected P = 0.05/10 = 0.005. We did not apply corrections for multiple testing for the number of inheritance models that we ran, as these tests were not independent of each other, and instead tested related hypotheses. Similarly, we did not apply further multiple testing corrections across all the phenotypes investigated, as a number of sleep measures are at least partially correlated and thus are not fully independent tests. If we were to correct stringently for all sleep measures investigated, then we would require a Bonferroni-adjusted P-value of 0.001. None of the analyses reported here would survive this correction, but this would be a very conservative correction given that the sleep measures are not completely independent of each other.
Results
The genotype counts and percentages for the 10 SNPs investigated are summarized in Table 1. Table 2 summarizes the means and standard deviations for each measure by genotype.
Table 2.
Means and standard deviations for all sleep measures by genotype
| Gene | SNP ID | Allele | Diurnal preference | Global PSQI | Sleep duration | Sleep latency | Sleep quality |
|---|---|---|---|---|---|---|---|
| ARNTL2 | rs922270 | TT | 48.4 (8.2) | 5.9 (3.2) | 7.5 (1.2) | 28.7 (23.2) | 1.2 (0.7) |
| TC | 48.8 (8.2) | 5.6 (2.9) | 7.5 (1.1) | 26.1 (24.3) | 1 (0.7) | ||
| CC | 52.6 (6.6) | 6 (2.6) | 7.6 (0.9) | 20.7 (13.3) | 1.1 (0.6) | ||
| Total n | 945 | 927 | 932 | 934 | 941 | ||
| CLOCK | rs2070062 | TT | 48.6 (8.2) | 5.8 (3.2) | 7.5 (1.2) | 28.3 (24.6) | 1.1 (0.7) |
| TG | 48.7 (8.1) | 5.9 (2.9) | 7.4 (1.2) | 26.9 (21.2) | 1.1 (0.7) | ||
| GG | 47.7 (9.1) | 5.8 (3.1) | 7.4 (1.2) | 25.7 (16.3) | 1.2 (0.7) | ||
| Total n | 942 | 924 | 929 | 931 | 938 | ||
| DBP | rs3848543 | CC | 48.7 (8.3) | 5.9 (3.1) | 7.4 (1.2) | 28.8 (24.9) | 1.1 (0.7) |
| CT | 48.4 (7.8) | 5.5 (3.2) | 7.6 (1.2) | 24.9 (18.2) | 1.1 (0.7) | ||
| TT | 46.2 (9.4) | 6.3 (4) | 6.9 (1.3) | 28.9 (25.7) | 1.2 (0.8) | ||
| Total n | 916 | 899 | 903 | 905 | 913 | ||
| FBXL3 | 825679097 | GG | 48.5 (8.2) | 5.9 (3.1) | 7.5 (1.2) | 27.8 (23.5) | 1.1 (0.7) |
| GT | 52 (11.3) | 5 (1.4) | 8.3 (1.1) | 22.5 (10.6) | 1 (0.0) | ||
| Total n | 938 | 920 | 925 | 927 | 934 | ||
| GNβ3 | rs5443 | CC | 48.3 (8.2) | 5.9 (3) | 7.5 (1.2) | 27.3 (20.3) | 1.2 (0.7) |
| CT | 48.6 (8.5) | 6 (3.3) | 7.4 (1.3) | 28.6 (27) | 1.1 (0.8) | ||
| TT | 49.5 (7.4) | 5.1 (2.8) | 7.7 (1) | 26.8 (21.5) | 1 (0.7) | ||
| Total n | 942 | 924 | 929 | 931 | 938 | ||
| PER1 | rs2735611 | TT | 48.7 (8.3) | 5.9 (3.1) | 7.5 (1.2) | 28 (23.9) | 1.1 (0.7) |
| TC | 48.2 (8) | 5.8 (3) | 7.6 (1.2) | 27.2 (21.9) | 1.2 (0.7) | ||
| CC | 48.8 (6.4) | 6.3 (3.8) | 7 (1.1) | 31.7 (28.2) | 1 (0.7) | ||
| Total n | 932 | 914 | 919 | 921 | 928 | ||
| PER2 | rs934945 | GG | 48.6 (8.4) | 5.8 (3.1) | 7.5 (1.2) | 27.6 (24.1) | 1.1 (0.7) |
| GA | 48.6 (7.9) | 6.1 (3.1) | 7.4 (1.2) | 29.1 (22.9) | 1.2 (0.7) | ||
| AA | 49.8 (9.2) | 6.1 (3.4) | 7.3 (1.1) | 24.9 (15.9) | 1.2 (0.8) | ||
| Total n | 939 | 922 | 927 | 928 | 935 | ||
| PER2 | rs2304672 | CC | 48.7 (8.1) | 5.8 (3.1) | 7.5 (1.2) | 27 (21.2) | 1.1 (0.7) |
| CG | 48 (8.7) | 6 (3.3) | 7.5 (1.4) | 31.5 (30.2) | 1.1 (0.7) | ||
| GG | 42.3 (7.5) | 4.3 (1.5) | 8.2 (0.3) | 21.7 (7.6) | 1 (0) | ||
| Total n | 941 | 923 | 928 | 930 | 937 | ||
| PER3 | rs2797687 | CC | 48.4 (8.5) | 5.9 (3.1) | 7.5 (1.2) | 28.1 (23.2) | 1.1 (0.7) |
| CA | 48.6 (7.6) | 5.8 (3.1) | 7.5 (1.3) | 27.4 (22.6) | 1.2 (0.7) | ||
| AA | 49.4 (8.4) | 5.8 (2.8) | 7.3 (1) | 26.1 (23.8) | 1.1 (0.6) | ||
| Total n | 908 | 891 | 895 | 898 | 905 | ||
| PER3 | rs10462020 | TT | 48.2 (8.3) | 5.9 (3.1) | 7.4 (1.2) | 28.1 (22.9) | 1.1 (0.7) |
| TG | 48.5 (8) | 6 (3.2) | 7.6 (1.3) | 27.2 (21.1) | 1.2 (0.7) | ||
| GG | 51.3 (7.7) | 5.3 (2.7) | 7.6 (1.2) | 29.7 (34.9) | 1 (0.6) | ||
| Total n | 941 | 923 | 928 | 930 | 937 |
ARNTL2, aryl hydrocarbon receptor nuclear translocator-like 2; CLOCK, circadian locomotor output cycles kaput; DBP, D site of albumin promoter (albumin D-box) binding protein; PSQI, Pittsburgh Sleep Quality Index; PER, period homologue (PER1, PER2, PER3); FBXL3, F-box and leucine-rich repeat protein 3; GNβ3, guanine nucleotide binding protein beta 3.
The mean scores with standard deviations (in parentheses) are listed for all sleep measures by genotype, including: diurnal preference (range of 16–86, a higher score indicates greater preference for morningness), global PSQI score (range from 0 to 21, with higher scores indicating poorer overall sleep quality); sleep duration (h), sleep latency (min) and sleep quality (range 0–3, higher scores indicate poorer sleep quality).
Diurnal preference
There was a significant association between the PER3 SNP rs10462020 genotype and the mean diurnal preference score using a recessive model of inheritance (P = 0.003) (see Table 3 for a summary of the linear regression scores). These results indicate that the GG individuals (n = 63) had a higher diurnal preference score, and thus an increased morning preference (mean = 51.3, SD = 7.7), than the TT and TG individuals (mean = 48.3, SD = 8.2) (see Fig. 1a). There was also a nominally significant association between this SNP and mean diurnal preference using an additive model, P = 0.02). As this SNP was not in HWE in the total sample, but was in HWE in the male subsample, we repeated the regression analyses in the male subsample. We found that there was still a significant association between rs10462020 genotype and the mean diurnal preference in the male subsample using a recessive model of inheritance (β = 5.2, P = 0.003).
Table 3.
Standardized regression coefficients β (SE) from linear regression analyses for main effects of genotype on all sleep measures
| Gene | SNP | Diurnal reference | Global PSQI | Sleep duration | Sleep latency | Sleep quality | |
|---|---|---|---|---|---|---|---|
| ARNTL2 | rs922270 | Additive | 0.76 (0.53) | −0.23 (0.2) | 0.02 (0.08) | −2.96 (1.47)* | −0.1 (0.05)* |
| Recessive | 3.72 (1.55)* | 0.07 (0.56) | 0.06 (0.21) | −7.19 (3.11)* | −0.02 (0.14) | ||
| Dominant | 0.59 (0.59) | −0.28 (0.23) | 0.02 (0.09) | −2.97 (1.76) | −0.12 (0.05)* | ||
| CLOCK | rs2070062 | Additive | −0.27 (0.44) | 0.02 (0.17) | −0.08 (0.06) | −1.37 (1.09) | 0.03 (0.04) |
| Recessive | −0.74 (1.11) | 0.05 (0.4) | −0.1 (0.16) | −2.42 (2.16) | 0.06 (0.09) | ||
| Dominant | −0.23 (0.53) | 0.02 (0.21) | −0.1 (0.08) | −1.55 (1.49) | 0.04 (0.05) | ||
| DBP | rs3848543 | Additive | −0.54 (0.56) | −0.31 (0.23) | 0.03 (0.09) | −2.99 (1.51)* | −0.06 (0.05) |
| Recessive | −2.58 (2.33) | 0.44 (0.94) | −0.63 (0.33) | 1.02 (6.65) | 0.09 (0.2) | ||
| Dominant | −0.45 (0.6) | −0.41 (0.25) | 0.09 (0.09) | −3.65 (1.59)* | −0.08 (0.06) | ||
| FBXL3 | 825679097 | Additive | 2.33 (5.88) | −1.1 (0.82) | 0.81 (0.44) | −4.76 (5.03) | −0.15 (0.04)** |
| Dominant | 2.33 (5.88) | −1.1 (0.82) | 0.81 (0.44) | −4.76 (5.03) | −0.15 (0.04)** | ||
| GNβ3 | rs5443 | Additive | 0.53 (0.4) | −0.23 (0.15) | 0.04 (0.06) | 0.35 (1.11) | −0.07 (0.03)* |
| Recessive | 0.95 (0.81) | −0.9 (0.32)** | 0.24 (0.11)* | −1.03 (2.34) | −0.2 (0.08)* | ||
| Dominant | 0.59 (0.56) | −0.09 (0.21) | 0 (0.08) | 0.96 (1.6) | −0.06 (0.05) | ||
| PER1 | rs2735611 | Additive | −0.28 (0.49) | 0 (0.22) | 0.01 (0.08) | −0.08 (1.58) | 0.02 (0.05) |
| Recessive | −0.13 (1.38) | 0.38 (0.88) | −0.49 (0.27) | 3.96 (6.2) | −0.14 (0.17) | ||
| Dominant | −0.34 (0.58) | −0.04 (0.23) | 0.06 (0.09) | −0.51 (1.69) | 0.03 (0.05) | ||
| PER2 | rs934945 | Additive | 0.29 (0.47) | 0.28 (0.19) | −0.13 (0.07) | 0.24 (1.24) | 0.04 (0.04) |
| Recessive | 1.43 (1.34) | 0.31 (0.57) | −0.21 (0.17) | −3.32 (2.64) | 0.09 (0.13) | ||
| Dominant | 0.15 (0.56) | 0.34 (0.22) | −0.14 (0.08) | 0.96 (1.61) | 0.05 (0.05) | ||
| PER2 | rs2304672 | Additive | −1.16 (0.75) | 0.04 (0.28) | 0.01 (0.11) | 3.93 (2.44) | −0.04 (0.06) |
| Recessive | −5.66 (3.68) | −1.47 (0.79) | 0.78 (0.15)** | −6.04 (4) | −0.12 (0.03)** | ||
| Dominant | −1.1 (0.78) | 0.08 (0.3) | −0.01 (0.12) | 4.34 (2.58) | −0.04 (0.06) | ||
| PER3 | rs2797687 | Additive | 0.32 (0.48) | −0.06 (0.18) | −0.01 (0.07) | −0.85 (1.37) | 0.01 (0.04) |
| Recessive | 0.81 (1.38) | −0.09 (0.49) | −0.25 (0.17) | −1.95 (4.23) | −0.04 (0.11) | ||
| Dominant | 0.32 (0.56) | −0.06 (0.22) | 0.02 (0.09) | −0.87 (1.59) | 0.02 (0.05) | ||
| PER3 | rs10462020 | Additive | 1.02 (0.43)* | −0.09 (0.16) | 0.11 (0.06) | 0.02 (1.52) | −0.01 (0.04) |
| Recessive | 2.99 (1.01)** | −0.6 (0.35) | 0.14 (0.15) | 1.85 (4.41) | −0.11 (0.08) | ||
| Dominant | 0.87 (0.55) | 0.01 (0.21) | 0.14 (0.08) | −0.47 (1.63) | 0.02 (0.05) | ||
ARNTL2, aryl hydrocarbon receptor nuclear translocator-like 2; CLOCK, circadian locomotor output cycles kaput; DBP, D site of albumin promoter (albumin D-box) binding protein; PSQI, Pittsburgh Sleep Quality Index; PER, period homologue (PER1, PER2, PER3); FBXL3, F-box and leucine-rich repeat protein 3; GNβ3, guanine nucleotide binding protein beta 3; SE, standard error.
The table presents the standardized regression coefficients for linear regression analyses using additive, recessive and dominant models of inheritance for the various sleep measures. Significant results are highlighted in bold type.
Nominally significant results (P < 0.05).
Significant result following multiple testing correction (P < 0.005).
Figure 1.
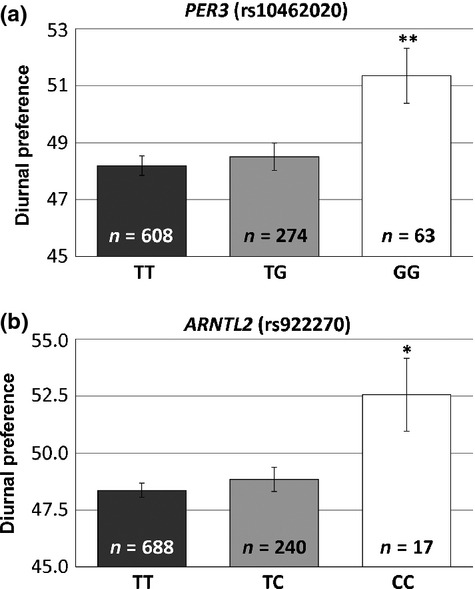
Period homologue 3 (PER3) and aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) single nucleotide polymorphisms (SNPs) are associated with diurnal preference.
There was also a nominally significant association between the ARNTL2 SNP rs922270 and mean diurnal preference score using a recessive model (P = 0.02) (see Fig. 1b). These results indicate that the CC individuals (n = 17) had a higher diurnal preference, and thus an increased morning preference (mean = 52.6, SD = 6.6), than the TT and TC individuals (mean = 48.5, SD = 8.2).
In the combined analysis (including all 10 SNPs investigated), the PER3 SNP remained significant (β = 3.3, P = 0.003, recessive model), although the ARNTL2 SNP did not (β = 2.8, P = 0.013, recessive model). We also found two novel associations not seen in the individual analysis: the rare FBXL3 SNP 825679097 (β = 11.7, P < 0.0001, additive model) and the GNβ3 SNP rs5443 (β = 0.9, P = 0.04, additive model).
Global PSQI score
There was a significant association between the GNβ3 SNP rs5443 and the global PSQI score using a recessive model (P = 0.005) (see Fig. 2a). These results indicate that the TT individuals (n = 88) had a lower global PSQI score, and thus less overall sleep disturbance (mean = 5.10, SD = 2.8), than the CC and CT individuals (mean = 5.97, SD = 3.1). In the combined analysis (including all 10 SNPs investigated), this SNP was still nominally significant in a recessive model (β = −0.7, P = 0.04).
Figure 2.
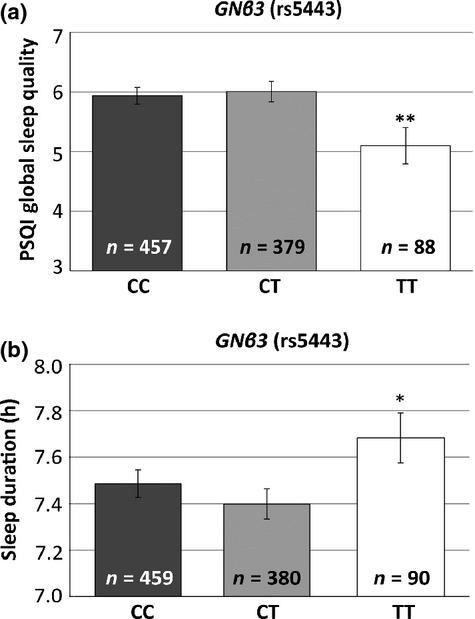
Guanine nucleotide binding protein beta 3 (GNβ3) is associated with variations in global sleep quality and sleep duration in recessive models.
Sleep duration
There was a significant association between the PER2 SNP rs2304672 and sleep duration using a recessive model (P < 0.001). These results indicate that the rare GG individuals (n = 3) had a longer sleep duration (mean = 8.2 h, SD = 0.3) than the CC and CG individuals (mean = 7.5 h, SD = 1.2).
There was also a nominally significant association between the GNβ3 SNP rs5443 and sleep duration using a recessive model (P = 0.03) (see Fig. 2b). These results indicate that the TT individuals (n = 88) had a longer sleep duration (mean = 7.7 h, SD = 1.0) than the CC and CT individuals (mean = 7.4 h, SD = 1.2).
In the combined analysis (including all 10 SNPs investigated), the associations with both the PER2 SNP rs2304672 (β = 0.6, P < 0.001, recessive) and the GNβ3 SNP rs5443 (β = 0.29, P = 0.03, recessive) remained mainly unchanged. We additionally found a novel association with the rare FBXL3 SNP 825679097 (β = 1.3, P < 0.0001, additive model).
Sleep latency
There was a nominally significant association between the ARNTL2 SNP rs922270 and sleep latency in both an additive model (P = 0.045) and a recessive model (P = 0.02) (see Fig. 3a). These results indicate that the CC individuals (n = 17) had a lower sleep latency, thus taking a shorter period of time to fall asleep (mean = 20.7 min, SD = 13.3) than the TC (mean = 26.1 min, SD = 24.3) and TT individuals (mean = 28.7 min, SD = 23.2).
Figure 3.
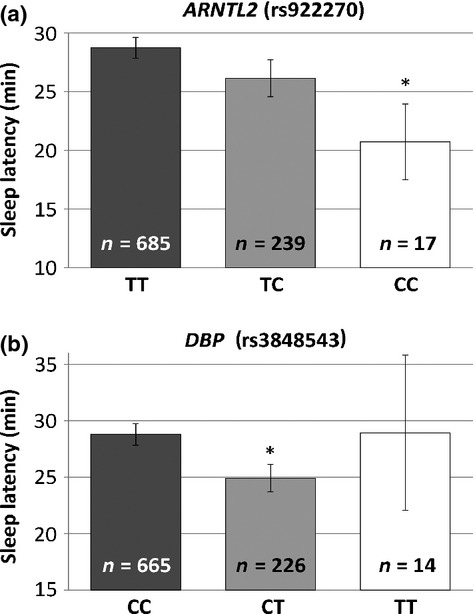
Aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) and D site of albumin promoter (albumin D-box) binding protein (DBP) are associated with variations in sleep latency.
There was also a nominally significant association between the DBP SNP rs3848543 and sleep latency in both an additive model (P = 0.047) and a dominant model (P = 0.02) (see Fig. 3b). These results indicate that the CT individuals (n = 231) had a lower sleep latency, thus taking a shorter period of time to fall asleep (mean = 24.9 min, SD = 18.2) than the CC (mean = 28.8 min, SD = 24.9) and TT individuals (mean = 28.9 min, SD = 25.7). In the combined analysis (including all 10 SNPs investigated), we found only a nominal trend towards significance for the DBP SNP (β = −2.7, P = 0.09, additive model).
Sleep quality
There was a significant association between the FBXL3 SNP 825679097 and sleep quality using an additive model (P < 0.001). These results indicate that the rare GT individuals (n = 2) had a lower sleep quality score, thus better sleep quality (mean = 1.0, SD = 0.0), than the GG individuals (mean = 1.1, SD = 0.7).
There was also a significant association between the PER2 SNP rs2304672 and sleep quality using a recessive model (P < 0.001). These results indicate that the rare GG individuals (n = 3) had a lower sleep quality score, thus better sleep quality (mean = 1.0, SD = 0.0), than the CC and CG individuals (mean = 1.1, SD = 0.7).
There were nominally significant associations between the ARNTL2 SNP rs922270 and sleep quality in both additive (P = 0.03) and dominant models (P = 0.02) and the GNβ3 SNP rs5443 and sleep quality in both additive (P = 0.03) and recessive models (P = 0.01) (see Fig. 4a,b). The ARNTL2 CC individuals (n = 17) and the GNβ3 TT individuals (n = 89) had lower sleep quality scores, and thus better sleep quality (mean = 1.1, SD = 0.6 and mean = 1.0, SD = 0.7), respectively, than the other genotypes (see Table 2 for details).
Figure 4.
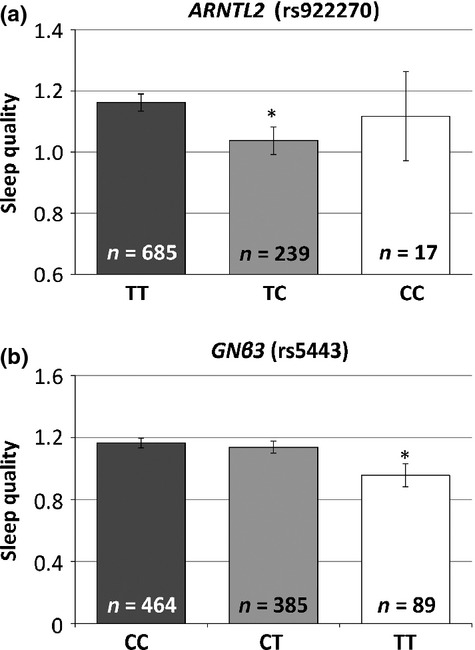
Aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) and guanine nucleotide binding protein beta 3 (GNβ3) are associated with variations in sleep quality.
In the combined analysis (including all 10 SNPs investigated), only the rare FBXL3 SNP 825679097 was nominally significant (β = −0.14, P = 0.04, additive model).
Discussion
Diurnal preference
We found a significant association of a PER3 SNP rs10462020 with diurnal preference. This finding is in line with a wide range of studies showing that genetic variation in the PER3 gene is associated with diurnal preference (Archer et al., 2003; Ebisawa et al., 2001), including a study that also found the G allele to be associated with increased morning preference (Johansson et al., 2003). This SNP is also part of a haplotype associated with delayed sleep phase syndrome (Ebisawa et al., 2001). Taken together, these studies suggest that this gene may play a role in regulating diurnal preference.
The PER3 SNP was found to be significantly out of HWE in the total sample, but not in the male subsample. As the association between this SNP and diurnal preference was significant in both the total sample and the male subsample, this suggests that this association is unlikely to be due to genotyping error. Failure to reach HWE could be due to a number of other factors, including non-random mating, which occurs with morningness–eveningness (Randler and Kretz, 2011).
The mechanism by which the PER3 gene modulates diurnal preference is uncertain, as diurnal preference can be affected by both circadian rhythms and sleep homeostasis. Period genes act as negative regulators of the molecular clock, with Per1 and Per2 playing more central roles than Per3. Per1 and Per2 knockout mice have a shorter circadian period, while the Per3 knockout mice have only subtle (Bae et al., 2001) or no differences in circadian period (Van der Veen and Archer, 2010). Additionally, while Per1/Per2 double knockout mice are arrhythmic in constant darkness, Per1/Per3 or Per2/Per3 double knockout mice are not (Bae et al., 2001). More recently, the PER3 gene has also been shown to play a role in sleep homeostasis in humans (Viola et al., 2007) and its orthologue plays the same role in mice (Hasan et al., 2011). It is not known whether this missense SNP is functional, but as this residue is conserved across most period homologues and is proximal to predicted Csnk1e target sites, it may directly underlie this association (Ebisawa et al., 2001).
We also found a nominally significant association between an ARNTL2 SNP rs922270 and diurnal preference. This is the first time a SNP in the ARNTL2 gene has been associated with diurnal preference. The C allele for this SNP had previously been associated with better afternoon/evening mood in a bipolar sample (Shi et al., 2008). As better afternoon and evening moods are associated with morning and evening preferences, respectively (Kerkhof, 1998), it is difficult to relate these findings to our own. ARNTL2 was thought to be unnecessary for the core circadian clock, as its paralogue Arntl is the only circadian gene that when knocked out on its own causes arrhythmic locomotor activity in mice (Bunger et al., 2000). Recently, it has been found that Arntl knockout mice have decreased Arntl2 mRNA expression and that the arrhythmic locomotor activity can be rescued by Arntl2 if it is driven by a constitutively activated promoter (Shi et al., 2010). The ARNTL2 protein may play an important role in the molecular clock, and thus genetic variation in ARNTL2 could directly impact diurnal preference by affecting the core molecular clock. As this SNP is intronic, and not known to affect splicing, it is possible that it is in linkage disequilibrium with another functional SNP that, in turn, may underlie this association.
The associations with PER3 and ARNTL2 were still significant in the combined analysis including all 10 SNPs, suggesting that they are at least partially independent. This analysis also found associations of SNPs in FBXL3 and GNβ3, with diurnal preference not revealed in the individual analyses. For the GNβ3 association the T allele was associated with increased morningness, which matches a previous study (Johansson et al., 2004), while this is the first association of a FBXL3 SNP with diurnal preference in humans, although Fbxl3 mutant mice are known to have an altered circadian period (Godinho et al., 2007).
Global PSQI score
We found a novel significant association between the functional GNβ3 SNP rs5443 and the global PSQI sleep quality score. This SNP is part of a haplotype that was associated previously with diurnal preference (Lee et al., 2007). Additionally, two other SNPs in this gene have been associated with increased wakefulness after sleep onset in an elderly population (Evans et al., 2013). Although this SNP does not cause an amino acid change, it has been shown to lead to alternative splicing, leading in turn to the shorter Gnβ3-s isoform which causes increased signal transduction (Siffert et al., 1998). This gene codes for the beta-subunit of the membrane-associated heterotrimeric guanine nucleotide binding proteins (G-proteins). G-proteins are integral to a number of secondary messenger pathways, including those for serotonin and noradrenaline receptors (Millan et al., 2008), both of which are involved in sleep mechanisms (Murillo-Rodriguez et al., 2012). It is thus possible that this SNP directly underlies these associations by altering the signalling pathways of one of these key neurotransporter systems.
Sleep duration
Both the PER2 SNP rs2304672 and the GNβ3 SNP rs5443 were associated with sleep duration. Genetic variation in PER2 has been associated previously with advanced sleep phase syndrome (ASPS) (Toh et al., 2001); additionally, Per2 knockout mice have initially diminished delta activity in response to sleep deprivation (Kopp et al., 2002). Of the two large genome-wide association studies (GWAS) conducted for sleep measures, one found a nominally significant association of genetic variation in PER2 with sleep duration (Allebrandt et al., 2013), while the other found that genetic variation in PER3 was nominally associated with sleep duration and sleep latency (Byrne et al., 2013). Part of the discrepancy between these studies and our findings may be due to the fact that different questionnaires were used to measure subjective sleep characteristics. The PER2 association is due to a rare genotype and thus may be due to chance, although it is worth noting that rare SNPs are thought to explain a portion of the heritability that is missing from GWAS studies (Dickson et al., 2010; Manolio et al., 2009).
Both these associations remained mainly unchanged in a combined analysis of these two SNPs, suggesting that two associations are independent of each other. This analysis also found an association between the FBXL3 SNP with sleep duration not revealed in the individual analyses, which is the first reported association of FBXL3 with sleep duration.
Sleep latency
We found nominally significant associations of both ARNTL2 and DBP with sleep latency. The effects of ARNTL2 may be related to its potential role in regulating sleep timing discussed above. The DBP SNP was associated previously with worse evening mood (Shi et al., 2008). Interestingly, there is a wealth of evidence that Dbp gene expression decreases following sleep deprivation in both mice and rats (Curie et al., 2013; Franken et al., 2007; Wisor et al., 2002). These findings suggest that DBP may directly play a role in sleep homeostasis.
In the combined analysis, the DBP association remained, while the ARNTL2 association disappeared. This suggests that the ARNTL2 association is not independent of the DBP association.
Sleep quality
Four SNPs were associated with sleep quality, two of which were very rare: FBLX3 (n = 2) and PER2 (n = 3). This is the first study suggesting that FBXL3 may play a role in the regulation of sleep. Animal models with mutations in Fbxl3 have a longer circadian period (Godinho et al., 2007), but no study has investigated this gene's role in sleep homeostasis. In the combined analysis only the FBLX3 association was significant.
Candidate gene association studies
Genome-wide association studies approaches demonstrate that there are likely to be a large number of genes that affect sleep measures, most of which have small effects. Candidate gene approaches can be useful in determining genetic variants with small effects as they do not require the same scale of multiple testing corrections required in GWAS approaches, but they have limitations (Lewis and Knight, 2012). Any candidate gene approach requires that the a priori rationale for its selection is valid. Additionally, as with GWAS studies, it is difficult to determine the causal variant underlying the association and significant associations may be false positives. Finally, any candidate gene association study needs to be replicated in additional and varied samples (Lewis and Knight, 2012).
In summary, we found that genetic variation in genes with circadian expression is associated with both diurnal preference (PER3 and ARNTL2) and specific subjective sleep measures (GNβ3). These findings are in line with previous studies that found circadian gene involvement in sleep homeostasis (Franken, 2013; Franken and Dijk, 2009; Wisor et al., 2002), and stress the importance of further studies aimed at dissecting the specific roles that individual circadian genes play in these two interrelated but distinct behaviours. Further studies combining new technologies, such as next-generation sequencing that allows for detection of all SNPs, and the use of more refined phenotypic measurements, such as employing actigraphy or polysomnography, will be critical in dissecting the specific roles of circadian genes in these behaviours.
Acknowledgments
Wave 4 of the G1219 study was supported by grants from the Economic and Social Research Council (RES-000-22-2206) and the Institute of Social Psychiatry to Alice M. Gregory. Funding for DNA extraction was provided by the Goldsmiths Early Career Award to Alice M. Gregory. Funding for the genotyping was provided by a Medical Research Council Core Grant (Nolan). Waves 1–3 of the G1219 study were supported by grants from the Wellcome Trust Grant Foundation and a Medical Research Council Training Fellowship and Career Development Award to Thalia C. Eley.
Author Contributions
AMG, KJL, MJP, PMN & TCE designed the study. SNA found the rare FBXL3 SNP. KJL, MJP & NLB conducted the genotyping and analysis. All authors helped to prepare the manuscript.
Conflict of Interest
No conflicts of interest declared.
References
- Allebrandt KV, Amin N, Müller-Myhsok B, et al. A K(ATP) channel gene effect on sleep duration: from genome-wide association studies to function in Drosophila. Mol. Psychiatry. 2013;18:122–132. doi: 10.1038/mp.2011.142. [DOI] [PubMed] [Google Scholar]
- Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- Archer SN, Carpen JD, Gibson M, et al. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. 2010;33:695–701. doi: 10.1093/sleep/33.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: same genes? A study of young adult twins. Chronobiol. Int. 2010a;27:278–296. doi: 10.3109/07420521003663801. [DOI] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Buysse DJ, Rijsdijk FV, Gregory AM. Genetic and environmental influences on different components of the Pittsburgh Sleep Quality Index and their overlap. Sleep. 2010b;33:659–668. doi: 10.1093/sleep/33.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Mill J, et al. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3 and CLOCK 3111. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156:681–690. doi: 10.1002/ajmg.b.31210. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two-process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:192–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Byrne EM, Gehrman PR, Medland SE, et al. A genome-wide association study of sleep habits and insomnia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013;162:439–451. doi: 10.1002/ajmg.b.32168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpen JD, von Schantz M, Smits M, Skene DJ, Archer SN. A silent polymorphism in the PER1 gene associates with extreme diurnal preference in humans. J. Hum. Genet. 2006;51:1122–1125. doi: 10.1007/s10038-006-0060-y. [DOI] [PubMed] [Google Scholar]
- Curie T, Mongrain V, Dorsaz S, Mang GM, Emmenegger Y, Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;51:1122–1125. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;26:e1000294. doi: 10.1371/journal.pbio.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Uchiyama M, Kajimura N, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO J. 2001;2:342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Liang HL, Plomin R, et al. Parental familial vulnerability, family environment and their interactions as predictors of depressive symptoms in adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:298–306. doi: 10.1097/00004583-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Evans DS, Parimi N, Nievergelt CM, et al. Common genetic variants in ARNTL and NPAS2 and at chromosome 12p13 are associated with objectively measured sleep traits in the elderly. Sleep. 2013;36:431–446. doi: 10.5665/sleep.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken P. A role for clock genes in sleep homeostasis. Curr. Opin. Neurobiol. 2013;23:864–872. doi: 10.1016/j.conb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur. J. Neurosci. 2009;29:1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- Franken P, Thomason R, Heller HC, O'Hara BF. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 2007;8:87. doi: 10.1186/1471-2202-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho SI, Maywood ES, Shaw L, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- Hasan S, van der Veen DR, Winsky-Sommerer R, Dijk DJ, Archer SN. Altered sleep and behavioral activity phenotypes in PER3-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1821–R1830. doi: 10.1152/ajpregu.00260.2011. [DOI] [PubMed] [Google Scholar]
- Horne JA, Oostburg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hughes ME, DiTacchio L, Hayes KR, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Willeit M, Smedh C, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- Johansson C, Willeit M, Aron L, et al. Seasonal affective disorder and the G-protein beta-3-subunit C825T polymorphism. Biol. Psychiatry. 2004;55:317–319. doi: 10.1016/s0006-3223(03)00640-1. [DOI] [PubMed] [Google Scholar]
- Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA. Differences between morning-types and evening-types in the dynamics of EEG slow wave activity during night sleep. Electroencephalogr. Clin. Neurophysiol. 1991;78:197–202. doi: 10.1016/0013-4694(91)90033-z. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA. The 24-hour variation of mood differs between morning- and evening-type individuals. Percept. Mot. Skills. 1998;86:264–266. doi: 10.2466/pms.1998.86.1.264. [DOI] [PubMed] [Google Scholar]
- Kopp C, Albrecht U, Zheng B, Tobler I. Homeostatic sleep regulation is preserved in mPer1 and mPer2 mutant mice. Eur. J. Neurosci. 2002;16:1099–1106. doi: 10.1046/j.1460-9568.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Paik JW, Kang SG, Lim SW, Kim L. Allelic variants interaction of CLOCK gene and G-protein beta3 subunit gene with diurnal preference. Chronobiol. Int. 2007;24:589–597. doi: 10.1080/07420520701534632. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim L, Kang SG, et al. PER2 variation is associated with diurnal preference in a Korean young population. Behav. Genet. 2011;41:273–277. doi: 10.1007/s10519-010-9396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Knight J. Introduction to genetic association studies. Cold Spring Harb. Protoc. 2012;2012:297–306. doi: 10.1101/pdb.top068163. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams TA, Gregory AM, Rowe R, et al. The genesis 12–19 (G1219) study: a twin and sibling study of gene–environment interplay and adolescent development in the UK. Twin Res. Hum. Genet. 2013;16:134–143. doi: 10.1017/thg.2012.83. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep–wake cycle and underlying circadian rhythms in morningness–eveningness. J. Biol. Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness–eveningness. J. Sleep Res. 2006;15:162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Arias-Carrion O, Zavala-Garcia A, et al. Basic sleep mechanisms: an integrative review. Cent. Nerv. Syst. Agents Med. Chem. 2012;12:38–54. doi: 10.2174/187152412800229107. [DOI] [PubMed] [Google Scholar]
- Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–185. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli M, Louzada FM, Pereira DS, et al. Clock polymorphisms and circadian rhythms phenotypes in a sample of the Brazilian population. Chronobiol. Int. 2007;24:1–8. doi: 10.1080/07420520601139789. [DOI] [PubMed] [Google Scholar]
- Randler C, Kretz S. Assortative mating in morningness–eveningness. Int. J. Psychol. 2011;46:91–96. doi: 10.1080/00207594.2010.518237. [DOI] [PubMed] [Google Scholar]
- Rogers WH. Regression standard errors in clustered samples. Stata Tech. Bull. 1993;13:19–23. [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- Shi J, Wittke-Thompson JK, Badner JA, et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1047–1055. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr. Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffert W, Rosskopf D, Siffert G, et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat. Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 8. StataCorp LP, College Station, TX: 2002. [Google Scholar]
- Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J. Sleep Res. 2003;12:275–282. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- van der Veen DR, Archer SN. Light-dependent behavioral phenotypes in PER3-deficient mice. J. Biol. Rhythms. 2010;25:3–8. doi: 10.1177/0748730409356680. [DOI] [PubMed] [Google Scholar]
- Viola AU, Archer SN, James LM. PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- Wisor JP, O'Hara BF, Terao A, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]


