Abstract
Pregabalin is a commonly used therapy currently recommended as first-line treatment for a number of neuropathic pain (NeP) conditions. Since licensure, a number of clinical trials of pregabalin in different NeP conditions have been completed from which additional data on safety and tolerability can be drawn. In this analysis, patient-level data from 31 randomized clinical trials of pregabalin in peripheral NeP sponsored by Pfizer were pooled and assessed for incidence of adverse events (AEs). Incidence by age, disease condition, and race, together with risk differences and time to onset and resolution of AEs, was assessed. In total, 7,510 patients were included: 4,884 on pregabalin (representing 805 patient-years treatment) and 2,626 on placebo. Pregabalin vs. placebo risk analysis identified 9 AEs with a risk difference, for which the lower limit of the 95% confidence interval (CI) was > 1%: dizziness (risk difference [95% CI]: (17.0 [15.4 to 18.6]), somnolence (10.8 [9.5 to 12.1]), peripheral edema (5.4 [4.3 to 6.4]), weight increase (4.7 [3.9 to 5.5]), dry mouth (2.9 [2.1 to 3.8]), constipation (2.3 [1.5 to 3.2]), blurred vision (2.2 [1.6 to 2.9]), balance disorder (2.0 [1.5 to 2.5]), and euphoric mood (1.6 [1.2 to 2.0]). The most common AEs, dizziness and somnolence, typically emerged within the first 1 to 2 weeks of treatment and resolved 1 to 2 weeks later, without resulting in cessation of treatment. The data from this review provide information, indicating which AEs may be expected in patients treated with pregabalin, and suggest that careful dose titration to the highest tolerable dose is the most appropriate approach in clinical practice.
Keywords: pregabalin, adverse events, safety, postherpetic neuralgia, peripheral neuropathic pain, neuralgia, diabetic, pain
Introduction
Neuropathic pain (NeP) is a common condition1 which, due to its chronicity, severity, and associated comorbidities, can be challenging to treat.2–4 In particular, difficulties in managing NeP frequently arise if the patient is resistant to treatment, if they receive inappropriate or subtherapeutic doses, or if they are unable to tolerate medication.2
Pregabalin is well established as one of the most commonly used treatment options for chronic pain therapy and is recommended as a first-line treatment for most NeP conditions.5–8 In Europe, pregabalin is indicated for the treatment of peripheral and central NeP, generalized anxiety disorder, and as adjunctive therapy for partial seizures.9 In the United States, pregabalin is indicated for the management of NeP associated with diabetic peripheral neuropathy (DPN), postherpetic neuralgia (PHN), and spinal cord injury, for the treatment of fibromyalgia, and for adjunctive therapy for partial seizures.10 To date, the worldwide exposure to pregabalin is in excess of 19 million patient-years.11
It is always important for the physician to have access to up-to-date information on any adverse events (AEs) associated with a drug in order to guide decision-making. Since licensure, many randomized, double-blind trials have assessed the safety and efficacy of pregabalin in NeP conditions, providing a wealth of information on the safety profile of pregabalin in this patient population. A number of recent publications have taken information from published clinical trials to gain new insights into the AE profile of pregabalin.3,12–15 However, these studies were limited to an analysis of population-level data available in the literature. This current analysis pooled patient-level data from 31 randomized, placebo-controlled studies of pregabalin in peripheral NeP, allowing for a more detailed and robust analysis of pregabalin's safety profile. In this comprehensive assessment, analyses were undertaken to stratify the pooled data by pregabalin dose and patients' disease condition, age, and race. The time to onset and time to resolution of the most common AEs were also evaluated. This analysis provides a detailed assessment of what physicians and other healthcare providers may expect when treating patients with peripheral NeP with pregabalin.
Methods
Source Data
For this analysis, data were pooled from all 31 phase II, III, and IV randomized, controlled trials of pregabalin in peripheral NeP sponsored by Pfizer. Trials were conducted between March 1998 and May 2012 in Asia, Australia, Canada, Europe, Latin America, the Middle East, South Africa, and the United States. The studies were conducted in patients with DPN, PHN, chronic lower back pain (CLBP), human immunodeficiency virus (HIV) neuropathy, cancer-related NeP (chemotherapy-induced neuropathy and cancer-induced bone pain), posttraumatic peripheral NeP (PT), and other NeP conditions (idiopathic trigeminal neuralgia [TGN] and disturbed sleep concurrent with NeP) (Table 1). The duration of these studies ranged from 2 to 18 weeks. Pregabalin dosing included fixed doses of 75, 150, 300, 450, and 600 mg/day and flexible dose of either 100 to 600 mg/day or 150 to 600 mg/day. AEs were assessed throughout each trial including clinically significant symptoms and signs, in addition to abnormal laboratory test values and changes in physical examination findings. AEs were coded in accordance with the Medical Dictionary for Regulatory Activities' medical terminology.
Table 1.
Studies Included in the Analysis
| Disease | Studies* |
|---|---|
| DPN | 1008-01440; 1008-02941; 1008-04042; 1008-13143; 1008-14944; 1008-15525; 1008-173; A0081030 (NCT00156078); A0081037 (NCT00141219)45; A0081060 (NCT00159679)24; A0081071 (NCT00143156); A0081081 (NCT00301223)46; A0081163 (NCT00553475)47 |
| PHN | 1008-03J; 1008-03042; 1008-04548; 1008-12749; 1008-132; 1008-15525; 1008-19650; A0081004 (NCT00159666)26; A0081081 (NCT00301223)46; A0081120 (NCT00394901)51; A6061026 (NCT00288652); A0081037 (NCT00141219)45 |
| CLBP | 1008-032; 1008-104 |
| HIV neuropathy | A0081066 (NCT00232141)52; A0081244 (NCT01049217) |
| Cancer NeP | A0081124 (NCT00380874)53; A0081128 (NCT00381095)54 |
| PT | A0081037 (NCT00141219)45; A0081064 (NCT00292188)28 |
| Other NeP | 1008-04J; 1008-160 |
DPN, diabetic peripheral neuropathy; PHN, postherpetic neuralgia; CLBP, chronic lower back pain; HIV, human immunodeficiency virus; NeP, neuropathic pain; PT, post-traumatic peripheral neuropathic pain.
Some studies were conducted across multiple diseases and appear under more than 1 disease heading. ClinicalTrials.gov identifying numbers shown where available (some historical trials are not recorded at ClinicalTrials.gov). CLBP studies included patients with nonspecific chronic lower back pain; no steps were taken to diagnose or characterize NeP. Cancer NeP includes studies in chemotherapy-induced neuropathy and cancer-induced bone pain. Other NeP includes studies in idiopathic trigeminal neuralgia and disturbed sleep concurrent with NeP.
Three randomized withdrawal trials of pregabalin in NeP, conducted between April 2005 and January 2012 in patients with NeP of mixed etiology,16 chronic lumbosacral radiculopathy,17 and refractory DPN,18 were analyzed separately to the full data set. AEs were reported in the first phase in which they occurred unless they worsened in severity or frequency in a later phase. AEs that began or worsened in severity after the end of the single-blind pregabalin run-in are included. Data from randomized withdrawal trials were not pooled with those from parallel, double-blind trials as the trial methodologies are not compatible.
Data Analysis
The full data set was analyzed for incidence of AEs, with the most common AEs identified as those with a risk difference, for pregabalin all-doses compared with placebo, for which the lower limit of the confidence interval (CI) was > 1%. For each AE, 95% CI calculations for the risk differences between pregabalin and placebo were adjusted by study. Risk difference is calculated by subtracting the absolute risk with placebo from the absolute risk with pregabalin, to determine the level of risk that can be attributed to treatment. The AE profile was also determined for patients grouped by pregabalin dose and patient age, race, and disease condition. Time to onset and time to resolution of AEs were also assessed using survival estimates and the LIFETEST procedure for censored values. All analyses were descriptive.
Results
Patient Population
In total, 7,510 patients were included in the data analysis: 4,884 on pregabalin (a total of 805 patient-years of pregabalin treatment) and 2,626 on placebo (Table 2). Of these, safety data were available for 7,509 patients: 4,883 on pregabalin (859 on ≤ 150 mg/day; 1,212 on > 150 to ≤ 300 mg/day; 129 on > 300 to ≤ 450 mg/day; 1,211 on > 450 to ≤ 600 mg/day; 1,472 on flexible dose) and 2,626 on placebo. Of the patients included, 53.1% were male and 40.6% were aged ≥ 65 years (Table 2). The median duration of treatment across all studies was 59.0 days with pregabalin and 63.0 days with placebo. There were notable differences between races in the median duration of treatment with both pregabalin and placebo: White patients, 55 days with pregabalin and 56 days with placebo; Asian patients, 88 days and 90 days; Black patients, 92 days and 99 days; and other patients, 57.5 days and 75 days. This difference was likely due to larger proportions of Asian and Black patients in the more recent studies, which tended to be of longer duration.
Table 2.
Patient Characteristics
| Pregabalin (N = 4,884) | Placebo (N = 2,626) | |
|---|---|---|
| Sex, n (%) | ||
| Male | 2,569 (52.6) | 1,419 (54.0) |
| Female | 2,315 (47.4) | 1,207 (46.0) |
| Age (years), n (%) | ||
| 18 to 64 | 2,857 (58.5) | 1,602 (61.0) |
| 65 to 74 | 1,271 (26.0) | 682 (26.0) |
| ≥ 75 | 756 (15.5) | 342 (13.0) |
| Mean (SD) | 60.6 (13.3) | 59.1 (13.7) |
| Range | 18 to 100 | 19 to 96 |
| Race, n (%) | ||
| White | 3,253 (66.6) | 1,679 (63.9) |
| Asian | 1,018 (20.8) | 544 (20.7) |
| Black | 339 (6.9) | 270 (10.3) |
| Other | 224 (4.6) | 119 (4.5) |
| Unspecified | 50 (1.0) | 14 (0.5) |
| Weight (kg) | ||
| N | 4,830 | 2,610 |
| Mean (SD) | 80.2 (20.9) | 79.8 (20.8) |
| Range | 30.9 to 194.4 | 29.1 to 187.7 |
| Body mass index (kg/m2) | ||
| N | 4,823 | 2,605 |
| Mean ± SD | 28.5 (6.5) | 28.3 (8.1) |
| Range | 13.5 to 78.8 | 11.4 to 247.4 |
| Median duration of treatment (days) | 59.0 | 63.0 |
SD, standard deviation.
In the flexible-dose studies, the mean dose of pregabalin (across the duration of the study, including the titration and maintenance phases) was approximately 280 mg/day for most conditions (279 mg/day for DPN, 269 mg/day for PHN, 275 mg/day for HIV neuropathy, and 288 mg/day for PT), with a median dose of 300 mg/day for DPN, HIV neuropathy and PT, and 294 mg/day for PHN. However, in cancer pain, the mean dose of pregabalin was lower, 203 mg/day (median 196 mg/day), potentially due to most patients continuing to receive concomitant pain medications during these trials. Mean (and median) doses of pregabalin in each condition were also similar across racial groups (median dose of 300 mg/day in the White, Black, and Asian groups).
Incidence of AEs
Table 3 summarizes the incidence of AEs occurring in > 2% of patients treated with pregabalin (all-doses combined). Due to the low number of patients in the > 300 to ≤ 450 mg/day group (n = 129), this group was combined with the > 450 to ≤ 600 mg/day group. The lower number of patients in the 450 mg/day group was due to the design of the trials; earlier trials tended to use doses of 150, 300, and 600 mg/day, whereas later trials tended to use flexible dosing. Incidence of AEs typically increased with higher fixed doses of pregabalin and was lowest in the ≤ 150 mg/day group, followed by the flexible-dosing group. The most common AEs with pregabalin were dizziness, somnolence, peripheral edema, and weight increase. Headache, nausea, and diarrhea were less frequent with pregabalin (all-doses combined) than with placebo, while nasopharyngitis had a similar incidence with pregabalin and placebo. The incidence of euphoric mood in the pregabalin all-doses group was 1.6%, compared with 0.2% in the placebo group.
Table 3.
Incidence of Adverse Events by Pregabalin Dose
| Pregabalin | Placebo (n = 2,626) | |||||
|---|---|---|---|---|---|---|
| ≤ 150 mg/day (n = 859) | > 150 to ≤ 300 mg/day (n = 1,212) | > 300 to ≤ 600 mg/day (n = 1,340) | Flexible Dose (n = 1,472) | All-doses (n = 4,883) | ||
| Dizziness | 109 (12.7) | 315 (26.0) | 463 (34.6) | 298 (20.2) | 1,185 (24.3) | 191 (7.3) |
| Somnolence | 78 (9.1) | 208 (17.2) | 258 (19.3) | 193 (13.1) | 737 (15.1) | 122 (4.6) |
| Peripheral edema | 32 (3.7) | 118 (9.7) | 154 (11.5) | 116 (7.9) | 420 (8.6) | 95 (3.6) |
| Headache | 53 (6.2) | 58 (4.8) | 104 (7.8) | 94 (6.4) | 309 (6.3) | 182 (6.9) |
| Weight increase | 20 (2.3) | 86 (7.1) | 118 (8.8) | 78 (5.3) | 302 (6.2) | 33 (1.3) |
| Dry mouth | 34 (4.0) | 57 (4.7) | 104 (7.8) | 51 (3.5) | 246 (5.0) | 53 (2.0) |
| Constipation | 38 (4.4) | 62 (5.1) | 68 (5.1) | 66 (4.5) | 234 (4.8) | 63 (2.4) |
| Fatigue | 30 (3.5) | 31 (2.6) | 88 (6.6) | 61 (4.1) | 210 (4.3) | 81 (3.1) |
| Nausea | 21 (2.4) | 40 (3.3) | 71 (5.3) | 77 (5.2) | 209 (4.3) | 133 (5.1) |
| Blurred vision | 25 (2.9) | 45 (3.7) | 79 (5.9) | 26 (1.8) | 175 (3.6) | 33 (1.3) |
| Diarrhea | 28 (3.3) | 35 (2.9) | 41 (3.1) | 54 (3.7) | 158 (3.2) | 108 (4.1) |
| Nasopharyngitis | 39 (4.5) | 49 (4.0) | 27 (2.0) | 41 (2.8) | 156 (3.2) | 85 (3.2) |
| Balance disorder | 9 (1.0) | 26 (2.1) | 57 (4.3) | 17 (1.2) | 109 (2.2) | 7 (0.3) |
| Edema* | 14 (1.6) | 34 (2.8) | 46 (3.4) | 20 (1.4) | 114 (2.3) | 18 (0.7) |
Number (percentage) of patients experiencing each adverse event. Adverse events with an incidence ≥ 2% in the pregabalin all-doses group are shown. The > 300 to ≤ 600 mg/day group includes the > 300 to ≤ 450 (n = 129) and > 450 to ≤ 600 groups (n = 1,211). The median flexible dose of pregabalin was 300 mg/day.
Italics indicate adverse events in which incidence with pregabalin all-doses was equal or less than placebo.
Whole-body edema, as distinct from edema of the peripheral limbs.
Analysis of the overall pooled population identified 9 AEs with a risk difference for pregabalin (all-doses combined) compared with placebo for which the lower limit of the 95% CI was > 1% (Figure 1): dizziness (risk difference [95% CI]: 17.0 [15.4 to 18.6]), somnolence (10.8 [9.5–12.1]), peripheral edema (5.4 [4.3 to 6.4]), weight increase (4.7 [3.9 to 5.5]), dry mouth (2.9 [2.1 to 3.8]), constipation (2.3 [1.5 to 3.2]), blurred vision (2.2 [1.6 to 2.9]), balance disorder (2.0 [1.5 to 2.5]), and euphoric mood (1.6 [1.2 to 2.0]).
Figure 1.
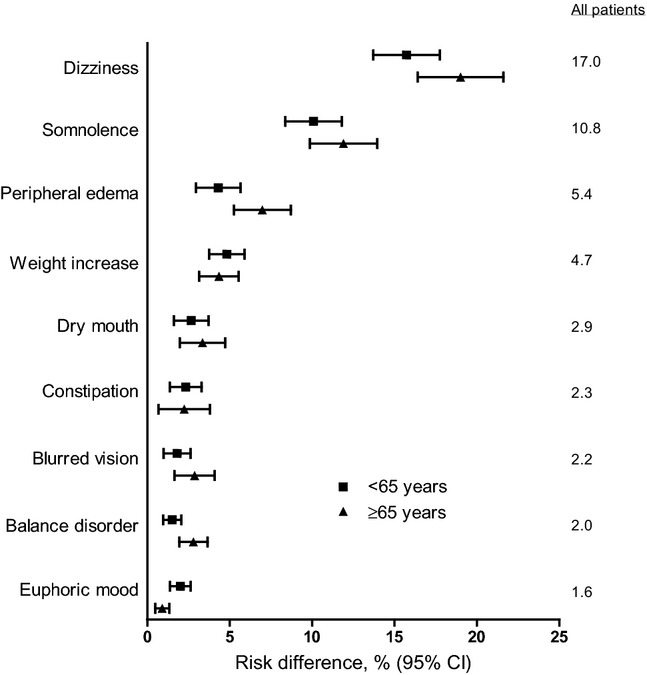
Risk difference for common adverse events by age group. Risk difference with 95% confidence interval (CI) for patients < 65 years and ≥ 65 years for each AE (pregabalin, n = 4,883; placebo, n = 2,626). The risk difference for each AE, for all patients (of any age), is also shown. Those AEs with a risk difference for which the lower limit of the 95% CI was > 1% (for pregabalin all-doses) are shown.
The incidence of AEs with pregabalin varied by patient age (Figure 1, Table 4). Most AEs were more common with increased age, with the exception of weight increase and euphoric mood, which were more common in younger patients. While incidence of constipation with pregabalin was higher in older patients (Table 4), there was also a higher incidence with placebo, resulting in a lower risk difference than in younger patients (Figure 1).
Table 4.
Incidence of Adverse Events by Age Group
| 18–64 Years | 65–74 Years | ≥ 75 Years | ||||
|---|---|---|---|---|---|---|
| Pregabalin (n = 2,856) | Placebo (n = 1,602) | Pregabalin (n = 1,271) | Placebo (n = 682) | Pregabalin (n = 756) | Placebo (n = 342) | |
| Dizziness | 639 (22.4) | 113 (7.1) | 333 (26.2) | 45 (6.6) | 213 (28.2) | 33 (9.6) |
| Somnolence | 414 (14.5) | 78 (4.9) | 194 (15.3) | 27 (4.0) | 129 (17.1) | 17 (5.0) |
| Peripheral edema | 218 (7.6) | 62 (3.9) | 123 (9.7) | 26 (3.8) | 79 (10.4) | 7 (2.0) |
| Weight increase | 185 (6.5) | 21 (1.3) | 70 (5.5) | 11 (1.6) | 47 (6.2) | 1 (0.3) |
| Dry mouth | 134 (4.7) | 31 (1.9) | 69 (5.4) | 16 (2.3) | 43 (5.7) | 6 (1.8) |
| Constipation | 115 (4.0) | 27 (1.7) | 70 (5.5) | 23 (3.4) | 49 (6.5) | 13 (3.8) |
| Blurred vision | 86 (3.0) | 16 (1.0) | 48 (3.8) | 8 (1.2) | 41 (5.4) | 9 (2.6) |
| Balance disorder | 46 (1.6) | 4 (0.2) | 29 (2.3) | 2 (0.3) | 34 (4.5) | 1 (0.3) |
| Euphoric mood | 63 (2.2) | 4 (0.2) | 9 (0.7) | 0 | 8 (1.1) | 0 |
Number (percentage) of patients experiencing each adverse event. Adverse events with a risk difference for which the lower limit of the 95% CI was >1% (all-doses pregabalin) are shown.
The incidence of AEs also varied across disease conditions (Table 5). In DPN and PHN patients, the AE profile was similar to the pooled population (all conditions) with slightly higher incidences of somnolence and constipation in PHN patients than in DPN patients. In CLBP patients, dizziness, dry mouth, and euphoric mood were more common, with peripheral edema and constipation less common, than the pooled population. Euphoric mood was also more common in HIV neuropathy patients, while other AEs were less common. In patients with cancer NeP, dizziness, peripheral edema, weight increase, and dry mouth were all less common than in the pooled population. Dizziness was more common in patients with PT and Other NeP.
Table 5.
Incidence of Adverse Events by Disease Condition
| Dizziness | Somnolence | Peripheral Edema | Weight Increase | Dry Mouth | Constipation | Blurred Vision | Balance Disorder | Euphoric Mood | |
|---|---|---|---|---|---|---|---|---|---|
| DPN | |||||||||
| Pregabalin (n = 2,175) | 485 (22.3) | 286 (13.2) | 227 (10.4) | 165 (7.6) | 77 (3.5) | 78 (3.6) | 60 (2.8) | 53 (2.4) | 20 (0.9) |
| Placebo (n = 1,088) | 60 (5.5) | 46 (4.2) | 65 (6.0) | 18 (1.6) | 11 (1.0) | 16 (1.5) | 11 (1.0) | 4 (0.4) | 1 (0.1) |
| PHN | |||||||||
| Pregabalin (n = 1,604) | 408 (25.4) | 272 (17.0) | 141 (8.8) | 98 (6.1) | 87 (5.4) | 111 (6.9) | 70 (4.4) | 37 (2.3) | 10 (0.6) |
| Placebo (n = 718) | 61 (8.5) | 35 (4.9) | 13 (1.8) | 5 (0.7) | 19 (2.6) | 25 (3.5) | 15 (2.1) | 2 (0.3) | 0 |
| CLBP | |||||||||
| Pregabalin (n = 465) | 146 (31.4) | 83 (17.8) | 13 (2.8) | 25 (5.4) | 49 (10.5) | 13 (2.8) | 30 (6.4) | 11 (2.4) | 34 (7.3) |
| Placebo (n = 193) | 17 (8.8) | 8 (4.2) | 0 | 3 (1.6) | 10 (5.2) | 4 (2.1) | 3 (1.6) | 0 | 2 (1.0) |
| HIV neuropathy | |||||||||
| Pregabalin (n = 334) | 54 (16.2) | 48 (14.4) | 23 (6.9) | 6 (1.8) | 15 (4.5) | 10 (3.0) | 5 (1.5) | 4 (1.2) | 15 (4.5) |
| Placebo (n = 343) | 26 (7.6) | 17 (5.0) | 11 (3.2) | 1 (0.3) | 2 (0.6) | 3 (0.9) | 1 (0.3) | 1 (0.3) | 1 (0.3) |
| Cancer NeP | |||||||||
| Pregabalin (n = 104) | 18 (17.3) | 18 (17.3) | 5 (4.8) | 0 | 2 (1.9) | 10 (9.6) | 0 | 0 | 0 |
| Placebo (n = 109) | 11 (10.1) | 6 (5.5) | 3 (2.8) | 3 (2.8) | 3 (2.8) | 10 (9.2) | 0 | 0 | 0 |
| PT | |||||||||
| Pregabalin (n = 175) | 65 (37.1) | 27 (15.4) | 9 (5.1) | 7 (4.0) | 16 (9.1) | 11 (6.3) | 9 (5.1) | 4 (2.3) | 1 (0.6) |
| Placebo (n = 155) | 16 (10.3) | 10 (6.4) | 3 (1.9) | 3 (1.9) | 8 (5.2) | 5 (3.2) | 3 (1.9) | 0 | 0 |
| Other NeP* | |||||||||
| Pregabalin (n = 26) | 9 (34.6) | 3 (11.5) | 2 (7.7) | 1 (3.8) | 0 | 1 (3.8) | 1 (3.8) | 0 | 0 |
| Placebo (n = 20) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
DPN, diabetic peripheral neuropathy; PHN, postherpetic neuralgia; CLBP, chronic lower back pain; HIV, human immunodeficiency virus; NeP, neuropathic pain; CI, confidence interval; PT, post-traumatic peripheral NeP.
Number (percentage) of patients experiencing each adverse event. Adverse events with a risk difference for which the lower limit of the 95% CI was > 1% (all-doses pregabalin) are shown.
Including idiopathic trigeminal neuralgia and disturbed sleep concurrent with NeP.
There were some differences in the incidence of AEs across racial groups (Table 6). Dry mouth was notably rare (< 1%) in Asian patients, as was blurred vision, while there was only 1 instance of balance disorder and none of euphoric mood. At the same time, incidence of somnolence, peripheral edema, weight increase, and constipation was slightly higher than in other racial groups. In Black patients, dizziness, weight increase, dry mouth, blurred vision, and balance disorder were less common than in all racial groups combined.
Table 6.
Incidence of Adverse Events by Race
| White | Asian | Black | Other | |||||
|---|---|---|---|---|---|---|---|---|
| Pregabalin (n = 3,252) | Placebo (n = 1,679) | Pregabalin (n = 1,018) | Placebo (n = 544) | Pregabalin (n = 339) | Placebo (n = 270) | Pregabalin (n = 224) | Placebo (n = 119) | |
| Dizziness | 827 (25.4) | 116 (6.9) | 225 (22.1) | 42 (7.7) | 64 (18.9) | 19 (7.0) | 56 (25.0) | 14 (11.8) |
| Somnolence | 458 (14.1) | 72 (4.3) | 177 (17.4) | 30 (5.5) | 49 (14.5) | 11 (4.1) | 47 (21.0) | 9 (7.6) |
| Peripheral edema | 270 (8.3) | 58 (3.5) | 101 (9.9) | 19 (3.5) | 26 (7.7) | 11 (4.1) | 23 (10.3) | 7 (5.9) |
| Weight increase | 200 (6.2) | 19 (1.1) | 84 (8.3) | 8 (1.5) | 12 (3.5) | 4 (1.5) | 6 (2.7) | 2 (1.7) |
| Dry mouth | 218 (6.7) | 43 (2.6) | 8 (0.8) | 5 (0.9) | 10 (2.9) | 4 (1.5) | 10 (4.5) | 1 (0.8) |
| Constipation | 150 (4.6) | 40 (2.4) | 66 (6.5) | 16 (2.9) | 12 (3.5) | 4 (1.5) | 4 (1.8) | 3 (2.5) |
| Blurred vision | 144 (4.4) | 23 (1.4) | 17 (1.7) | 6 (1.1) | 6 (1.8) | 0 | 8 (3.6) | 4 (3.4) |
| Balance disorder | 99 (3.0) | 6 (0.4) | 1 (0.1) | 0 | 5 (1.5) | 0 | 4 (1.8) | 1 (0.8) |
| Euphoric mood | 65 (2.0) | 3 (0.2) | 0 | 0 | 12 (3.5) | 1 (0.4) | 3 (1.3) | 0 |
Number (percentage) of patients experiencing each adverse event. Adverse events with a risk difference for which the lower limit of the 95% CI was > 1% (all-doses pregabalin) are shown.
Patient Outcomes Following AEs
Across all studies and doses of pregabalin, 195 patients (4%) withdrew from their trial due to dizziness (compared with 19 patients [0.7%] on placebo). Ninety-five patients (1.9%) withdrew due to somnolence (compared with 4 patients [0.2%] on placebo). No other AE led to the withdrawal of ≥ 1% of all patients. Limited to patients with that specific AE, balance disorder was the most likely to lead to withdrawal. Of a total of 109 incidences of balance disorder, 17.4% (19 patients) withdrew from the study as a consequence. The percentage of incidences leading to withdrawal for other identified AEs was as follows: dizziness, 16.5%; blurred vision, 14.3%; somnolence, 12.9%; peripheral edema, 10.5%; euphoric mood, 7.5%; dry mouth, 5.7%; weight increase, 5.0%; and constipation, 3.8%.
The AEs with the highest relative risk with pregabalin were examined to determine the point at which they emerged and the point at which the event resolved. Kaplan–Meier plots of time to onset and time to resolution of the most common AEs with flexible-dose pregabalin are shown in Figure 2. Survival distributions for flexible-dose pregabalin are shown, but data followed similar trends for other doses of pregabalin and for placebo, both for onset and resolution.
Figure 2.
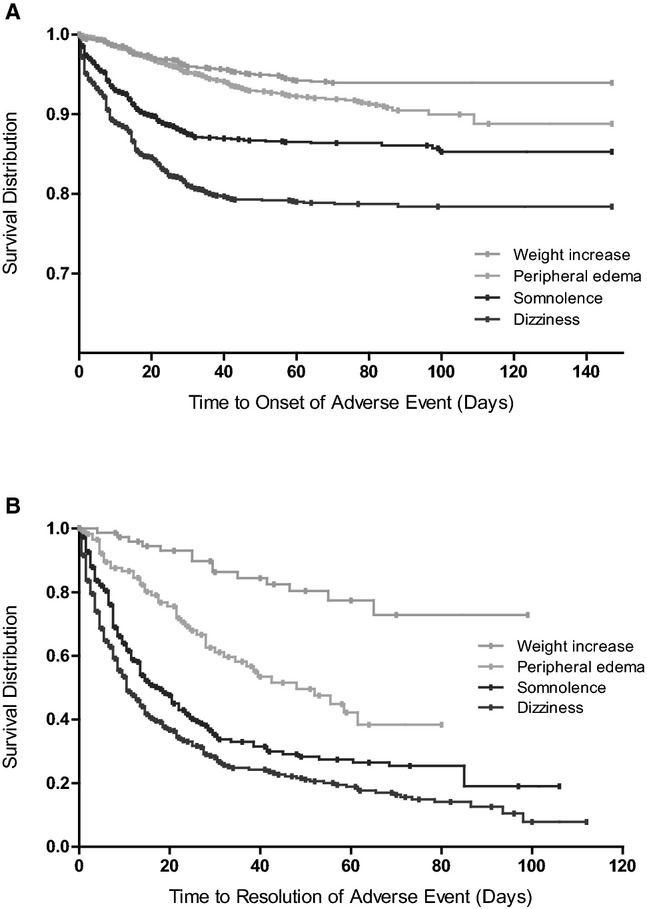
Time to onset and resolution of common adverse events in studies of flexible-dose pregabalin. Kaplan–Meier plots of (A) time to onset and (B) time to resolution of common adverse events (those with a risk difference > 3) in the pregabalin flexible-dose group. Data followed similar trends for other doses of pregabalin and for placebo, for both time to onset and time to resolution.
Median times to onset and times to resolution for AEs with placebo and flexible-dose pregabalin, together with the proportion of AEs that resolved prior to the end of the trial, are shown in Table 7. Both dizziness and somnolence typically emerged within the first 1 to 2 weeks of starting pregabalin treatment, with a median time to onset of 9 and 10 days, respectively. Most instances resolved before the conclusion of the trial, typically within 9 to 14 days of onset. With the exception of weight increase, the other identified AEs also tended to be transient and most instances resolved prior to the end of the study (within 9 to 24 days).
Table 7.
Time to Onset and Resolution of Adverse Events with Placebo and Flexible-Dose Pregabalin
| Adverse Event | Treatment | Incidence (n) | Time to Onset (Days) | Number Resolved | Percentage Resolved | Time to Resolution (Days) |
|---|---|---|---|---|---|---|
| Dizziness | Placebo | 191 | 10.0 | 122 | 63.9 | 4.0 |
| Pregabalin | 298 | 9.0 | 222 | 74.5 | 9.0 | |
| Somnolence | Placebo | 122 | 6.0 | 62 | 50.8 | 27.0 |
| Pregabalin | 193 | 10.0 | 125 | 64.8 | 14.0 | |
| Peripheral edema | Placebo | 95 | 29.0 | 34 | 35.8 | 25.0 |
| Pregabalin | 116 | 28.0 | 47 | 40.5 | 22.0 | |
| Weight increase | Placebo | 33 | 40.0 | 2 | 6.1 | 41.0 |
| Pregabalin | 78 | 21.0 | 14 | 17.9 | 49.5 | |
| Dry mouth | Placebo | 53 | 7.0 | 18 | 34.0 | 28.0 |
| Pregabalin | 51 | 16.0 | 23 | 45.1 | 17.0 | |
| Constipation | Placebo | 63 | 16.0 | 38 | 60.3 | 12.0 |
| Pregabalin | 66 | 14.0 | 38 | 57.6 | 21.5 | |
| Blurred vision | Placebo | 33 | 12.0 | 12 | 36.4 | 15.0 |
| Pregabalin | 26 | 24.5 | 15 | 57.7 | 9.0 | |
| Balance disorder | Placebo | 7 | 15.0 | 5 | 71.4 | 7.0 |
| Pregabalin | 17 | 16.0 | 10 | 58.8 | 24.0 | |
| Euphoric mood | Placebo | 4 | 3.5 | 4 | 100 | 1.5 |
| Pregabalin | 18 | 5.0 | 15 | 83.3 | 8.5 |
Incidence, resolution, and median time to onset and resolution with placebo and flexible-dose pregabalin. Adverse events with a risk difference for which the lower limit of the 95% CI was > 1% (all-doses pregabalin) are shown.
Incidence of AEs in Randomized Withdrawal Trials
Adverse events from randomized withdrawal trials were not included in the full data set due to the unique nature of this type of trial. However, 3 randomized withdrawal trials of pregabalin in NeP (mixed etiology,16 chronic lumbosacral radiculopathy,17 and refractory DPN18) were assessed separately from the full analysis population. Across the 3 trials, 337 patients were treated with pregabalin and 331 with placebo. The most common treatment-emergent AEs (occurring in ≥ 2% of patients) were peripheral edema (9.2% with pregabalin and 5.7% with placebo), dizziness (4.5% and 3.3%), increased weight (4.5% and 1.8%), and somnolence (3.0% and 2.1%). The median dose of pregabalin in randomized withdrawal trials was approximately 200 mg/day.
Discussion
No drug is without AEs, and the more information available about which AEs might be expected and when they may emerge, or resolve, the greater will be the ability of the physician to anticipate complications, set expectations, and improve patient management. Improved discussion between physician and patient regarding potential AEs has been credited for the low rates of AEs seen in observational studies of pregabalin.19 This may be due to reduced anxiety regarding expected AEs or patients' experience of mild AEs. Previous publications have investigated the incidence of AEs with pregabalin, through pooled analyses of published data from trials3,12–15,20 and patient-reported AEs.21 This analysis of patient-level data from 31 clinical trials of pregabalin across a number of NeP conditions provides comprehensive data on the incidence of the most common AEs, including when they are most likely to occur and resolve (Table 8). The use of patient-level data in pooled analyses is recognized as the least biased and most reliable means of addressing questions not answered by individual trials.22
Table 8.
Adverse Events with Pregabalin Treatment
| Adverse events in clinical trials of pregabalin |
| The most common adverse events are dizziness (24.3% of patients) and somnolence (15.1%) |
| 4% of patients withdraw from clinical trials due to dizziness |
| 1.9% due to somnolence |
| The majority of instances of dizziness and somnolence emerge within the first 1 to 2 weeks of treatment |
| The majority of instances of dizziness and somnolence resolve within 1 to 2 weeks |
| Most adverse events are transient and resolve prior to the end of the study |
| Most adverse events are mild or moderate in severity3,20 |
| Patients with a lower baseline body mass index are more likely to gain weight12 |
| Managing adverse events with pregabalin |
| Carefully titrate pregabalin to the highest tolerable dose (a minimum of 150 mg/day6,27) |
| The initial dose, and any dose increase, should be given at night |
| To set expectations, potential AEs should be discussed with the patient ahead of treatment |
The incidences of most common AEs in this analysis were lower than those reported in the current prescribing/product information for pregabalin.9,10,23 The scientific discussion for the approval of pregabalin from the European Medicines Agency reports incidences with all-doses of pregabalin of 29.1% for dizziness, 22.6% for somnolence, 5.6% for weight gain, and 9.1% for dry mouth23 compared with 24.3%, 15.1%, 6.2%, and 5.0% in this analysis. In contrast, the scientific discussion reports an incidence of 5.6% for peripheral edema,23 compared with 8.6% in this analysis. This may be due to an ascertainment bias in some more recent DPN trials.24 In these trials, study investigators were required, at each visit, to check for peripheral edema and report any increase in peripheral edema as an AE, leading to higher reported incidences with both pregabalin and placebo.24 Consistent with this, the incidence of peripheral edema with placebo reported in the scientific discussion was 1.4%,10,23 compared with 3.6% in this analysis.
The lower incidence of many AEs in this analysis, compared with the prescribing/product information, may reflect the greater proportion of flexible-dose studies conducted more recently. In this analysis, the incidences of AEs were lower in studies with flexible dosing. While the median dose of pregabalin in the flexible-dosing studies was 300 mg/day for most conditions, the incidence of AEs with flexible dosing was notably lower than in the fixed-dose, > 150 to ≤ 300 mg/day group (or any fixed-dose group > 150 mg/day) for all of the most common AEs. In those trials with both flexible- and fixed-dose arms, the incidence of AEs was notably higher in the fixed-dose arm. For example, in the 1008-155 study,25 incidence of dizziness was 28.8% with 600 mg/day fixed dose, compared with 19.1% with flexible dosing. In the A0081004 study,26 incidence of dizziness was 30.7% with 300 mg/day fixed dose and 24.2% with flexible dosing. These data suggest that replicating the flexible-dosing strategy, through careful dose titration to the highest tolerable dose, would be the most appropriate approach in clinical practice. This conclusion is supported by the current European guidelines for the treatment of NeP, which recommend treating with pregabalin between 150 and 600 mg/day,6 with 150 mg/day being the lowest effective dose.27
In this analysis, the incidence of dizziness in PT studies was notably higher than in other disease conditions (37.1% compared with 24.3% overall). This difference is most likely a consequence of the dosing schedule in the A0081064 clinical trial, which represented the majority of PT patients.28 In this trial, patients were given their initial dose, and any subsequent dose increase, in the morning, while in other trials, and in clinical practice, it is given at night.28 To reduce the likelihood of dizziness (and potentially other AEs), patients should, whenever possible, take their first dose of pregabalin or any change in dose at night.
Previous studies have suggested that somnolence and dizziness are frequently transient and are most commonly reported in the first week of treatment, with a fall in incidence from week 2.29 This finding is largely supported by this analysis in which more than half the cases emerged in the first 2 weeks. The majority of cases (> 90%) emerged in the first 5 weeks for dizziness and the first 7 weeks for somnolence. Dizziness and somnolence were often quick to resolve, with median times to resolution of 9 and 14 days, respectively. These data indicate that the most common AEs with pregabalin, dizziness, and somnolence, typically emerge soon after starting treatment and resolve soon after that, without having to cease treatment. In fact, instances of AEs were generally transient and resolved prior to the end of the study without withdrawing from treatment. Time to onset and time to resolution of the AE were often broadly similar for both pregabalin and placebo. This suggests that, typically, continued exposure to pregabalin did not significantly affect the progression, or resolution, of the AE.
A recent analysis of weight gain in more than 40 studies of patients treated with pregabalin for up to 1 year indicated that weight increase was more likely in patients with lower baseline body mass index.12 Weight increase more commonly emerged following a longer period of treatment (> 56 days) with a median weight increase of 5 to 6 kg, while the majority of patients (82%) did not have any significant weight change.12 These data are consistent with those reported here, where weight increase emerged later than other common AEs.
Incidence of euphoric mood across all pregabalin groups was low (80 incidences in 4,883 patients; 1.6%), with similar incidences across all pregabalin doses > 150 mg/day. The majority of cases were from trials in CLBP and HIV neuropathy with 34 and 15 cases, respectively (incidences of 7.3% and 4.5%, respectively). Incidence of euphoric mood was < 1% in all other conditions. For HIV patients, there were no cases of euphoric mood in the A0081244 trial, which excluded patients who failed a urine screen for drug abuse, and 15 cases in the A0081066 trial, which did not include a urine screen and only excluded patients who disclosed current and active drug abuse. It is thought that this observation may also apply to the CLBP population, where neither of the 2 trials in this analysis included a urine screen for illicit substances. While there have been some reports of potential abuse of pregabalin in patients with a substance abuse history,30 a recent large-scale drug utilization study did not detect a signal of widespread pregabalin abuse.31
It is recommended that pregabalin be gradually discontinued over a minimum of 1 week,9 although there are limited data showing the most effective way to discontinue this type of medication. Discontinuation symptoms have been observed in some patients, but there are no data on their incidence or severity in relation to dose or duration of pregabalin use.9 This analysis did not incorporate AEs reported following discontinuation of pregabalin in these trials (which may be considered discontinuation symptoms) due to the lack of consistent data collection methods and the potential for underreporting of AEs. Pregabalin has also been proposed as a treatment option for withdrawal symptoms in alcohol, benzodiazepine and, more recently, opioid dependence, but this use is not supported by Level I clinical evidence and remains controversial.32,33
There were a number of potential limitations of this analysis. The trials included were of different durations. There were significant differences in dose-escalation protocols across these studies, ranging from no escalation period, to a 6-week escalation period, and flexible-dose trials. Data are not included for longer-term, open-label, follow-up of patients. The majority of patients included in the analysis were DPN or PHN patients making it more challenging to draw conclusions about other disease conditions. This analysis did not consider pregabalin in combination with other pain medications; however, published data suggest combination with nonsteroidal anti-inflammatory drugs (eg, COX-2 inhibitors)34,35 and serotonin-norepinephrine reuptake inhibitors36 does not negatively impact pregabalin's overall tolerability profile. Pregabalin has also shown efficacy in combination with opioids,37,38 but caution should be taken as there may be the potential for additive central nervous system-related AEs.39
This analysis of patient-level data from 31 clinical trials of pregabalin in NeP provides extensive information for physicians and other healthcare providers on both which types of AE may be expected in a peripheral NeP population and when they are most likely to emerge and resolve. These data highlight the importance of monitoring the dose of pregabalin used, particularly in the first weeks of treatment when AEs are most likely to emerge. Careful dose titration to the highest tolerable dose is the most appropriate approach in patients with NeP treated with pregabalin. Potential AEs with pregabalin should be discussed with the patient, as greater awareness of what might be expected can help physicians find the right balance between management of AEs and improving patient outcomes.
Acknowledgments
This study was funded by Pfizer Inc. All authors were responsible for the decision to submit for publication. Medical writing support was provided by Joshua Fink PhD, of Engage Scientific Solutions, and funded by Pfizer Inc.
References
- 1.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toth C. Drug safety evaluation of pregabalin. Expert Opin Drug Saf. 2012;11:487–502. doi: 10.1517/14740338.2012.677026. [DOI] [PubMed] [Google Scholar]
- 4.Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ. 2009;339:b3002. doi: 10.1136/bmj.b3002. [DOI] [PubMed] [Google Scholar]
- 5.Bril V, England J, Franklin GM, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy. Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 7.Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–965. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Lyrica® Summary of Product Characteristics. Sandwich, UK; Pfizer Ltd. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf (accessed April 10, 2012)
- 10.Pfizer Inc. LYRICA prescribing information http://www.pfizer.com/files/products/uspi_lyrica.pdf (accessed November 30, 2012)
- 11. IMS MIDAS data from quarter ending June 2004 till quarter ending December 2012. Average daily dose based on IMS MIDAS data Q4 2012. Data on file.
- 12.Cabrera J, Emir B, Dills D, Murphy TK, Whalen E, Clair A. Characterizing and understanding body weight patterns in patients treated with pregabalin. Curr Med Res Opin. 2012;28:1027–1037. doi: 10.1185/03007995.2012.684044. [DOI] [PubMed] [Google Scholar]
- 13.Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52:826–836. doi: 10.1111/j.1528-1167.2010.02966.x. [DOI] [PubMed] [Google Scholar]
- 14.Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68:903–912. doi: 10.1007/s00228-012-1213-x. [DOI] [PubMed] [Google Scholar]
- 15.Semel D, Murphy TK, Zlateva G, Cheung R, Emir B. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85. doi: 10.1186/1471-2296-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilron I, Wajsbrot D, Therrien F, Lemay J. Pregabalin for peripheral neuropathic pain: a multicenter, enriched enrollment randomized withdrawal placebo-controlled trial. Clin J Pain. 2011;27:185–193. doi: 10.1097/AJP.0b013e3181fe13f6. [DOI] [PubMed] [Google Scholar]
- 17.Baron R, Freynhagen R, Tölle TR, et al. on behalf of the A0081007 Investigators. The efficacy and safety of pregabalin in the treatment of neuropathic pain associated with chronic lumbosacral radiculopathy. Pain. 2010;150:420–427. doi: 10.1016/j.pain.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Raskin P, Huffman C, Toth C, et al. Pregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: a randomized withdrawal trial. Clin J Pain. 2013;30:379–390. doi: 10.1097/AJP.0b013e31829ea1a1. [DOI] [PubMed] [Google Scholar]
- 19.Toelle TR, Varvara R, Nimour M, Emir B, Brasser M. Pregabalin in neuropathic pain related to DPN, cancer and back pain: analysis of a 6-week observational study. Open Pain J. 2012;5:1–11. [Google Scholar]
- 20.Ogawa S, Satoh J, Arakawa A, Yoshiyama T, Suzuki M. Pregabalin treatment for peripheral neuropathic pain: a review of safety data from randomized controlled trials conducted in Japan and in the west. Drug Saf. 2012;35:793–806. doi: 10.2165/11632660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Härmark L, Puijenbroek E, Grootheest K. Longitudinal monitoring of the safety of drugs by using a web-based system: the case of pregabalin. Pharmacoepidemiol Drug Saf. 2011;20:591–597. doi: 10.1002/pds.2135. [DOI] [PubMed] [Google Scholar]
- 22.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency. Scientific Discussion for the Approval of Lyrica http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000546/WC500046600.pdf (accessed November 30, 2012)
- 24.Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. doi: 10.1186/1471-2377-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115:254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Stacey BR, Barrett JA, Whalen E, Phillips KF, Rowbotham MC. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9:1006–1017. doi: 10.1016/j.jpain.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31:1448–1454. doi: 10.2337/dc07-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Seventer R, Bach FW, Toth CC, et al. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol. 2010;17:1082–1089. doi: 10.1111/j.1468-1331.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- 29.Gil-Nagel A, Zaccara G, Baldinetti F, Leon T. Add-on treatment with pregabalin for partial seizures with or without generalisation: pooled data analysis of four randomised placebo-controlled trials. Seizure. 2009;18:184–192. doi: 10.1016/j.seizure.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Gahr M, Freudenmann RW, Hiemke C, Kolle MA, Schönfeldt-Lecuona C. Pregabalin abuse and dependence in Germany: results from a database query. Eur J Clin Pharmacol. 2013;69:1335–1342. doi: 10.1007/s00228-012-1464-6. [DOI] [PubMed] [Google Scholar]
- 31.Asomaning K, Abramsky S, Liu Q, Zhou X, Sobel R, Watt S. Pregabalin prescriptions and substance abuse history-report from a drug utilization study [APS abstract 454] J Pain. 2013;14(Suppl):S89. [Google Scholar]
- 32.Oulis P, Konstantakopoulos G. Efficacy and safety of pregabalin in the treatment of alcohol and benzodiazepine dependence. Expert Opin Investig Drugs. 2012;21:1019–1029. doi: 10.1517/13543784.2012.685651. [DOI] [PubMed] [Google Scholar]
- 33.Kammerer N, Lemenager T, Grosshans M, Kiefer F, Hermann D. Pregabalin for the reduction of opiate withdrawal symptoms [Article in German] Psychiatr Prax. 2012;39:351–352. doi: 10.1055/s-0032-1305042. [DOI] [PubMed] [Google Scholar]
- 34.Romanò CL, Romanò D, Bonora C, Mineo G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J Orthop Traumatol. 2009;10:185–191. doi: 10.1007/s10195-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saldaña MT, Navarro A, Pérez C, Masramón X, Rejas J. Patient-reported-outcomes in subjects with painful lumbar or cervical radiculopathy treated with pregabalin: evidence from medical practice in primary care settings. Rheumatol Int. 2010;30:1005–1015. doi: 10.1007/s00296-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: High-dose monotherapy or their combination? The “COMBO-DN study” - a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154:2616–2625. doi: 10.1016/j.pain.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 37.Gatti A, Sabato AF, Occhioni R, Colini BaldeschiG, Reale C. Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: results of a multicenter Italian study. Eur Neurol. 2009;61:129–137. doi: 10.1159/000186502. [DOI] [PubMed] [Google Scholar]
- 38.Zin CS, Nissen LM, O'Callaghan JP, Duffull SB, Smith MT, Moore BJ. A randomized, controlled trial of oxycodone versus placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin. J Pain. 2010;11:462–471. doi: 10.1016/j.jpain.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Steigerwald L, Kern KU, Buunen M, Baron R, Falke D. Effectiveness of tapentadol prolonged release (PR) versus a combination of tapentadol (PR) and pregabalin for managing severe, chronic low back pain with a neuropathic component [ASRA abstract A059] Reg Anesth Pain Med. 2013;38:77–88. [Google Scholar]
- 40.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 42.Sharma U, Griesing T, Emir B, Young JP., Jr Time to onset of neuropathic pain reduction: A retrospective analysis of data from nine controlled trials of pregabalin for painful diabetic peripheral neuropathy and postherpetic neuralgia. Am J Ther. 2010;17:577–585. doi: 10.1097/MJT.0b013e3181d5e4f3. [DOI] [PubMed] [Google Scholar]
- 43.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12:203–213. doi: 10.1016/j.ejpain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Moon DE, Lee DI, Lee SC, et al. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther. 2010;32:2370–2385. doi: 10.1016/j.clinthera.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Guan Y, Ding X, Cheng Y, et al. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther. 2011;33:159–166. doi: 10.1016/j.clinthera.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28:109–116. doi: 10.1111/j.1464-5491.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- 48.Sabatowski R, Galvez R, Cherry DA, et al. The 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109:26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Dworkin RH, Corbin AE, Young JP, Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 50.van Seventer R, Feister HA, Young JP, Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22:375–384. doi: 10.1185/030079906x80404. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa S, Suzuki M, Arakawa A, Araki S, Yoshiyama T. Efficacy and tolerability of pregabalin for postherpetic neuralgia: a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Japan Soc Pain Clin. 2010;17:141–152. [Google Scholar]
- 52.Simpson DM, Schifitto G, Clifford DB, et al. 1066 HIV Neuropathy Study Group. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74:413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karthaus M, Karapetis CS, Brown MP, et al. Pregabalin-Study Group. A randomized, double-blind, placebo-controlled trial for prevention and treatment of chemotherapy-induced peripheral neuropathy symptoms with pregabalin in patients with advanced colorectal cancer [ASCO abstract e19573] J Clin Oncol (Meeting Abstracts) 2010;28(Suppl):e19573. [Google Scholar]
- 54.Sjölund K-F, Yang R, Lee K-H, Resnick M. Randomized study of pregabalin in patients with cancer-induced bone pain. Pain Ther. 2013;2:1–12. doi: 10.1007/s40122-013-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


