Abstract
As a potent neurotrophic agent, the sesquiterpenoid jiadifenolide represents a valuable small-molecule lead for the potential therapeutic treatment of neurodegenerative diseases. A stereocontrolled total synthesis of this densely functionalized natural product is reported, central to which is an adventurous samarium-mediated cyclization reaction to establish the tricyclic core and the adjacent C5 and C6 quaternary stereocenters.
Keywords: cyclization, neurological agents, samarium, terpenoids, total synthesis
Jiadifenolide (1, Scheme 1) is an architecturally complex sesquiterpenoid first isolated from the pericarps of the Chinese plant Illicium jiadifengpi by Fukuyama and co-workers in 2009.1 Preliminary biological investigation revealed potent neurotrophic activity, promoting neurite outgrowth in primary cultured rat cortical neurons at concentrations as low as 10 nm. Given the important regulatory role of neurotrophins in the central nervous system, jiadifenolide represents a valuable small-molecule lead for the potential therapeutic treatment of neurodegenerative conditions such as Alzheimer’s disease.2 Its low natural abundance (1.5 mg kg−1 plant material) makes total synthesis of particular importance for the preparation of sufficient quantities for further biological evaluation and to enable access to a range of analogues for structure–activity relationship (SAR) profiling.3
Figure 1.
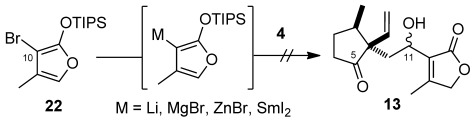
a) X-ray crystal structure of alcohol 17. b) Possible samarium-chelate transition structure leading to 16.
Our interest in jiadifenolide derives not only from its potent bioactivity, but also from its complex seco-prezizaane skeleton, placing it amongst the Illicium family of sesquiterpenoids,4 which has garnered substantial synthetic attention.2a, 5 Jiadifenolide’s intricate and densely functionalized caged pentacyclic structure (1) comprises four rings emanating from a central, highly substituted B-ring cyclohexane. This poses particular synthetic challenges, most notably the controlled introduction of five contiguous quaternary stereocenters, which we aimed to address as part of a concise and efficient total synthesis,6 following that first reported by Theodorakis in 2011 and most recently by Sorensen (2014).7
Our conceptually unique synthetic solution sought to forge the central B-ring from a functionalized A,C-ring precursor. As outlined retrosynthetically in Scheme anie0053-7048-f1, we envisaged late-stage unveiling the D- and E-rings through oxidation of the C13-pendant vinyl group of ABC-tricycle 2. A critical transformation would thus be the reductive cyclization of keto-butenolide 3 to establish the central B-ring and the adjacent C5 and C6 quaternary stereocenters in a single operation. Cyclization substrate 3 would be prepared through aldol coupling of butenolide 5 with aldehyde 4. Notably, a single C5 stereocenter would template for all remaining stereochemistry under substrate-based control.
The preparation of aldehyde 4 commenced with Luche reduction of cyclopentenone 6 (Scheme anie0053-7048-f2).8 The ensuing allylic alcohol then underwent hydroxy-directed epoxidation with m-CPBA and TBS protection to deliver epoxide 7 in 65 % yield over the three steps. The C2 methyl-bearing stereocenter was then established through Lewis-acid mediated rearrangement of 7 to selectively deliver the 2,5-syn-configured cyclopentanone 8 (78 %, 19:1 d.r.).9, 10 Horner–Wadsworth–Emmons (HWE) homologation of 8 (73 %, >19:1 E:Z) followed by reduction and acylation of 10 then smoothly delivered allylic acetate 11 in readiness for an Ireland–Claisen rearrangement to install the key C13 quaternary stereocenter.11 In the event, heating the corresponding TBS ketene acetal (LDA, TBSCl) in anhydrous benzene led to the selective (10:1 d.r.) formation of a mixture of rearranged products arising from preferential reaction at the less hindered alkene π-face.12 This material was subjected directly to reduction with LiAlH4 to deliver alcohol 12 in 63 % yield from 11. Finally, hydrolysis of the TBS ether and double oxidation under Swern conditions gave the targeted aldehyde 4 in 74 % yield (13 % overall from 6).
Scheme 1.
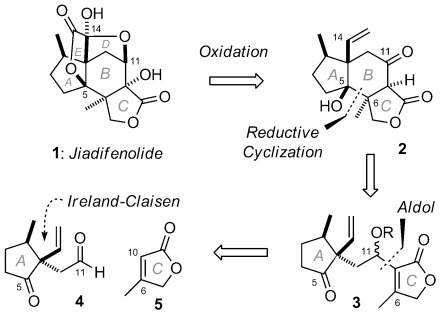
Retrosynthetic analysis of jiadifenolide (1).
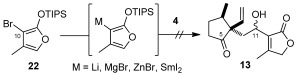
Initial approaches at appending the C-ring butenolide through addition of 3-metallated furyl derivatives to 4 all met with failure, with optimally only trace amounts of adducts observed.13 By contrast (Scheme 3), it was found that butenolide 514 and aldehyde 4 could be smoothly coupled through a boron-mediated aldol reaction, which provided adducts 13 as a 2:1 mixture of diastereomeric alcohols in almost quantitative yield.15 At this stage, with a view to effecting the crucial reductive cyclization reaction to close the central B-ring, alcohols 13 were oxidized to provide tricarbonyl 14. Disappointingly however, no productive cyclization of 14 could be induced under a range of conditions utilizing either SmI216 or alternative reagents,17 returning only starting material or, under more forcing conditions, decomposition products. A similar situation was observed for alcohols 13. The apparent instability of 14 with respect to potential reagents for cyclization led to the examination of TES ethers 15 and 11-epi-15 as alternative substrates, which were chromatographically separable. Now, gratifyingly, it was found that addition of 15 to a freshly prepared solution of SmI2 (ca. 6 equiv) in THF and heating to 65 °C, led to the generation of a single cycloadduct (51 %), identified as 16 on the basis of NMR and computational analysis.10, 18 More rigorous structural proof was subsequently obtained through X-ray crystallographic analysis of alcohol 17, formed upon acid-mediated desilylation of 16 (Figure 1 a).19 Subsequent oxidation with PCC then delivered the targeted ABC-ketone 2 (81 %)19 along with diol 18 (11 %), containing the requisite oxygenation at C10.20 Attempts to encourage complete oxidation to 18 were unsuccessful however.
Scheme 2.
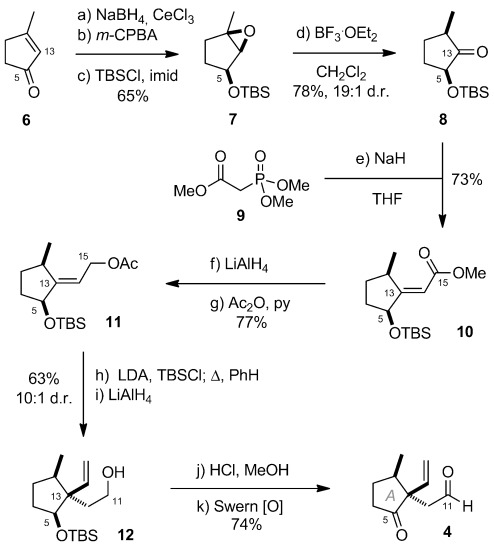
Preparation of A-ring keto-aldehyde 4. Reagents and conditions: a) NaBH4, CeCl3, MeOH, −78 °C to 0 °C; b) m-CPBA, CH2Cl2, 0 °C; c) TBSCl, imid, CH2Cl2, 0 °C to RT, 65 % over 3 steps; d) BF3⋅OEt2, CH2Cl2, −20 °C, 78 %, 19:1 d.r.; e) NaH, RT, 72 h, 73 %, >19:1 (E):(Z); f) LiAlH4, Et2O, 0 °C; g) Ac2O, py, DMAP, CH2Cl2, RT, 77 % over 2 steps; h) LDA, TBSCl, THF, −78 °C to RT; PhH, reflux, 16 h, 10:1 d.r.; i) LiAlH4, Et2O, 0 °C, 63 % over 2 steps; j) 3 n HCl, MeOH, (1:3), RT; k) DMSO, (COCl)2, Et3N, CH2Cl2, −78 °C to RT, 74 % over 2 steps. m-CPBA=3-chloroperoxybenzoic acid, DMAP=N,N-dimethyl-4-aminopyridine, DMSO=dimethylsulfoxide, imid=imidazole, LDA=lithium diisopropylamide, py=pyridine, TBS=tert-butyldimethylsilyl, THF= tetrahydrofuran.
The desired stereochemical outcome of the SmI2-mediated reductive cyclization reaction of 15 is consistent with a chelated boat-type transition structure such as 19 (Figure 1 b), in which the C11 substituent is equatorially disposed.21 This may explain the failure of the epimeric C11 TES ether (natural configuration, pseudo-axially disposed) to undergo analogous cyclization, attesting, along with failed substrates 13 and 14, to the challenge of this adventurous transformation.
With the requisite carbon skeleton of jiadifenolide secured, completion of the total synthesis was accomplished through controlled oxygenation at C10, C11, C14, and C15, with attendant cyclization to form the D,E-rings (Scheme 4). Accordingly, chemoselective dihydroxylation (OsO4, NMO) of the TMS-enol ether of 2 (TMSOTf, Et3N), cleanly provided the C10 tertiary alcohol (18, 99 %). This was then utilized to set the adjacent C11 alcohol stereocenter through hydroxy-directed reduction with Me4NBH(OAc)3 (87 %, >19:1 d.r.).22 TES protection proved necessary to facilitate the subsequent unveiling of the D,E-rings (20, 96 %). In the event, pyridine-accelerated dihydroxylation of the C13-pendant alkene of 20 provided a diol which then underwent smooth oxidative lactonization upon treatment with TPAP, NMO to form the E-ring (84 %).23 Finally, desilylation occurred with concomitant hemiacetalization to form the D-ring and provided synthetic (±)-jiadifenolide (1, 86 %), which exhibited spectral characteristics (1H/13C NMR, IR, HRMS) identical in all respects to that reported for the natural sample.1
Scheme 3.
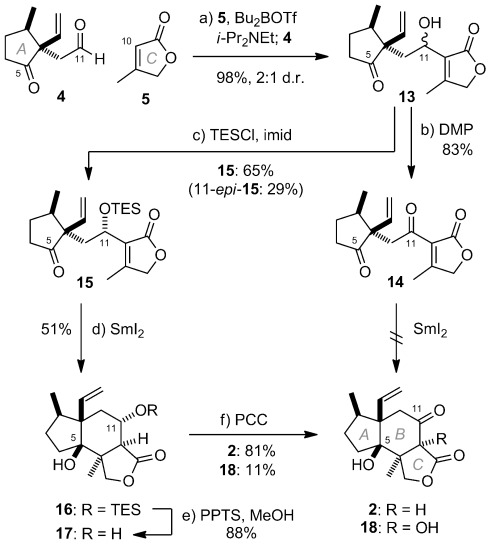
Preparation of ABC-tricycle 2. Reagents and conditions: a) Bu2BOTf, iPr2NEt, THF, −78 °C to −20 °C, 98 %, 2:1 d.r.; b) DMP, NaHCO3, CH2Cl2, 83 %; c) TESCl, imid, DMF, RT, 15: 65 %, 11-epi-15: 29 %; d) SmI2, 65 °C, 2 h, 51 %; e) PPTS, MeOH, CH2Cl2, RT, 88 %; f) PCC, NaOAc, SiO2, CH2Cl2, 2: 81 %, 18: 11 %. DMF=N,N-dimethylformamide, DMP=Dess–Martin periodinane, PCC=pyridinium chlorochromate, PPTS=pyridinium para-toluenesulfonate, TES= triethylsilyl.
Scheme 4.
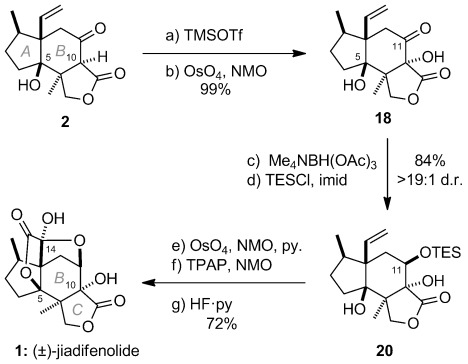
Total synthesis of jiadifenolide (1). Reagents and conditions: a) TMSOTf, Et3N, THF, −78 °C; b) OsO4, NMO, tBuOH/H2O, RT; 99 % over 2 steps; c) Me4NBH(OAc)3, AcOH, MeCN, −20 °C, 87 %, >19:1 d.r.; d) TESCl, imid, DMF, RT, 96 %; e) OsO4, NMO, py, tBuOH/H2O, RT; f) TPAP, NMO, 4 Å MS, CH2Cl2, RT, 84 % over 2 steps; g) HF⋅py, THF, RT, 86 %. NMO=N-methylmorpholine-N-oxide, TMS=trimethylsilyl, TPAP=tetrapropylammonium perruthenate.
In summary, we have completed the total synthesis of the neurotrophic agent jiadifenolide in 2.3 % yield over 23 steps, showcasing a pivotal SmI2-mediated reductive cyclization reaction to establish the tricyclic core. Notably, the synthesis demonstrates a high degree of solely substrate-based stereocontrol to establish this densely functionalized structure, wherein the full relative configuration is templated for by a single C5 alcohol stereocenter. Starting from known (S)-3-methyl-cyclopenten-1-ol should thus render this approach asymmetric.24 Work to prepare quantities of this valuable scaffold for further biological evaluation and analogue synthesis7b will be reported in due course.
Dedicated to Professor Richard J. K. Taylor on the occasion of his 65th birthday
Supporting Information
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201404224.
References
- [1].Kubo M, Okada C, Huang J-M, Harada K, Hioki H, Fukuyama Y. Org. Lett. 2009;11:5190. doi: 10.1021/ol9021029. [DOI] [PubMed] [Google Scholar]
- [2a].Xu J, Lacoske MH, Theodorakis EA. Angew. Chem. 126 doi: 10.1002/anie.201302268. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2014;53:909. [Google Scholar]
- [2b].Wootla B, Denic A, Warrington AE, Rodriguez M. Expert Rev. Neurother. 2012;12 doi: 10.1586/ern.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2c].Mitran SI, Catalin B, Sfredel V, Balseanu T-A. J. Mol. Psychiatry. 2014;1 doi: 10.1186/2049-9256-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2d].Wilson RM, Danishefsky SJ. Acc. Chem. Res. 2006;39 doi: 10.1021/ar068018n. [DOI] [PubMed] [Google Scholar]
- [3a].Paterson I, Anderson EA. Science. 2005;310:451. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- [3b].Nicolaou KC, Chen JS, Dalby SM. Bioorg. Med. Chem. 2009;17 doi: 10.1016/j.bmc.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3c].Danishefsky SJ. Nat. Prod. Rep. 2010;27 doi: 10.1039/c003211p. [DOI] [PubMed] [Google Scholar]
- [4].Fukuyama Y, Huang J-M. In: Studies in Natural Products Chemistry, Vol. 32. Atta-ur-Rahman, editor. Amsterdam: Elsevier; 2005. pp. 395–429. (Ed.:, pp. . [Google Scholar]
- [5a].Urabe D, Inoue M. Tetrahedron. 2009;65:6271. seco For a review, see: For total syntheses of majucin-type -prezizaane sesquiterpenoids, see. [Google Scholar]
- [5b].Carcache DA, Cho YS, Hua Z, Tian Y, Li Y-M, Danishefsky SJ. J. Am. Chem. Soc. 2012;128 doi: 10.1021/ja056980a. [DOI] [PubMed] [Google Scholar]
- [5c].Yang Y, Fu X, Chen J, Zhai H. Angew. Chem. 2006;124 doi: 10.1002/anie.201203176. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [5d].Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Org. Lett. 2011;13 doi: 10.1021/ol201742j. and Ref. [7b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6a].Paterson I, Ng KK-H, Williams S, Millican DC, Dalby SM. Angew. Chem. 2014;126 doi: 10.1002/anie.201310164. For recent work from our group, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2014;53:2730. doi: 10.1002/anie.201308675. [DOI] [PubMed] [Google Scholar]
- [6b].Dalby SM, Goodwin-Tindall J, Paterson I. Angew. Chem. 2013;125 doi: 10.1002/anie.201301978. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52 [Google Scholar]
- [6c].Xuan M, Paterson I, Dalby SM. Org. Lett. 2012;14 doi: 10.1021/ol302570k. [DOI] [PubMed] [Google Scholar]
- [7a].Xu J, Trzoss L, Chang WK, Theodorakis EA. Angew. Chem. 123 doi: 10.1002/anie.201100313. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50:3756. [Google Scholar]
- [7b].Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Chem. Eur. J. 2013;19 doi: 10.1002/chem.201300198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7c].Siler DA, Mighion JD, Sorensen EJ. AngewChem. 2014. DOI: 10.1002/anie.201402335. [DOI] [PMC free article] [PubMed]; Angew. Chem. Int. Ed. 2014. DOI: 10.1002/ange.201402335.
- [8].Bagnell L, Bliese M, Cablewski T, Strauss CR, Tsanaktsidis J. Aust. J. Chem. 1997;50:921. Prepared from 2,5-pentanedione: [Google Scholar]
- [9].Kita Y, Kitagaki S, Yoshida Y, Mihara S, Fang D-F, Kondo M, Okamoto S, Imai R, Akai S, Fujioka H. J. Org. Chem. 1997;62:4991. 8The relative configuration of was inferred from an nOe contact observed between H2 and H5. See Ref. [10] [Google Scholar]
- [10]. See the Supporting Information for further details.
- [11].Ireland RE, Mueller RH, Willard AK. J. Am. Chem. Soc. 1976;98:2868. 32Attempted Johnson orthoester Claisen rearrangement (MeC(OEt), EtCO H, reflux) of the corresponding primary alcohol led only to 1,3-allylic transposition, presumably via a cationic pathway. [Google Scholar]
- [12]. An inconsequential mixture of silyl ester and carboxylic acid products was obtained. The stereochemical outcome of the reaction was determined by nOe-based NMR analysis. See Ref. [10]
-
[13].He W, Huang J, Sun X, Frontier AJ. J. Am. Chem. Soc. 2008;130:300. doi: 10.1021/ja0761986.
 [DOI] [PubMed] [Google Scholar]
[DOI] [PubMed] [Google Scholar] - [14].Gogoi S, Argade NP. Tetrahedron. 2006;62:2715. Prepared from citraconic anhydride. [Google Scholar]
- [15a].Varvogli A-AC, Karagiannis IN, Koumbis AE. Tetrahedron. 2009;65:1048. [Google Scholar]
- [15b].Jefford CW, Jaggi D, Boukouvalas J. J. Chem. Soc. Chem. Commun. 1988 5The corresponding Li enolate (LDA, THF, −78 °C) of provided a ca. 3:2 mixture of α- and γ-adducts. [Google Scholar]
- [16a].Edmonds D, Johnston D, Procter DJ. Chem. Rev. 2009;104:3371. doi: 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]
- [16b].Nicolaou KC, Ellery SP, Chen JS. Angew. Chem. 2004;121 doi: 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48 [Google Scholar]
- [17a].Streuff J. Synthesis. 2013:281. [Google Scholar]
- [17b].Lee GH. Curr. Org. Chem. 2004;8 [Google Scholar]
- [17c].Zhou L, Hirao T. Tetrahedron. 2001;57 [Google Scholar]
- [17d].Corey EJ, Payne SG. Tetrahedron Lett. 1983;24 [Google Scholar]
- [17e].Lee GH, Choi EB, Lee E, Pak CS. J. Org. Chem. 1994;59 [Google Scholar]
- [18].Smith SG, Goodman JM. J. Am. Chem. Soc. 2010;132:12946. doi: 10.1021/ja105035r. [DOI] [PubMed] [Google Scholar]
- [19]. CCDC 993662 (172 and 993663 ( ) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- [20].Mehta G, Shindea HM, Kumarana RS. Tetrahedron Lett. 2012;53:4320. [Google Scholar]
- [21]. Alternative chelated transition structures leading to the trans-A,B-fused product are expected to be of significantly higher energy due to incurred ring strain and steric interaction with the C13 vinyl group.
- [22a].Saksena AK, Mangiaracina P. Tetrahedron Lett. 1983;24:273. [Google Scholar]
- [22b].Evans DA, Chapman KT, Carreira EM. J. Am. Chem. Soc. 1988;110 [Google Scholar]
- [23].Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994:639. [Google Scholar]
- [24].Zhou X, De Clercq PJ, Gawronski J. Tetrahedron: Asymmetry. 1995;6:1551. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


