Abstract
The soft rot Enterobacteriaceae (SRE) Pectobacterium and Dickeya species (formerly classified as pectinolytic Erwinia spp.) cause important diseases on potato and other arable and horticultural crops. They may affect the growing potato plant causing blackleg and are responsible for tuber soft rot in storage thereby reducing yield and quality. Efficient and cost-effective detection and identification methods are essential to investigate the ecology and pathogenesis of the SRE as well as in seed certification programmes. The aim of this review was to collect all existing information on methods available for SRE detection. The review reports on the sampling and preparation of plant material for testing and on over thirty methods to detect, identify and differentiate the soft rot and blackleg causing bacteria to species and subspecies level. These include methods based on biochemical characters, serology, molecular techniques which rely on DNA sequence amplification as well as several less-investigated ones.
Keywords: Biovar, isolation, molecular techniques, PCR, pectinolytic Erwinia spp, phylogeny, potato, serology
Introduction
Soft rot Enterobacteriaceae (SRE) Pectobacterium atrosepticum (Gardan et al., 2003) (formerly Erwinia carotovora subsp. atroseptica (Van Hall) Dye) (Pba), Pectobacterium carotovorum subsp. carotovorum (Gardan et al., 2003) [Erwinia carotovora subsp. carotovora (Jones) Bergey et al.] (Pcc), Pectobacterium carotovorum subsp. brasiliense (Erwinia carotovora subsp. brasiliense) (Pcb) (Duarte et al., 2004), Pectobacterium wasabiae (Erwinia carotovora subsp. wasabiae) (Pwa) (Pitman et al., 2008) and several Dickeya spp. (Erwinia chrysanthemi), including D. dianthicola (Erwinia chrysanthemi pv. dianthicola), Dickeya dadantii, Dickeya zeae (Erwinia chrysanthemi pv. zeae) and the new species Dickeya solani (Toth et al., 2011; van der Wolf et al., 2013) are responsible for causing potato blackleg in the field and tuber soft rots in storage and in transit as well as in the field worldwide (Fig. 1). Additionally, some reports suggest that P. carotovorum subsp. odoriferum (Pco) (Waleron et al., 2014) and P. betavasculorum (Pbt) (Nabhan et al., 2012) can also cause soft rot disease in potato. SRE are recognised among the top 10 most important bacterial pathogens in agriculture limiting crop yield and quality (Mansfield et al., 2012).
Figure 1.
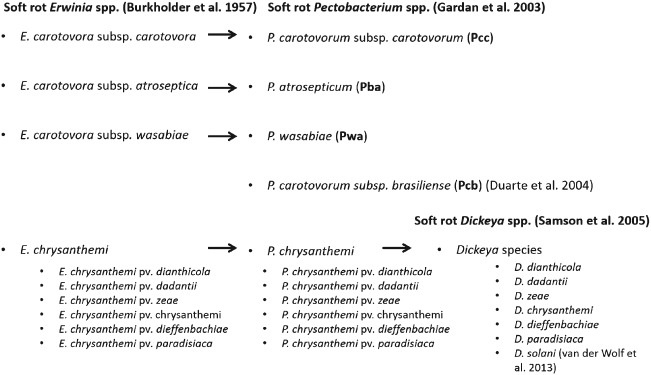
Taxonomic classification of Pectobacterium spp. and Dickeya spp. associated with tuber soft rot and blackleg disease of potato (Solanum tuberosum L.).
Of all Pectobacterium spp. infecting potato, Pcc has the widest host range worldwide, whereas Pba is found mainly on potato grown in temperate regions (Pérombelon, 2002). In 2004, Pcb, a highly aggressive bacterium, was shown to cause severe infection of potato crops in tropical and subtropical regions (viz. Brazil and South Africa) (Duarte et al., 2004; van der Merwe et al., 2010). Pwa which was first associated with horse radish in Japan (Pitman et al., 2008) was later found also on potato in New Zealand, South Africa, Canada and various European countries, where it is responsible for severe economic losses (Nykyri et al., 2012; Waleron et al., 2013).
In 2005, P. chrysanthemi species was elevated to the genus level and renamed Dickeya which was divided into six genomo-species (D. dianthicola, D. dadantii, D. zeae, D. chrysanthemi, D. dieffenbachia and D. paradisiaca) and nine biovars which largely resembled the initial Erwinia chrysanthemi biovar division (Samson et al., 2005). Recently, D. dieffenbachiae was reclassified as a subspecies of D. dadantii (Brady et al., 2012).
Dickeya spp. can affect a number of host species in different temperature zones (Toth et al., 2011). Over the last decade, most Dickeya spp. strains in Europe belong to biovars 1 and 7, and were classified as D. dianthicola (Janse & Ruissen, 1988). Subsequently, isolates belonging to the new genetic clade of biovar 3 have been found on potato in Europe (Sławiak et al., 2009). These strains constitute possibly a new Dickeya sp. named D. solani, which was found to be the prevalent Dickeya spp. on potato in Europe (Toth et al., 2011; van der Wolf et al., 2013).
During the last 50 years different aspects of Pectobacterium and Dickeya species causing blackleg and soft rot diseases have been extensively reviewed. However, procedures used to detect and identify the SRE have not been reviewed as much (Barras et al., 1994; De Boer, 2003; Charkowski, 2012; Czajkowski et al., 2012; Hauben et al., 1998). The purpose of this review is to present a comprehensive examination of the available methods. We present methods based on selective growth agar media, biochemical and physiological assays, serological methods and more recently developed nucleic acid sequence-based amplification methods. In addition, methods for species/strain differentiation and identification are examined including biochemical profiling, fingerprinting (REP-PCR), restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP), multi locus sequence tagging (MLST), random amplification of polymorphic DNA (RAPD), fatty acid methyl ester (FAME) analyses and full genome sequencing in order to find either major or subtle differences in nucleotide genome sequences. The review also presents less established methods used that could be potentially useful in some situations, namely volatile metabolic profiling, conductometry, flow cytometry, microsphere immunoassays and phage typing.
Finally, using the present literature data on the topic and research experience we would like to propose and recommend set of methods and procedures that in our view would prove useful in the isolation, detection, identification and differentiation of SRE from environmental samples with special emphasis on potato.
Seed potato certification system and visual field inspections
Classification systems were introduced in virtually every country producing seed potato tubers to ensure high quality of the propagation material. These systems and schemes set the levels of tolerance to soft rot and blackleg diseases based on the visual inspections of growing crops as well as on inspections of the harvested tubers after grading and dressing and before sale (Shepard & Claflin, 1975). Usually, inspectors visit potato fields at least two times during the season, looking for the presence of symptomatic plants. Records of the blackleg diseased plants provide some indications on the disease incidence and possible infection potential of the progeny tubers (seed) in the next generation but ignore latent progeny tuber contamination which tends to be more closely related to future disease development (Pérombelon & Hyman, 1995; Toth et al., 2011).
It is important to point out that the tolerance for blackleg in the European seed potato tubers differs from country to country as there is no uniform policy in the European Union and the rest of the world (Toth et al., 2011).
Sampling potato tubers for the presence of soft rot Enterobacteriaceae
A good sampling strategy is crucial for the fool-proof detection and identification of the bacteria present in environmental samples (Pickup, 1991). It is worth mentioning that in Europe there is no compulsory testing for Pectobacterium and Dickeya species in potato. In the case of potato seed stocks, we suggest to use a sampling scheme based on the one used to detect quarantine bacteria Ralstonia solanacearum and/or Clavibacter michiganensis subsp. sepedonicus in which routinely 200 tubers are tested per 25 tonne lots in four pooled samples of 50 tubers (alternatively 8 composite samples of 25 tubers each). Although this sampling scheme is not officially accepted, according to the data presented by the Euphresco Phytosanitary ERA-NET ‘Dickeya species in potato and management strategies’ (http://www.euphresco.org/), it allows estimation of infection level in (seed) tubers. This is based on the statistical assumption that there is a 95% probability of detecting at least one infected tuber in a seed lot if 1.5% tubers are infected, according to the Poisson distribution (EPPO, http://www.eppo.int/). This strategy is more suited to high than lower seed grades which can be extensively latently contaminated (Pérombelon, 2002).
Isolation of Pectobacterium and Dickeya species
Most methods used for identification and differentiation of soft rot and blackleg causing bacteria require isolation of viable cells from samples, and growth and purification of the bacteria prior to analyses.
Isolation from plant material
From symptomatic tissues, where bacterial densities are often greater than 106 cells g−1, it is best to sample from the advancing front of the rot or from newly diseased tissue to avoid interference and growth suppression by contaminating saprophytes. The sample is usually suspended and diluted in sterile water or buffer and a loopful streaked on a growth medium selective for SRE. To protect bacterial cells from oxidative stress due to the release of the plant compounds during tissue preparation or enrichment, an antioxidant 0.05% DIECA (diethyldithiocarbamic acid) is commonly used (Toth et al., 2011).
Plates are incubated at different temperatures as the pathogens have different optimal growth temperatures (Pérombelon & van der Wolf, 2002). Depending on the medium, bacterial colonies appear after 24–48 h at 21–37°C.
In latent infection, the bacteria may be found in all tissues, stems, roots, leaves and (progeny) tubers; however, their density is usually low, rarely exceeding 103 cells g−1 plant tissue. In stems, the bacteria are more frequently found in the first 15–20 cm above ground level (Hélias et al., 2000), whereas in tubers they are more commonly present in the stolon end (Czajkowski et al., 2009), but are also frequently found in lenticels and suberized wounds (Pérombelon, 2002). As the diseases are seed-borne, (seed) tubers are tested in seed certification programmes for the bacteria either qualitatively or quantitatively. Extraction of the bacteria from tubers varies and is either from ground peel strips encompassing the stolon end or the stolon end tuber section of individual or replicated bulked tuber lots (Pérombelon & van der Wolf, 2002). However, what has not yet been agreed on is whether it is more useful: (a) to determine the presence or absence of contaminated tubers in a seed lot, (b) the average tuber contamination level of a seed lot or (c) the distribution frequency of individual tuber with different contamination levels, as disease development is related to the latter (Tsror et al., 2012). It can be speculated that the number of highly contaminated tubers is more important than the average level of lot contamination, as even relatively small population of heavily infected tubers may already result in diseased plants.
Isolation from soil, rain and irrigation water
Soft rot and blackleg bacteria survive poorly in the environment (Pérombelon & Kelman, 1980), and their survival depends mostly on temperature, humidity level and pH. Survival can be longer in potato debris postharvest and in the rhizosphere of certain plants and weeds, but even under favourable conditions, survival is restricted (Pérombelon & Hyman, 1989). The detection of low numbers of the bacteria in soil is further hampered by the presence of other (antagonistic) microorganisms that can overgrow the target bacteria during isolation.
In general, soil to be tested for the presence of SRE can be processed by suspending in sterile water or buffer and shaking for a couple of hours, or better still, first enriched by incubating in a selective broth (pectate enrichment broth – PEB; see below) (Pérombelon & van der Wolf, 2002) before dilution and plating onto agar selective media (see below).
Soft rot and blackleg bacteria can survive for more than 200 days in sterile water (Cother & Gilbert, 1990), and have been detected in surface water (Quinn et al., 1980). The use of contaminated surface water during irrigation can result in infection of potato crops (Cappaert et al., 1988). Because of the low numbers present, often <101 cells mL−1, test samples have to be enriched by incubation in a PEB or the bacteria need to be concentrated by centrifugation prior to plating (Pérombelon & van der Wolf, 2002).
Detection, identification and differentiation
For many years, detection and identification of pectinolytic Pectobacterium and Dickeya species bacteria have depended solely on the isolation of viable bacterial cells on (semi-selective) culture agar media followed by serological and biochemical analyses, bioassays and microscopic observations including Gram staining. Later, molecular techniques based on detection of nucleic acids have been introduced, which avoid the need for live cells and now are used routinely due to their high specificity and reproducibility. What is more, they are much faster than traditional methods and allow qualitative and (semi) quantitative detection of bacteria in complex environments.
It is often difficult to discriminate among the methods used exclusively for detection and identification of SRE from those used to differentiate the isolates of Pectobacterium and Dickeya into species and subspecies, as some may be used for both objectives simultaneously. Therefore, we decided, for the purpose of this review, to divide the methods according to their (technical) background; (a) morphological and biological methods, (b) serological (immunological) methods, (c) molecular detection methods and (d) other methods. They are critically assessed in terms of their practical applications in detection systems.
Morphological and biological methods
Immunomagnetic separation of soft rot Enterobacteriaceae before growth on selective media
Overcrowding of the target bacteria by saprophytes can be a real drawback with semi-selective media but can be avoided by introducing an immunomagnetic separation (IMS) step prior to plating (Van Der Wolf et al., 1996a). In IMS, complexes are formed consisting of the target bacterial cells bound to the target bacteria-specific surface-directed antibodies which then bind to protein A coupled with paramagnetic particles. These complexes can be recovered when subjected to a strong magnetic force, thereby allowing rapid and efficient recovery and concentration of the target bacterial cells, which can then be dilution-plated on selective media such as crystal violet pectate (CVP; see below). The recovery is affected by several factors including the antibodies, nature and type of the magnetic particles, the IMS system and the number of wash cycles applied to remove non-target components. Van der Wolf et al. (1996a, b)1996 reported a recovery sensitivity of <102 Pba cells mL−1 tuber peel extract with little interference from saprophytic bacteria, which is a significant improvement on direct plating on CVP.
Artificial media used for isolation and identification of Pectobacterium and Dickeya species
Different agar media have been developed for the isolation of SRE from plant tissue, soil and water; however, till present only one medium –CVP– has been used extensively worldwide (Cuppels & Kelman, 1974). Detection of SRE on CVP depends on the formation of characteristic deep cavities by the bacterial colonies. The selectivity of the CVP medium is based on the presence of crystal violet which inhibits growth of most Gram-positive bacterial species, and the use of polypectate as the sole carbon source. At present, only one source appears to be available commercially (Hélias et al., 2011) that can be used also in single and double layer forms (Pérombelon & Burnett, 1991). Crystal violet pectate remains the preferred diagnostic selective medium for the isolation of SRE from plants and the environment (Laurila et al., 2008; Tsror et al., 2009).
The selectivity of the medium is sufficient for the isolation of the pathogen from symptomatic stems, but may be insufficient for an efficient isolation from tuber extracts with a high microbial background. Occasionally some Pectobacterium and Dickeya species isolates may not grow on the CVP medium or do not produce cavities; this phenomenon is not yet entirely understood (R. Czajkowski, unpublished observations).
Several other selective media for the isolation of SRE have been developed, but are not widely used. The most promising is that of Pierce (1992) who modified Miller-Schroth's medium. In this medium, agar was replaced with sodium polypectate and NaOH and MOPS (3-morpholinopropane-1-sulfonic acid) were used to buffer the pH. Pectinolytic Pectobacterium and Dickeya species colonies growing on this medium form pink-red-orange cavities, whereas growth of other saprophytic bacteria is either suppressed or failed to produce cavities. Surprisingly, despite the better recovery rate for SRE than CVP (Pierce, 1992), it has not become popular, possibly due to the customary practice of the researchers.
Artificial media used for enrichment
When the pathogen populations are low, they need to be enriched above detection level (Pérombelon, 2002). The test material is incubated under anaerobic conditions in a liquid enrichment medium, PEB, containing sodium polypectate as the sole carbon source (Pérombelon & van der Wolf, 2002). The basic principle here relies on creating conditions that stimulate natural selection for SRE at the expense of other (saprophytic) bacteria. These procedures allow multiplication of the pathogens which facilitates isolation of the bacteria as well as the bacterial genomic DNA.
Biochemical characterisation of Pectobacterium and Dickeya species
In the past, biochemical tests were commonly used to differentiate Pectobacterium and Dickeya species from other bacteria. As the procedures are cumbersome and time consuming, they tended to be replaced by more rapid serological and molecular methods described below. Biochemical methods often are based on a restricted number of characters and, by ignoring the natural variability within taxa, run the risk of closely related species or subspecies being missed. Nowadays, the biochemical approach is mainly used to confirm results obtained with other less cumbersome methods. The tests used are usually those previously described (Hyman et al., 1998). The most commonly applied biochemical and physiological tests are summarised in Table 1. These tests, however, can give rise to ambiguous results, which can be avoided by using freshly grown, pure cultures of the test bacteria. It is worth mentioning that at the moment there are no reliable biochemical tests available for distinguishing Pcb from Pcc and Pwa due to high strain variation (van der Merwe et al., 2010).
Table 1.
Selected physiological and biochemical tests used to discriminate the most common soft rot Enterobacteriaceae (Pba, Pcc, Pwa, Pcb and Dickeya spp.) (after Pérombelon & van der Wolf, 2002; Baghaee-Ravari et al., 2011)
| Test | Pba | Pcc | Pwa | Pcb | Dickeya spp. |
|---|---|---|---|---|---|
| Cavity formation on CVP (24 h, at 28°C) | + | + | + | + | + |
| Growth in nutrient agar at 37°C | − | + | −/+ | + | + |
| Growth in 5% NaCl | + | + | −/+ | + | − |
| Sensitivity to erythromycin | − | − | − | − | + |
| Production of reducing substances from sucrose | + | − | − | − | − |
| Production of indole | − | − | − | − | + |
| Production of phosphatase | − | − | − | − | + |
| Acid production from lactose | + | + | − | + | + |
| Acid production from maltose | + | − | − | + | − |
| Acid production from α-methyl glucoside | + | − | − | + | − |
| Acid production from trehalose | + | + | + | + | − |
| Acid production from sorbitol | − | − | − | − | − |
| Utilisation of malonate | − | − | − | − | + |
‘+’, indicates positive reaction; ‘−’, indicates negative reaction; ‘−/+’, indicates ambiguous reaction.
As a shortcut, it is common to apply only a restricted number of key tests when the presumed identity of the target isolates is known: catalase and oxidase activity and oxidation/fermentation tests (oxidative/fermentative metabolism of carbohydrates; Pérombelon & van der Wolf, 2002). Pectobacterium and Dickeya species possess catalase but not oxidase activity and are able to utilise carbohydrates both via fermentation and oxidation. In addition, it is advisable to determine the ability to macerate potato tuber tissue in potato slice or tuber assays as well as to perform the Gram staining test.
Determination of Dickeya spp. biovars with biochemical methods
Former classification of Dickeya spp. was based on physiological/biochemical tests which allowed dividing the previous Erwinia chrysanthemi (Dickeya spp.) species into seven biovars (biochemically distinct groups). Later, two additional biovars (biovars 8 and 9) of Dickeya spp. were described (Samson & Nassan-Agha, 1978). Palacio-Bielsa et al. (2006) introduced a modified Dickeya sp. biovar determination system and later two further biochemical tests were added (Palacio-Bielsa et al., 2006; Sławiak et al., 2009). The results of the tests, however, can sometimes be ambiguous.
Although identification of biovars may still play an important role in the differentiation of the bacterial isolates in some cases, the biochemical tests' data cannot be relied on their own and other complementary tests are desirable, preferably based on molecular methods (Boccara et al., 1991). Nowadays, the biovar classification system tends to receive less attention due to the development of much more precise molecular detection and differentiation techniques (Pritchard et al., 2012).
NGM medium for differentiation of Dickeya spp. from Pectobacterium spp
In 1966, it was first reported that Dickeya spp. produce a blue pigment called indigoidine, which gives the bacteria protection from reactive oxygen species during plant infection (Starr et al., 1966). This observation led Lee & Yu (2005) to develop the NGM medium, containing glycerol and manganese chlorite, on which Dickeya spp. form blue colonies. Other SRE do not produce the blue pigment (Lee & Yu, 2005). The medium was used successfully to differentiate D. solani and D. dianthicola from Pectobacterium spp. isolated from symptomatic plants (Van Vaerenbergh et al., 2012). However, not all strains of Dickeya spp. produce the blue pigment when grown on this medium (R. Czajkowski, unpublished observations), therefore its usefulness may be questionable and results must be confirmed by another method [e.g. species-specific (real-time) PCRs].
Fatty acid methyl ester analysis
Pectobacterium spp. was reported to produce at least 10 different fatty acids, with chain lengths between 12 and 18 carbon atoms (Dawyndt et al., 2006), which have been used to develop an identification system (FAME). This allows differentiation of the various Pectobacterium spp. and identification of unknown pectinolytic bacterial strains based on the ratios between different fatty acids (de Boer & Sasser, 1986). Cother et al. (1992) used FAME to identify Dickeya spp. isolates in alpine water: all isolates contained cis-9-hexadecanoic, hexadecanoic, tetradecanoic acids and most strains contained also dodecanoic acid (Cother et al., 1992). Although FAME analyses allow distinction of Dickeya spp. from Pectobacterium spp., the assay is not suitable to differentiate Dickeya to species and subspecies (van Der Wolf et al., 2013)
Volatiles profiling
Volatile metabolites have been used for the detection of Pectobacterium and Dickeya species (Varns & Glynn, 1979). Several volatiles were reported to be produced by SRE, for example acetaldehyde, ethanol, 1-propanol, acetone 3-hydroxy-2-butanone, 2-butanone and ethanol (Lui et al., 2005). However, not all can be attributed to Pectobacterium and Dickeya species as some were also detected in uninfected plant tissue. Only few could be related solely to the presence of the Pectobacterium spp. bacteria. Liu et al. (2005) reported that acetic acid ethenyl ester (vinyl acetate) was uniquely associated with Pba and cyclohexene, diazene and methoxy-(1,1-dimethyl-2-hydroxyethyl)-amine were exclusively associated with the Pcc presence on potato. Although several other specific volatile metabolites have been described, it is difficult to assess the practical impact of this discovery. The first electronic sensor system (so-called ‘electronic nose’) was developed in 2000 and contains three independent sensors to detect compounds produced by Pectobacterium spp. during potato tuber infections in storage. The sensor system was reported to detect even latent infection in potato tubers artificially contaminated with Pectobacterium spp. at 85% relative humidity and at 4°C (de Lacy Costello et al., 2000). If this is confirmed, the method could be useful in commercial bulk-stored potatoes where massive tuber soft rot within the stock is a constant threat.
Serological (immunological) methods
In general, immunological methods for detection of Pectobacterium and Dickeya species are based on a comprehensive knowledge of the existing serogroups. For serological grouping of Pectobacterium and Dickeya species, LPS (lipopolysaccharides) O antigens have been considered as the most useful. For Pba, eight different serogroups have been described with the prevalence of the serogroup I strains in Scotland and the Netherlands (de Boer & McNaughton, 1987). For Dickeya spp., nine serogroups have been described although only serogroup O1 Dickeya spp. strains were isolated from potato in Europe (Samson & Nassan-Agha, 1978). In the case of Pcc, the situation is more complex due to occurrence of a large number of different O serogroups present in strains infecting potato in Europe. So far, this heterogeneity of O serogroups has prevented the development of good Pcc detection methods based on antibodies and antigens interactions (de Boer et al., 1979). The major problems related to the use of polyclonal and monoclonal antibodies are listed below.
For polyclonal antibodies, the major drawback is that they show low specificity as they can cross-react with some non-target pathogens due to the presence of shared somatic and flagella antigens as well as other cellular antigens/antibodies. Furthermore, Pectobacterium and Dickeya species are serologically heterogeneous and in consequence not all isolates would react specifically with polyclonal antibodies. In contrast to the polyclonal, monoclonal antibodies exhibit unique, overall high, specificity to a single epitope. This may, however, involve an increasing risk of false-negative reactions, in case of serological variation of the pathogen. The relatively high production costs of monoclonal antibodies are considered as another important obstacle for its use (de Boer et al., 1987).
Monoclonal antibodies have been produced, evaluated and used in several studies relating to detection and identification of Pectobacterium spp. in general but specifically of Pba (Gorris et al., 1994). Polyclonal and monoclonal antibodies specific to extracellular pectate lyases (PL) from Pcc have been produced, and tested for their potential in the detection and identification of soft rot and blackleg causing Pcc, Pba and Dickeya spp. (Klopmeyer & Kelman, 1988).
The polyclonal antibodies raised against PL from Pba not only reacted readily with the enzyme from Pectobacterium spp. and Dickeya spp. but also cross-reacted with PL from pectinolytic Pseudomonas spp. and Xanthomonas spp. However, the PL monoclonal antibodies showed high specificity to SRE but not with other PLs, neither from different genera, nor with commercially available enzyme preparations (Klopmeyer & Kelman, 1988). Detection is possible only when PL has been produced, hence latent infection would be missed.
In general, the immunological assays do not require expensive laboratory equipment, therefore they can be used everywhere, where simple, cheap and relatively sensitive and specific detection on a large scale is desirable, especially under field conditions. Different antisera are available from several commercial sources.
Early use of antibodies: double immunodiffusion
Polyclonal antibodies have long been used for the detection and differentiation of soft rot and blackleg causing bacteria in agglutination and precipitation tests (Graham, 1964). One of the initially popular methods is double immunodiffusion (Crowle, 1961). Double immunodiffusion assays have been used to characterise SRE with pure cultures and in symptomatic tissues (De Boer, 1983). The technique is simple and may still be of value in field studies if specialised laboratories and procedures cannot be used. However, with the improvement in immunological methods which followed, these tests are rarely if ever used at present.
Immunofluorescence staining and immunofluorescence colony staining
Immunofluorescence staining (IF) or fluorescent antibody staining (FAS) method is based on the application of the antibodies conjugated to a fluorophore (e.g. fluorescein and its derivatives – isothiocyanate FITC; rhodamin) which bind to bacterial cell walls (e.g. proteins, exopolymeric substances, lipopolysaccharides) hence facilitating recognition.
Immunofluorescence staining was used in soft rot plant pathogenic bacteria, such as Pba and Pcc. Its sensitivity is poor, ca. 106 cells mL−1, but it allows detection of SRE in mixed populations, 104 cells mL−1 of Pcc nd 4 × 103 cells cm−2 of Pba on potato plant leaf surface (Allan & Kelman, 1977, Phillips & Kelman, 1982). In addition, immunofluorescence stained Pcc cells were detected in spiked soil samples after 1 day of incubation and in artificially infected insects or rotting potato tubers (Allan & Kelman, 1977). Immunofluorescence staining was also used to study the role of insects in the dissemination of Pcc in potato fields (Phillips & Kelman, 1982).
Immunofluorescence colony staining (IFC) is a more sensitive variant of the immunofluorescence staining in which bacterial colonies, not individual bacterial cells, are stained with antibodies conjugated with fluorescent dyes. In addition, it allows isolation of viable bacteria if required while IF does not. Immunofluorescence colony staining was used to detect Pba in infested potato peel extracts with a detection limit of c. 10–50 cells mL−1 and to detect Dickeya spp. in cattle slurry with sensitivity of 102 cells mL−1 (Jones et al., 1994).
The major problem of applying IF and IFC methods for diagnostic purposes is that antibodies used for detection of Pectobacterium and Dickeya species may not be sufficiently specific, thereby they allow the detection of non-target (saprophytic) bacteria present in the same environment or fail to detect serological variants of the bacterial species. Therefore, although the risks of cross-reactions between anti-Dickeya and anti-Pectobacterium spp. antibodies and antigens of saprophytic bacteria are low, it is advisable to confirm the results using non serological tests (van der Wolf et al., 1996).
Enzyme-linked immunosorbent assay and enrichment enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) has been mainly used for detection of SRE in a double antibody sandwich format. More complex ELISA systems (e.g. with IF) have been developed but they all suffer from low sensitivity, with a detection lower limit of 105–106 cells g−1 of plant material (Pérombelon & Hyman, 1995). In addition, ELISA assays fail to distinguish between viable and nonviable Pectobacterium and Dickeya species cells and, as in case of IF and IFC, they are also prone to cross-reactions with non-target bacteria (van der Wolf & Gussenhoven, 1992).
The sensitivity could be increased 1000-fold by incubating the test sample in an enrichment medium selective for Pectobacterium and Dickeya species (usually PEB) prior to testing (Lopez et al., 1997). The enrichment-ELISA can be made quantifiable by a most probable number approach in which replicates of serially diluted test samples are verified. Up till now, ELISA and enrichment ELISA are used routinely in plant protection laboratories worldwide to detect bacteria in agriculture.
Nucleic acid-based molecular detection methods
Molecular detection methods based on the analysis of bacterial genomic DNA have become most frequently used to detect and differentiate tuber soft rot and blackleg pathogens in environmental samples. They consist of amplification of target-specific sequences by PCR assays of different formats including LAMP (loop-mediated isothermal amplification) (see below). The PCR-based assays enable faster and cheaper detection of the pathogen than the standard plating methods, but they require specific and often expensive equipment. In comparison with serological assays they are more specific as PCR primers can be designed with specificity for the target genus, or (sub) species level. There are, however, well-reported limitations. Firstly, the procedures are based on the detection of nucleic acids that can be often relatively more stable in the environment than viable bacterial cells (Willerslev & Cooper, 2005). Consequently, viable cells cannot be reliably distinguished from non-viable cells (Keer & Birch, 2003). This limitation has been partially overcome by the use of compounds that can specifically bind DNA released from dead cells such as propidium monoazide (Gorshkov et al., 2009). Secondly, there is still the possibility of false-positive reactions with non-targets with similar sequences, yet undetermined or not published in international databases. Furthermore, variation in the bacterial population genome may result in false-negative results.
As from the 1990s, several methods based on DNA amplification for detection of Pectobacterium and Dickeya species have been developed as well as new PCR variations, including multiplex PCR, real-time PCR, padlock probes and LAMP. DNA suitable for specific amplification of SRE sequences can be isolated from plant extracts by several standard DNA extraction procedures as well as by protocols using commercial kits, among which some are suitable for robotized DNA extraction able to process 96 samples simultaneously (Smit et al., 2001). Pure bacterial cell lysates obtained by boiling in water or 5 mM NaOH may also be used as templates for PCR reactions which would allow omitting the DNA isolation step. The detection threshold of procedures based on direct isolation of total bacterial genomic DNA from suspected plants or tubers is relatively high (Smit et al., 2001). Detection limits vary from 1 to 15 cells mL−1 bacterial suspensions in water (Pritchard et al., 2012) to 105 cells mL−1 from some potato cultivars possibly due to the presence of PCR inhibiting factors (Diallo et al., 2009). For increased sensitivity, bacteria may be enriched prior to DNA isolation as mentioned above (Pérombelon & van der Wolf, 2002).
Despite these drawbacks, DNA-based amplification methods are now the most widely used in modern laboratories for detection and differentiation of Pectobacterium and Dickeya species. New assays for specific detection of important species and subspecies of plant pathogenic SRE are continuously being developed. The most commonly used primer sets developed for detection of Pectobacterium and Dickeya species are listed in Tables 2 and 3.
Table 2.
Methods used for the isolation, detection and differentiation of soft rot Pectobacterium and Dickeya spp. in practical applications and research
| Method | Application | Sensitivity, Detection Level | Remarks | Reference |
|---|---|---|---|---|
| CVP plating | Isolation of pure bacterial colonies and viable bacterial cells | Depending on the sample source and presence of other microorganisms, recovery of bacteria on the medium up to 100% | Very rarely Pectobacterium and Dickeya spp. isolates may not grow on the medium or do not produce cavities; incubation up to 5 days at optimal temperature is required to assess the cavity formation; pectin source can affect the quality of the medium | Cuppels & Kelman (1974) and Helias et al. (2011) |
| Enrichment in PEB medium | Enrichment of low bacterial populations of Pectobacterium spp. and Dickeya spp., useful for isolation of bacteria from environmental samples | Frequently even 1–10 bacterial cell are enriched to the detectable densities: usually 102–103 cells mL−1 bacteria should be present in starting material | Despite medium selectivity, other bacterial species antagonistic to Pectobacterium and/or Dickeya spp. maybe also enriched, hence false-negative results | Pérombelon & van der Wolf (2002) |
| PCR with species-specific primers | Differentiation of Pectobacterium and Dickeya spp. from other bacteria, differentiation of Pectobacterium from Dickeya spp. Can be applied in a singlex and multiplex setting for detection of several pathogens | From 1 cfu mL−1 to 105 cells mL−1 depending on assay, primer sets, conditions; an average detection limit is 104 cells mL−1 in plant extracts | In general PCR works better on pure bacterial colonies or purified genomic DNA, false-positive reactions possible, the results should be confirmed with other methods, the assays detect also nonviable bacterial cells and DNA | Please see the Table 3 for reference |
| Real-time PCR (TaqMan™, SYBR green) | Quantitative and qualitative detection of bacteria, for TaqMan™, additionally assays with higher specificity due to the presence of probe complementary to the target DNA. TaqMan assays be applied in a singlex and multiplex setting for detection of several pathogens | Pure genomic DNA of good quality is prerequisite | Relatively expensive for routine use, samples need to be investigated in duplicates or triplicates together with positive and negative controls | Please see the Table 3 for reference |
| Repetitive Sequence-Based PCR (REP-PCR) | Differentiation of Pectobacterium and Dickeya spp. isolates | High concentration of pure genomic DNA of good quality is prerequisite, viable bacterial cells used for DNA isolation are necessary | High resolution, even closely related species can be differentiated from each other, results on two different gels cannot be directly compared, the assay should include control designated strains | Versalovic et al. (1994) |
| Pulsed field gel electrophoresis (PFGE) | Differentiation of Pectobacterium and Dickeya spp. isolates | High concentration of pure genomic DNA of good quality is prerequisite, | High resolution, even strains can be differentiated from each other, results on two different gels cannot be directly compared, the assay should include control designated strains | Lee et al. (2006) and Kim et al. (2009) |
| Restriction Fragment Length Polymorphism- PCR (PCR-RFLP) | Differentiation of Pectobacterium and Dickeya spp. isolates | High concentration of pure genomic DNA of good quality is prerequisite, viable bacterial cells used for DNA isolation are necessary | Results on two different gels cannot be directly compared, the assay should include control designated strains, relatively good resolution | Boccara et al. (1991) and Waleron et al. (2002) |
| Multi Locus Sequence Tagging (MLST) | Differentiation of Pectobacterium and Dickeya spp. isolates | Qualitative detection | Selection of good target genes may be difficult | Pitman et al. (2010), De Boer et al. (2012) and Waleron et al. (2013) |
Table 3.
PCR primer sets used to detect Pectobacterium spp. and Dickeya spp. bacteria with conventional, multiplex and real-time assays
| Species/Subspecies | Assay Type | Primers (5′→3′) (name, sequence) | PCR Product Size (bp) | References |
|---|---|---|---|---|
| Dickeya spp. and Pectobacterium spp. | ||||
| Conventional |
SR3F GGTGCAAGCGTTAATCGGAATG SR1cR AGACTCTAGCCTGTCAGTTTT |
119 | Toth et al. (1999b) | |
| Conventional RFLP |
G1 GAAGTCGTAACAAGG L1 CAAGGCATCCACCGT |
Toth et al. (2001) | ||
| Dickeya spp. | ||||
| Conventional RFLP |
ADE1 GATCAGAAAGCCCGCAGCCAGAT ADE2CTGTGGCCGATCAGGATGGTTTTGTCGTGC |
420 | Nassar et al. (1996) | |
| Conventional RFLP |
pelZ-1-F ATGAAACATACCCTTCTGTTTGC pelZ-1-R TTATTCCAGATCTTTGGCCAT |
1263 | Lee et al. (2006) | |
| Conventional |
5A GCGGTTGTTCACCAGGTGTTTT 5B ATGCACGCTACCTGGAAGTAT |
nd. | Chao et al. (2006) | |
| Real-time PCR |
Df AGAGTCAAAAGCGTCTTG Dr TTTCACCCACCGTCAGTC |
133 | Laurila et al. (2010) | |
| Real-time PCR (TaqMan) |
ECHf GAGTCAAAAGCGTCTTGCGAA ECHr CCCTGTTACCGCCGTGAA Probe ECH CTGACAAGTGATGTCCCCTTCGTCTAGAGG |
nd. | Pritchard et al. (2012) | |
| Dickeya dianthicola | Real-time PCR (TaqMan) |
DIA-Af GGCCGCCTGAATACTACATT DIA-Ar TGGTATCTCTACGCCCATCA Probe ATTAACGGCGTCAACCCGGC DIA-Cf CCAACGATTAGTCGGATCT DIA-Cr TAGTTGGTGCCAGGTTGGTA Probe DIA-C TCGACGTATGGGACGGTCGC |
nd. nd. |
Pritchard et al. (2012) |
| Dickeya solani | Real-time PCR (TaqMan) |
SOL-Cf GCCTACACCATCAGGGCTAT SOL-Cr ACACTACAGCGCGCATAAAC Probe Sol-C CCAGGCCGTGCTCGAAATCC SOL-Df GCCTACACCATCAGGGCTAT SOL-Dr CACTACAGCGCGCATAACT Probe SOL-D CCAGGCCGTGCTCGAAATCC |
nd. nd. |
Pritchard et al. (2012) |
| Dickeya solani | Real-time PCR (TaqMan) |
dsf GCGAACTTCAACGGTAAA dsr CAGAGCTACCAACAGAGA Probe CTCTGCTGGACGGTTC |
112 | Van Vaerenbergh et al. (2012) |
| Pectobacterium spp. | ||||
| Conventional |
Y1 TTACCGGACGCCGAGCTGTGGCGT Y2 CAGGAAGATGTCGTTATCGCGAGT |
434 | Darrasse et al. (1994b) | |
| Pba | Conventional |
Y45 TCACCGGACGCCGAACTGTGGCGT Y46 TCGCCAACGTTCAGCAGAACAAGT |
439 | Fréchon et al. (1998) |
|
Eca1 CGGCATCATAAAAACACG Eca2 GCACACTTCATCCAGCGA |
690 | de Boer & Ward (1995) | ||
|
PEAF CCGGTACTTCAAGCTAATCC PEAR CCTTACCTATCGCTTCTCCT |
904 | Park et al. (2006) | ||
| LAMP |
BIP (B1 TCCCCACAGTGTCACCAAGTTG + T linker + B2 TCGGCAGCCTATTCCTCTG) FIP (FIc GTCGCAGCCTCCGTTGAAGA + T linker + F2c AGCAAGCCTACCATCCCA) B3 GCATTCTGTAGGCGAAGCG F3 TGCTGTTGATAGCGGCAAT LoopF1 GTTACGCCGTTACTCAAAGA LoopB1 GCGTCCTCTTGGCTTAAATA |
Variable | Li et al. (2011) | |
| Pectobacterium carotovorum subsp. brasiliensis | Conventional |
BR1f GCGTGCCGGGTTTATGACCT L1r CA(A/G)GGCATCCACCGT |
690 | Duarte et al. (2004) |
| Pcc and Pwa | Conventional, nested |
ExpccF GAACTTCGCACCGCCGACCTTCTA ExpccR GCCGTAATTGCCTACCTGCTTAAG INPCCF GGCCAAGCAGTGCCTGTATATCC INPCCR TTCGATCACGCAACCTGCATTACT |
550 400 |
Kang et al. (2003) |
| Pwa | Conventional |
Contig 1F CCTGCTGGCGTGGGGTATCG Contig 1R TTGCGGAAGATGTCGTGAGTGCG Contig 3F GCATTGACCAGTTTCGCCAGTTAC Contig 3R CTTTTTGAGCAGCGCGGGTTGTG |
258 132 |
de Haan et al. (2008) |
| Conventional and real-time PCR |
PW7011F CTATGACGCTCGCGGGTTGCTGTT PW7011R CGGCGGCGTCGTAGTGGAAAGTC |
140 | Kim et al. (2011) | |
| Pba, Dickeya spp. | Conventional, multiplex |
ERWFOR ACGCATGAAATCGGCCATGC ATROREV ATCGATAATTTGATTGTCCT CHRREV AGTGCTGCCGTACAGCACGT |
389 | Smid et al. (1995) |
| Multiplex |
Y45 TCACCGGACGCCGAACTGTGGCGT Y46 TCGCCAACGTTCAGCAGAACAAGT Ech1 TGGCGCGTCAGGAAGTTTAT Ech1′ TCACCGGTCAGGGTGAAGTT |
420 600 |
Diallo et al. (2009) | |
| Multiplex |
Pca for GATCGGCATCATAAAAACACG Pca rev CGCACACTTCATCCAGCGAG Dcd Forw GAAAGCCCGCAGCCAGATC Dcd Rev TCAGGATGGTTTTGTCATGC |
690 420 |
Peters et al. (2007) | |
nd., not determined (the information is absent in the publication).
PCR-based detection with species-specific primers
Selection of appropriate target genes was performed with different approaches and in the past the most frequently selected genes were those associated with virulence including: pelADE (Nassar et al., 1996), pel genes (Darrasse et al., 1994), pelY (Fréchon et al., 1998), pelI (Diallo et al., 2009) and cfa6 (Li et al., 2011), or the housekeeping genes, for example rhsA taking part in cell wall biogenesis (Park et al., 2006). Others were determined as a result of an extensive phylogenetic study based on 16S rDNA and 16S-23S intergenic transcribed region (Duarte et al., 2004). Some primers were developed not from a defined gene of interest but rather on selected piece of bacterial genomic DNA of unknown function. For example, the Eca1f and Eca2r primers, specific for Pba (de Boer & Ward, 1995), were selected on the basis of the sequence of the DNA hybridization probes for detection of Pectobacterium spp. described earlier (Darrasse et al., 1994). Primers EXPCCF and EXPCCR, specific for Pcc and Pwa strains, were selected based on the URP-PCR fingerprinting result in which a common single band produced by all Pcc and Pwa strains was found (Kang et al., 2003). Recently, Pritchard et al. (2012) presented a bio-informatic tool allowing easy prediction of primer sets for specific detection of species and subspecies of bacteria based of the raw genome sequence information. In this approach, the algorithm is used to predict regions in the genome which are useful to design the specific primers (Pritchard et al., 2012). It seems that this approach may be advantageous over the classic one in which firstly the gene of interest needs to be found and then the primers developed on the basis of its nucleotide sequence. Notwithstanding the above, several PCRs developed in the early 1990s still remain widely used and recognised as the ‘gold standard’ in molecular detection of Pectobacterium and Dickeya species bacteria.
Multiplex PCR
There are a few multiplex PCR assays available for the detection of soft rot and blackleg pathogens (Peters et al., 2007; Diallo et al., 2009). The majority of the assays include simultaneous detection of several Dickeya spp. and Pba, which for a long time were considered as the foremost pathogens in both hot and temperate climatic regions, respectively. Now the situation is blurred due to worldwide potato seed trade and introduction of SRE from other hosts (Peters et al., 2007; Diallo et al., 2009). Recently, a more comprehensive multiplex PCR assay has been developed which allows simultaneous detection of the four major blackleg and tuber soft rot pathogens of potato but not all to subspecies level, namely Dickeya spp., Pba, Pcc and Pwa (M. Potrykus, unpublished information).
The detection limits of the multiplex PCRs are usually comparable with the conventional single pathogen detection assays. In the presence of other bacteria (e.g. saprophytes), however, the limits may sometimes decrease tenfold compared to simplex reactions (Smid et al., 1995).
Real-time PCR
Real-time PCR procedures for detection of SRE were developed to enhance specificity, reliability and for quantification of the target pathogen. At present, there are only two real-time PCR assays using SYBR green developed for detection of SRE, that is Dickeya spp. (Laurila et al., 2010) and Pwa (Kim et al., 2011) (Table 3).
Real-time PCR assay has a great potential as it combines identification and quantification of pectinolytic Pectobacterium and Dickeya species simultaneously and directly from the plant material (Table 3). In contrast to conventional end-point PCR, the real-time assay allows quantification of the template at each cycle. The new primer sets for the real-time assays developed by Pritchard et al. (2012) on the basis of the raw genome sequence from various Dickeya spp. strains is able to differentiate D. dianthicola from D. solani.
The attempts to design primers for specific detection of Pcc and Pba in the real-time PCR assays have been undertaken (Takle et al., 2007) and would be useful for the routine detection of the bacteria in environmental samples. Meanwhile, several TaqMan® (Roche Molecular Systems, Pleasanton, California, USA)-based assays have also been developed and used for generic detection of SRE and for the specific detection of Pba, but have not been published in peer reviewed journals (J.G. Elphinstone, FERA, York, UK, unpublished results, J. M. van der Wolf, PRI, Wageningen, the Netherlands, personal communication). Thus, in 2009, NAK (the Netherlands General Inspection Service for Agricultural Seeds and Seed Potatoes) introduced a multiplex TaqMan-based assay (BioPlex Real-Time PCR) for the simultaneous detection of Pectobacterium and Dickeya species (de Haan & van den Bovenkamp, 2009). The assay was designed in fourplex, meaning that it can detect four groups of bacteria: SRE (Pectobacterium and Dickeya species but without differentiation of the genus), Pba, Dickeya spp. and Pwa using four specific TaqMan probes. Unfortunately, no information was provided about the probes and target genes used. The assay is used routinely by the NAK to check high grade potato seed in the Netherlands for contamination with Pectobacterium and Dickeya species. It is claimed that it is possible to detect 104 cells mL−1 plant extracts and 102 cells mL−1 following enrichment in PEB medium prior to analysis (de Haan & van den Bovenkamp, 2009).
Loop-mediated isothermal amplification
Loop-mediated isothermal amplification (LAMP) assay was developed for the specific detection and identification of Pba (Li et al., 2011) and Pcc (Kim et al., 2011) but has not been used widely elsewhere. Li et al. (2011) developed the LAMP assay using a gene cluster coding for a pathogenicity-related phytotoxin similar to coronafacic acid of Pseudomonas syringae. The assay allowed differentiation of Pba from other Pectobacterium and Dickeya species and could detect down to 102 cells mL−1 bacteria.
Padlock probes
So far, there is only one publication describing the use of padlock probes (PLP) for the detection of SRE: Pba, Pwa and Dickeya spp. (Sławiak et al., 2013). Sławiak et al. (2013) designed PLP assays on the basis of recA and 16S rDNA gene sequences, which resulted in a very sensitive (detection limit 5 pg of bacterial genomic DNA and/or 500 bacterial cells per reaction) PLP assay. The PLP were also used to discriminate between all target bacteria simultaneously. The method seems to have many advantages over other molecular detection methods; however, it is too early to say whether the PLP assays would be used routinely to detect SRE bacteria in the future.
Molecular fingerprinting methods
Bacterial differentiation methods based on the comparison of the number and size (pattern) of the DNA fragments obtained from the bacterial genome following separation in and visualisation on agarose gels, polyacrylamide gels or nitrocellulose sheets are commonly known as DNA fingerprinting.
For each of the techniques, it is crucial to use a set of reference strains as controls, which should at least include the type strains of the (sub)species being studied. The use of a globally accepted set of reference strains is advocated.
Repetitive sequence-based PCR
Repetitive sequence-based PCR (REP-PCR) has been developed to target the repetitive sequences present in bacterial genomes, namely Repetitive Extragenic Palindromic elements (REP), Enterobacterial Repetitive Intergenic Consensus (ERIC-PCR) and BOX elements (BOX-PCR). Versalovic et al. (1994) developed primers specific to the various repetitive sequences present in bacterial genomes. The relative resolution of the generated patterns is high and allows phylogenetic classification of SRE from genus down to strain level. REP-PCR-based techniques have been used extensively for the classification and characterisation of Pectobacterium and Dickeya species strains. The REP-PCR analyses were used for example in studies on the classification of a new clade of Dickeya spp. biovar 3, D. solani (Degefu et al., 2013) and to characterise different Pectobacterium and Dickeya species in potato in South Africa and Zimbabwe (Ngadze et al., 2012). The technique is easy to perform and its resolution is high.
Pulsed field gel electrophoresis
The application of pulsed field gel electrophoresis (PFGE) allowed identification of specific patterns common for all Dickeya spp. isolates from Zantedeschia aethiopica in Taiwan (Lee et al., 2006). Pulsed field gel electrophoresis was also able to discriminate Pectobacterium spp. clades in diseased stems and tubers of potato plants originating from different fields in Korea (Kim et al., 2009). Pulsed field gel electrophoresis was also successfully applied to confirm the identity and homogeneity of D. solani strains isolated in Finland and Israel (Degefu et al., 2013; Tsror et al., 2013).
Restriction fragment length polymorphism of amplified PCR product
Restriction fragment length polymorphism of amplified PCR product (PCR-RFLP) may be applied specifically for the identification and differentiation of closely related isolates within species and subspecies. To differentiate between Pectobacterium and Dickeya species, two groups of genes were mainly used; those coding for virulence factors (e.g. pel genes, hrp genes) and the housekeeping genes (e.g. 16S rDNA, 23S rDNA, recA, gyrA, gyrB, rpoS, and dnaX). Boccara et al. (1991) were the first to use PCR-RFLP based on pectinase coding genes to differentiate Dickeya spp. isolates. In comparison with biochemical assays used to classify the SRE, the PCR-RFLP was more discriminative. Darrasse et al. (1994) applied pel gene-based PCR-RFLP to classify Pectobacterium spp. isolates. The pel PCR product was digested with several restriction endonucleases in independent reactions and the products were compared on agarose gel. It was shown that with the PCR-RFLP method, previous results were confirmed that Pba isolates form a homogenous group while isolates of P. carotovorum are more genetically diverse suggesting a polyphyletic origin. To differentiate Dickeya spp., a pelADE gene cluster was used on 78 Dickeya spp. strains which could be separated into 16 RFLP patterns grouped in six clusters almost exclusively correlated with the biovar and pathovar grouping, as well as with the geographical distribution and relation to the plant host (Nassar et al., 1996).
Waleron et al. (2002) applied a PCR-RFLP assay to differentiate pectinolytic Pectobacterium and Dickeya species on the basis of the housekeeping genes recA and rpoS. Restriction analyses of recA gene with four restriction endonucleases revealed the presence of 57 restriction groups (combined RFLP patterns). Additionally, application of the PCR-RFLP allowed the observation of two distinct RFLP profiles for Pba, 16 profiles for Pcc, one RFLP profile characteristic for P. betavasculorum, Pco and Pwa (Waleron et al., 2002). In the case of Dickeya spp., 14 separate recA PCR-RFLP restriction groups were identified. The high degree of differentiation within the Pcc and Dickeya spp. was later confirmed by other researchers and resulted in establishment of separate genera (Gardan et al., 2003; Samson et al., 2005).
Amplified fragment length polymorphism
Specific amplified fragment length polymorphism (AFLP) patterns were obtained for the majority of Pectobacterium and Dickeya species and subspecies, namely for Pcc, Pba, Pwa, P. betavasculorum, P. carotovorum subsp. odoriferum and Dickeya spp. (Avrova et al., 2002). Yishay et al. (2008) used the AFLP method to characterise Pcc isolates from monocot and dicot plants from diverse geographical locations, Nabhan et al. (2012) to differentiate strains belonging to Pectobacterium spp. isolated in Germany and in South Africa, and Ngadze et al. (2012) to identify Pectobacterium spp. isolates from Zimbabwe, such as Pcb, Pcc, Pba and D. dadantii. What is more, AFLP analysis allowed clear division of Pcc from Pcb and grouping the Pcb into 12 clusters, reflecting their geographical origins (Yishay et al., 2008; Nabhan et al., 2012; Ngadze et al., 2012).
In 2010 Pitman et al. introduced a modified AFLP method using fluorescent dye-labelled primers (fluorescent AFLP – fAFLP) for the differentiation of Pectobacterium spp. in New Zealand. Combining the detection of the fAFLP products with automatic DNA sequencing allowed mechanisation of the procedure without losing the resolution of the standard AFLP method (Pitman et al., 2010).
Random amplified polymorphic DNA
Random amplified polymorphic DNA (RAPD) technique was used to differentiate isolates of Pba and P. carotovorum (Mäki-Valkama & Karjalainen, 1994) and Parent et al. (1996) applied it to differentiate the Pcc and Pba isolates from the pectinolytic fluorescent Pseudomonas spp. strains that can potentially inhabit the same environments.
Phylogenetic analysis based on rDNA gene sequences
Kwon et al. (1997) used the 16S rDNA to assess the phylogenetic relationships between Erwinia amylovora, P. betavasculorum, P. carotovorum, Pwa, Dickeya spp., Escherichia coli, Pantoea agglomerans and Proteus vulgaris. Soft rot Enterobacteriaceae (SRE) bacteria cluster together whereas human and animal pathogenic Enterobacteriaceae show higher phylogenetic heterogeneity (Kwon et al., 1997). Fessehaie et al. (2002) used the 16S–23S intergenic spacer region to build Pectobacterium and Dickeya species phylogenetic trees and to compare this method with the use of 16S rDNA. The resolution of 16S–23S-based phylogeny was shown to be more efficient in separating Pectobacterium and Dickeya species subspecies than when 16S rDNA analysis was applied (Fessehaie et al., 2002).
Ribotyping
A specific PCR-RFLP analysis of the amplified 16S and 23S rDNA sequences encoding the ribosome subunits called ribotyping was also used to differentiate soft rot and blackleg causing bacteria. Nassar et al. used ribotyping to successfully classify Dickeya spp. isolates into biovars and by the host (monocots and dicots) (Nassar et al., 1994).
Multi locus sequence tagging
Multi locus sequence tagging has been applied to differentiate isolates of Pectobacterium and Dickeya species and it is recognised as a useful technique for this purpose (Pitman et al., 2010; de Boer et al., 2012; Waleron et al., 2013). The genes that are more commonly utilised in this assay are the housekeeping genes: for example acnA, gapA, mdh, pgi, mtlD, proA, rpoS, recA and dnaX (Zeigler, 2003).
Young & Park (2007) investigated the phylogenetic relationships of plant pathogenic Enterobacteriaceae, namely Brenneria spp., Dickeya spp., Enterobacter spp., Erwinia spp., Pectobacterium spp. and Samsonia spp. using MLST analysis of nucleotide and peptide sequences of atpD, carA and recA genes. Nabhan et al. (2012) used the MLST in combination with other methods to distinguish P. carotovorum isolates originated from various locations and to prove that some of them need to be reclassified as Pwa. Koh et al. (2012) classified atypical P. carotovorum isolates to new species P. carotovorum subsp. actinidiae subsp. nov. on the basis of the MLST analysis on atpD, carA and recA nucleotide sequences.
Whole-genome sequencing, comparative genome analysis and its application for SRE detection
Development of the whole-genome sequencing and (automatic) annotation services resulted in rapid rise of genomic data readily available for SRE detection and identification purposes (Toth et al., 2003; Pritchard et al., 2012). Until now (July 2014), there are 24 Dickeya spp. and 22 Pectobacterium spp. genomes published at GOLD Genomes Online Database (http://www.genomesonline.org/index) and National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/). Some of these are complete annotated data, but many others are just raw (draft) sequences (e.g. set of contigs) which often need some additional analysis. The average size of the genomes of Pectobacterium and Dickeya species is estimated to be c. 5 Mb which is within the range of most bacterial genomes sequenced so far. The genomic data have already proven to be useful in characterisation of (new) virulence factors (Bell et al., 2002, 2004) and development of new primer pairs for detection of D. dianthicola and D. solani (Pritchard et al., 2012). Genomics has also been successfully applied to properly identify strain Ecc SCC3193, which for years was a model strain to study molecular biology of Pcc (former Erwinia carotovora subsp. carotovora Pirhonen & Palva, 1988; Andersson et al., 2000; Koskinen et al., 2012) but recently was reclassified as Pwa (Nykyri et al., 2012). In line, fifteen Pectobacterium and Dickeya species genomes have been fully sequenced, annotated and used for the comparative analyses (Nykyri et al., 2012).
It is important to point out here, that the availability of complete and annotated genome sequences is expected to facilitate the future development of new whole-genome based detection and differentiation tools for Pectobacterium and Dickeya species (Pritchard et al., 2012).
Other less established methods used for detection and differentiation of Pectobacterium and Dickeya species
In this section we briefly summarise the methods that were either developed for detection and differentiation of pectinolytic Pectobacterium and Dickeya species in the past and have not been widely used or those that, although developed recently, have not become as yet popular. Nevertheless, in our view they are of interest and should be mentioned here.
Flow cytometry
To date, only a couple of publications describe the use of flow cytometry to detect Pectobacterium and Dickeya species. Golan et al. (2010) used the method to count the Pcc cells tagged with the green fluorescent protein (GFP) in artificially inoculated Ornithogalum dubium (sun star) plants. The combined use of flow cytometry and GFP-tagging facilitated screening plantlets of a large number of varieties of sun star for resistance to Pcc (Golan et al., 2010). Fröhling et al. (2012) used flow cytometry to measure the viability of Pcc after heat treatment to demonstrate that cells which cannot be resuscitated on agar media still remain viable and infectious.
Microsphere immunoassay (Luminex system)
The detection of Pectobacterium and Dickeya species via microsphere immunoassay (MIA) has been described in only one publication (Peters et al., 2007) and the method has not been widely used. Peters et al. (2007) utilised this system to compare the procedure with ELISA for the detection of Pectobacterium and Dickeya species bacteria. The microsphere immunoassay was superior to ELISA, being more sensitive with a detection limit of 102 cfu mL−1 instead of 105 cfu mL−1 and could be performed in 1 h instead of 6 h while detecting both pathogens simultaneously.
Conductometry-based detection of Pectobacterium and Dickeya species
In 1996, Fraaije et al. developed an automatic assay designed to detect viable Pectobacterium and Dickeya species cells in potato tuber peel extracts in the presence of other bacterial species including Pseudomonas, Bacillus, Enterobacter and Flavobacterium. The detection limits were 102–103 cells mL−1 and 103–105 cells mL−1 peel extract for Pba and Dickya spp. respectively (Fraaije et al., 1996). Although the potential success of this approach is linked to its simplicity, low running cost and robustness, it failed to be adopted in Pectobacterium spp. and Dickeya spp. research (Charkowski, 2006). It appears that it has also been ignored by the vegetable and ornamental industry and hence it is doubtful if the method had ever been validated under commercial production conditions.
Bacteriophage (phage) typing
The first bacteriophage typing system of Pectobacterium spp. and Dickeya spp. was described late in 1990s (Gross et al., 1991). In that instance, 13 different phages displaying distinct host range specificity against Pcc and Pba isolates were isolated from soil and rhizosphere of potato plants and screened against a collection of 389 Pectobacterium spp. strains of known serogroups. It was clear that the phage typing would be of limited use to differentiate the pectinolytic Pectobacterium spp. due to the high genetic diversity of the bacterial isolates and hence the need to utilise a large enough number of phages. Thus, Gross et al. (1991) concluded that Pectobacterium spp. differentiation by the phage typing is less efficient than by serological methods. There is also the risk of development of resistance to a phage which would require finding replacement discriminating phages. In contrast, Toth et al. (1999), when comparing RFLP, ERIC, RAPD and phage typing methods to differentiate the more genetically homogeneous Pba isolates, found that the resolution of phage typing methods was the highest. Despite difficulties in isolating suitable range of phages, the method was believed to have a potential to be the method of choice to study Pba ecology (Toth et al., 1999). To our knowledge, no phage typing system has been developed for the differentiation of Dickeya spp., and phage typing of Pectobacterium spp. has not been applied in ecological research.
A proposed set of procedures and methods for the isolation and characterisation of SRE
The protocol described below could be used as a blueprint for the reliable detection and characterisation of the SRE isolates. Briefly, once the test material has been selected, we advise to simultaneously isolate the target bacterial colonies on CVP and to enrich the possible low SRE numbers in latent infections in a selective PEB medium prior to application of PCRs followed by CVP plating or DNA extraction to identify the present Pectobacterium and Dickeya species and subspecies. This should be followed by the utilisation of the molecular techniques: MLST, PFGE and/or REP-PCR to determine the phylogenetic relationships of the isolates (Fig. 2).
Figure 2.
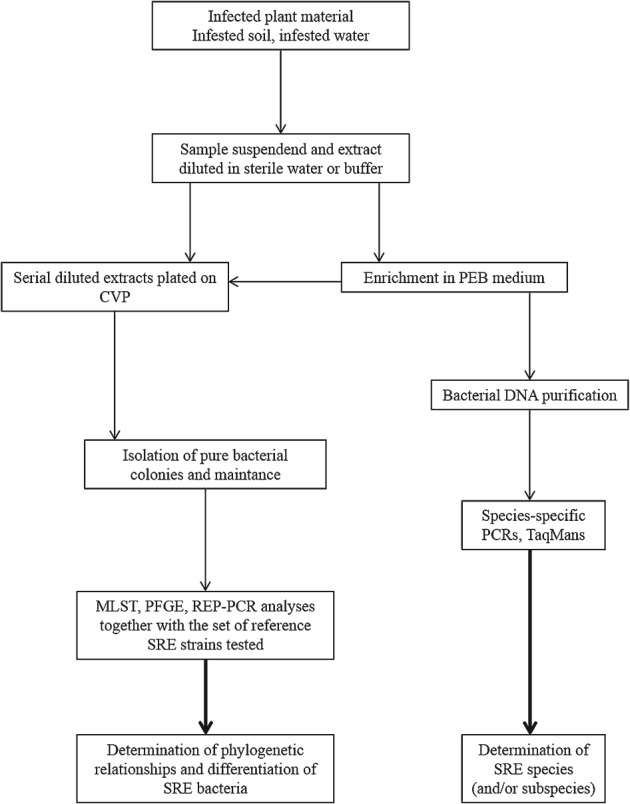
Protocol proposed for the detection and characterisation of SRE bacteria. Description of the methods is given in the text.
These methods are commonly used by researchers worldwide and have been published earlier. We hope that they would be adopted in future research concerning the isolation and characterisation of SRE.
Conclusions and perspectives
In the last 30 years there has been a migration from microbiological, serological and biochemical methods to molecular detection and differentiation techniques and in our view this trend will continue. Regardless of the approach, the overall aim has remained primarily to detect and characterise Pectobacterium spp. and Dickeya spp. populations under different conditions accurately and reliably. However, this goal has been achieved only partially, as most of the methods, especially the immunological and molecular ones, usually rely on the use of probes based on a narrow range of features, such as epitopes or DNA sequences. Since most bacterial species exhibit a range of variability, probe specificity is inevitably limited.
Despite those limitations, a wide range of methods and procedures described in this review is now available to tackle the still outstanding problems in the ecology, epidemiology and taxonomy of SRE associated with potatoes and the diseases they cause. Research on such topics as distribution, survival, relative virulence of the different taxa as well as the source and spread of crop contamination over several generations can now be undertaken with greater confidence. The difficulty is mainly in the choice of the methods that would be more appropriate in each instance. Therefore it is vital to choose them depending on the objectives of the study, the expertise and facilities as well as on the financial resources available.
When the objective is a disease control through monitoring of the seed tuber contamination in certification programmes, a general consensus has not yet been reached, largely because of the cost involved. The time honoured method of verifying seed health by field inspection and rogueing of blackleg-affected plants, which is still widely practiced, can at best provide a rough notion of the possible infection. As disease development is largely weather-dependent, the absence of disease symptoms in a crop does not necessarily signify that the crop would be pathogen-free (Pérombelon, 2002). A more meaningful approach would be to determine seed tuber contamination, preferably their incidence too, by different pathogens. At present this approach has been adopted in only a few national certification programmes and then usually is restricted to the higher grade seed. Although suitable methods are now available, the limiting factor remains the cost involved when applied on a large scale. Of the methods which could be deployed commercially for rapid and specific detection and characterisation of the key pathogens, multiplex real-time PCR-based methods appear to fit the bill best by allowing the simultaneous detection of several pathogens. Since direct detection of target DNA in plant material with PCR-based methods often lack sensitivity, multiplication of the target cells in or on growth media prior to DNA detection is desirable. Until the molecular assays based on complete genome sequence of individual taxon become more readily available, identification and differentiation of the soft rot bacteria will continue to rely on the simultaneous use of several complementary probes/methods whenever possible.
Acknowledgments
The work was financially supported by the National Science Centre, Poland (Narodowe Centrum Nauki, Polska) via a post-doctoral research grant (DEC-2012/04/S/NZ9/00018) to R. C., by the Ministry of Science and Higher Education, Poland (Ministerstwo Nauki i Szkolnictwa Wyższego, Polska) via a research grant Iuventus Plus 2012 (MNiSW 0241/IP/2013/72) to R. C. and by European Social Fund as a part of the project ‘Educators for the elite – integrated training program for PhD students, post-docs and professors as academic teachers at University of Gdansk’ within the framework of Human Capital Operational Programme, Action 4.1.1, Improving the quality of educational offer of tertiary education institutions to Robert Czajkowski. The work was additionally supported by the National Science Centre, Poland (Narodowe Centrum Nauki, Polska) via grant NCN 7322/B/P01/2011/40 to E. L. and via grant NCN DEC-2012/07/B/NZ9/01623 to S. J. The authors would like to thank M. Rajewska (Department of Biotechnology, Intercollegiate Faculty of Biotechnology, University of Gdansk and Medical University of Gdansk, Poland) for her comments on the manuscript and her editorial work.
Open access publication cost supported from the project MOBI4Health that has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement no 316094.
References
- Allan E, Kelman A. Immunofluorescent stain procedures for detection and identification of Erwinia carotovora var. atroseptica. Phytopathology. 1977;67:1305–1312. [Google Scholar]
- Andersson RA, Eriksson AR, Heikinheimo R, Mäe A, Pirhonen M, Kõiv V, Hyytiäinen H, Tuikkala A, Palva ET. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expR(Ecc) Molecular Plant–Microbe Interactions. 2000;13:384–393. doi: 10.1094/MPMI.2000.13.4.384. [DOI] [PubMed] [Google Scholar]
- Avrova AO, Hyman LJ, Toth RL, Toth IK. Application of amplified fragment length polymorphism fingerprinting for taxonomy and identification of the soft rot bacteria Erwinia carotovora and Erwinia chrysanthemi. Applied and Environmental Microbiology. 2002;68:1499–1508. doi: 10.1128/AEM.68.4.1499-1508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghaee-Ravari S, Rahimian H, Shams-Bakhsh M, Lopez-Solanilla E, Antúnez-Lamas M, Rodríguez-Palenzuela P. Characterization of Pectobacterium species from Iran using biochemical and molecular methods. European Journal of Plant Pathology. 2011;129:413–425. [Google Scholar]
- Barras F, Van Gijsegem F, Chatterjee A. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annual Review of Phytopathology. 1994;32:201–234. [Google Scholar]
- Bell KS, Avrova AO, Holeva MC, Cardle L, Morris W, De Jong W, Toth IK, Waugh R, Bryan GJ, Birch PRJ. Sample sequencing of a selected region of the genome of Erwinia carotovora subsp. atroseptica reveals candidate phytopathogenicity genes and allows comparison with Escherichia coli. Microbiology. 2002;148:1367–1378. doi: 10.1099/00221287-148-5-1367. [DOI] [PubMed] [Google Scholar]
- Bell KS, Sebaihia M, Pritchard L, Holden MTG, Hyman LJ, Holeva MC, Thomson NR, Bentley SD, Churcher LJC, Mungall K, Atkin R, Bason N, Brooks K, Chillingworth T, Clark K, Doggett J, Fraser A, Hance Z, Hauser H, Jagels K, Moule S, Norbertczak H, Ormond D, Price C, Quail MA, Sanders M, Walker D, Whitehead S, Salmond GPC, Birch PRJ, Parkhill J, Toth IK. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11105–11110. doi: 10.1073/pnas.0402424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara M, Vedel R, Lalo D, Lebrun MH, Lafay JF. Genetic diversity and host range in strains of Erwinia chrysanthemi. Molecular Plant–Microbe Interactions. 1991;4:293–299. [Google Scholar]
- Brady CL, Cleenwerck I, Denman S, Venter SN, Rodríguez-Palenzuela P, Coutinho TA, De Vos P. Proposal to reclassify Brenneria quercina (Hildebrand and Schroth 1967) Hauben et al. 1999 into a new genus, Lonsdalea gen. nov., as Lonsdalea quercina comb. nov., descriptions of Lonsdalea quercina subsp. quercina comb. nov., Lonsdalea quercina subsp. iberica subsp. nov. and Lonsdalea quercina subsp. britannica subsp. nov., emendation of the description of the genus Brenneria, reclassification of Dickeya dieffenbachiae as Dickeya dadantii subsp. dieffenbachiae comb. nov., and emendation of the description of Dickeya dadantii. International Journal of Systematic and Evolutionary Microbiology. 2012;62:1592–1602. doi: 10.1099/ijs.0.035055-0. [DOI] [PubMed] [Google Scholar]
- Cappaert MR, Powelson ML, Franc GD, Harrison MD. Irrigation water as a source of inoculum of soft rot erwinias for aerial stem rot of potatoes. Phytopathology. 1988;78:1668–1672. [Google Scholar]
- Chao YC, Feng CT, Ho WC. First report of Aglaonema bacterial blight caused by Erwinia chrysanthemi in Taiwan. Plant Disease. 2006;10:1358–1358. doi: 10.1094/PD-90-1358A. [DOI] [PubMed] [Google Scholar]
- Charkowski AO. Plant-Associated Bacteria. The Netherlands: Springer; 2006. The Soft Rot Erwinia; pp. 423–505. In. Ed. Gnanamanickam, S.S. Dordrecht. [Google Scholar]
- Charkowski A, Blanco C, Condemine G, Expert T, Franza T, Hayes C, Hugouvieux-Cotte-Pattat N, López SolanillaE, Low D, Moleleki L, Pirhonen M, Pitman A, Perna N, Reverchon S, Rodríguez PalenzuelaP, San FranciscoM, Toth I, Tsuyumu S, van der Waals J, van der Wolf J, Van Gijsegem F, Yang C-H, Yedidia I. The role of secretion systems and small molecules in soft-rot Enterobacteriaceae pathogenicity. Annual Review of Phytopathology. 2012;50:425–449. doi: 10.1146/annurev-phyto-081211-173013. [DOI] [PubMed] [Google Scholar]
- Cother EJ, Gilbert RL. Presence of Erwinia chrysanthemi in two major river systems and their alpine sources in Australia. Journal of Applied Bacteriology. 1990;69:729–738. [Google Scholar]
- Cother EJ, Bradley JK, Gillings MR, Fahy PC. Characterization of Erwinia chrysanthemi biovars in alpine water sources by biochemical properties, GLC fatty acid analysis and genomic DNA fingerprinting. Journal of Applied Bacteriology. 1992;73:99–107. [Google Scholar]
- Crowle AJ. Immunodiffusion. New York, NY, USA: Academic Press Inc; 1961. [Google Scholar]
- Cuppels D, Kelman A. Evaluation of selective media for isolation of soft-rot bacteria from soil and plant tissue. Phytopathology. 1974;64:468–475. [Google Scholar]
- Czajkowski R, Grabe G, van der Wolf JM. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. European Journal of Plant Pathology. 2009;125:263–275. [Google Scholar]
- Czajkowski R, Pérombelon MCM, van Veen JA, van der Wolf JM. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathology. 2012;60:999–1013. [Google Scholar]
- Darrasse A, Priou S, Kotoujansky A, Bertheau Y. PCR and restriction fragment length polymorphism of a pel gene as a tool to identify Erwinia carotovora in relation to potato diseases. Applied and Environmental Microbiology. 1994;60:1437–1443. doi: 10.1128/aem.60.5.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawyndt P, Vancanneyt M, Snauwaert C, De Baets B, De Meyer H, Swings J. Mining fatty acid databases for detection of novel compounds in aerobic bacteria. Journal of Microbiological Methods. 2006;66:410–433. doi: 10.1016/j.mimet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- De Boer SH. Evaluation of an agar immunodiffusion procedure for confirming bacterial ring rot diagnoses. American Potato Journal. 1983;60:661–669. [Google Scholar]
- De Boer SH. Characterization of pectolytic erwinias as highly sophisticated pathogens of plants. European Journal of Plant Pathology. 2003;109:893–899. [Google Scholar]
- De Boer SH, McNaughton ME. Monoclonal antibodies to the lipopolysaccharide of Erwinia carotovora subsp. atroseptica serogroup I. Phytopathology. 1987;77:828–832. [Google Scholar]
- De Boer SH, Sasser M. Differentiation of Erwinia carotovora ssp. carotovora and E. carotovora ssp. atroseptica on the basis of cellular fatty acid composition. Canadian Journal of Microbiology. 1986;32:796–800. [Google Scholar]
- De Boer SH, Copeman RJ, Vruggink H. Serogroups of Erwinia carotovora potato strains determined with diffusible somatic antigens. Phytopathology. 1979;69:316–319. [Google Scholar]
- De Boer SH, Verdonck L, Vruggink H, Harju P, Bang HO, De Ley J. Serological and biochemical variation among potato strains of Erwinia carotovora subsp. atroseptica and their taxonomic relationship to other E. carotovora strains. Journal of Applied Bacteriology. 1987;63:487–495. [Google Scholar]
- De Boer SH, Ward LJ. PCR detection of Erwinia carotovorora subsp. atroseptica associated with potato tissue. Phytopathology. 1995;85:854–858. [Google Scholar]
- De Boer SH, Li XZ, Ward LJ. Pectobacterium spp. associated with bacterial stem rot syndrome of potato in Canada. Phytopathology. 2012;102:937–947. doi: 10.1094/PHYTO-04-12-0083-R. [DOI] [PubMed] [Google Scholar]
- De Haan EG, Dekker-Nooren TCEM, van den Bovenkamp GW, Speksnijder AGCL, van der Zouwen PS, van der Wolf JM. Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. European Journal of Plant Pathology. 2008;122:561–569. [Google Scholar]
- De Haan EG, Van Den Bovenkamp GW. Test development in Erwinia at the NAK: BioPlex real-time PCR. Gewasbescherming. 2009;40:172–175. [Google Scholar]
- Degefu Y, Potrykus M, Golanowska M, Virtanen E, Lojkowska E. A new clade of Dickeya spp. plays a major role in potato blackleg outbreaks in North Finland. Annals of Applied Biology. 2013;162:231–241. [Google Scholar]
- van Der Merwe J, Coutinho T, Korsten L, Van Der Waals JE. Pectobacterium carotovorum subsp. brasiliensis causing blackleg on potatoes in South Africa. European Journal of Plant Pathology. 2010;126:175–185. [Google Scholar]
- Diallo S, Latour X, Groboillot A, Smadja B, Copin P, Orange N, Feuilloley MGJ, Chevalier S. Simultaneous and selective detection of two major soft rot pathogens of potato: Pectobacterium atrosepticumErwinia carotovora subsp. atrosepticum) and Dickeya spp. (Erwinia chrysanthemi. European Journal of Plant Pathology. 2009;125:349–354. [Google Scholar]
- Duarte V, De Boer SH, Ward LJ, De Oliveira AMR. Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. Journal of Applied Microbiology. 2004;96:535–545. doi: 10.1111/j.1365-2672.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- Fessehaie A, De Boer SH, Lévesque CA. Molecular characterization of DNA encoding 16S-23S rRNA intergenic spacer regions and 16S rRNA of pectolytic Erwinia species. Canadian Journal of Microbiology. 2002;48:387–398. doi: 10.1139/w02-026. [DOI] [PubMed] [Google Scholar]
- Fraaije BA, Birnbaum Y, Franken AJM, Van Den Bulk RW. The development of a conductimetric assay for automated detection of metabolically active soft rot Erwinia spp. in potato tuber peel extracts. Journal of Applied Bacteriology. 1996;81:375–382. [Google Scholar]
- Fréchon D, Exbrayat P, Helias V, Hyman LJ, Jouan B, Llop P, Lopez MM, Payet N, Pérombelon MCM, Toth IK, van Backhoven JRCM, van der Wolf JM, Bertheau Y. Evaluation of a PCR kit for the detection of Erwinia carotovora subsp. atroseptica on potato tubers. Potato Research. 1998;41:163–173. [Google Scholar]
- Fröhling A, Klocke S, Hausdorf L, Klocke M, Schlüter O. A method for viability testing of Pectobacterium carotovorum in postharvest processing by means of flow cytometry. Food and Bioprocess Technology. 2012;5:2871–2879. [Google Scholar]
- Gardan L, Gouy C, Christen R, Samson R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. International Journal of Systematic and Evolutionary Microbiology. 2003;53:381–391. doi: 10.1099/ijs.0.02423-0. [DOI] [PubMed] [Google Scholar]
- Golan A, Kerem Z, Tun OM, Luzzatto T, Lipsky A, Yedidia I. Combining flow cytometry and gfp reporter gene for quantitative evaluation of Pectobacterium carotovorum ssp. carotovorum in Ornithogalum dubium plantlets. Journal of Applied Microbiology. 2010;108:1136–1144. doi: 10.1111/j.1365-2672.2009.04517.x. [DOI] [PubMed] [Google Scholar]
- Gorris MT, Alarcon B, Lopez MM, Cambra M. Characterization of monoclonal antibodies specific for Erwinia carotovora subsp. atroseptica and comparison of serological methods for its sensitive detection on potato tubers. Applied and Environmental Microbiology. 1994;60:2076–2085. doi: 10.1128/aem.60.6.2076-2085.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorshkov VYu, Petrova OE, Mukhametshina NE, Ageeva MV, Mulyukin AL, Gogolev YuV. Formation of “nonculturable” dormant forms of the phytopathogenic Enterobacterium Erwinia carotovora. Microbiology. 2009;78:585–592. [Google Scholar]
- Graham DC. Taxonomy of the soft rot coliform bacteria. Annual Review of Phytopathology. 1964;2:13–42. [Google Scholar]
- Gross DC, Powelson ML, Regner KM, Radamaker GK. A bacteriophage-typing system for surveying the diversity and distribution of strains of Erwinia carotovora in potato fields. Phytopathology. 1991;81:220–226. [Google Scholar]
- Hauben L, Moore ERB, Vauterin L, Steenackers M, Mergaert J, Verdonck L, Swings J. Phylogenetic position of phytopathogens within the Enterobacteriaceae. Systematic and Applied Microbiology. 1998;21:384–397. doi: 10.1016/S0723-2020(98)80048-9. [DOI] [PubMed] [Google Scholar]
- Hélias V, Andrivon D, Jouan B. Internal colonization pathways of potato plants by Erwinia carotovora ssp. atroseptica. Plant Pathology. 2000;49:33–42. [Google Scholar]
- Hélias V, Hamon P, Huchet E, van der Wolf JM, Andrivon D. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathology. 2011;61:339–345. [Google Scholar]
- Hyman LJ, Toth IK, Pérombelon MCM. Methods for the Detection and Quantification of Erwinia carotovora subsp. atroseptica on Potato. Laboratory Manual. 1998. Isolation and identification; pp. 64–71. Scottish Crop Research Institute Annual Report, Dundee, Scotland, The United Kingdom. [Google Scholar]
- Janse JD, Ruissen MA. Characterization and classification of Erwinia chrysanthemi strains from several hosts in the Netherlands. Phytopathology. 1988;78:800–808. [Google Scholar]
- Jones DC, Hyman LJ, Tumeseit M, Smith P, Pérombelon MCM. Blackleg potential of potato seed: determination of tuber contamination by Erwinia carotovora subsp. atroseptica by immunofluorescence colony staining and stock and tuber sampling. Annals of Applied Biology. 1994;124:557–568. [Google Scholar]
- Kang HW, Kwon SW, Go SJ. PCR-based specific and sensitive detection of Pectobacterium carotovorum ssp. carotovorum by primers generated from a URP-PCR fingerprinting-derived polymorphic band. Plant Pathology. 2003;52:127–133. [Google Scholar]
- Keer JT, Birch L. Molecular methods for the assessment of bacterial viability. Journal of Microbiological Methods. 2003;53:175–183. doi: 10.1016/s0167-7012(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ma B, Perna NT, Charkowsky AO. Phylogeny and virulence of naturally occurring type III secretion system-deficient Pectobacterium strains. Applied and Environmental Microbiology. 2009;75:4539–4549. doi: 10.1128/AEM.01336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Cho MS, Kim BK, Choi HJ, Hahn JH, Kim CK, Kang MJ. Quantitative real-time polymerase chain reaction assay for detection of Pectobacterium wasabiae using YD repeat protein gene-based primers. Plant Disease. 2011;96:253–257. doi: 10.1094/PDIS-06-11-0511. [DOI] [PubMed] [Google Scholar]
- Klopmeyer MJ, Kelman A. Use of monoclonal antibodies specific for pectate lyase as serological probes in the identification of soft rot Erwinia spp. Phytopathology. 1988;78:1430–1434. [Google Scholar]
- Koh YJ, Kim GH, Lee YS, Sohn SH, Koh HS, Kwon S, Heu S, Jung JS. Pectobacterium carotovorum subsp. actinidiae subsp. nov., a new bacterial pathogen causing canker-like symptoms in yellow kiwifruit, Actinidia chinensis. New Zealand Journal of Crop and Horticultural Science. 2012;40:1–11. [Google Scholar]
- Koskinen JP, Laine P, Niemi O, Nykyri J, Harjunpää H, Auvinen P, Paulin L, Pirhonen M, Palva T, Holm L. Genome sequence of Pectobacterium sp. strain SCC3193. Journal of Bacteriology. 2012;194:6004. doi: 10.1128/JB.00681-12. Journal of Bacteriology194 Erratum in: 7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SW, Go SJ, Kang HW, Ryu JC, Jo JK. Phylogenetic analysis of Erwinia species based on 16S rRNA gene sequences. International Journal of Systematic Bacteriology. 1997;47:1061–1067. doi: 10.1099/00207713-47-4-1061. [DOI] [PubMed] [Google Scholar]
- de Lacy Costello BPJ, Ewen RJ, Gunson HE, Ratcliffe NM, Spencer-Phillips PTN. The development of a sensor system for the early detection of soft rot in stored potato tubers. Measurement Science and Technology. 2000;11:1685. [Google Scholar]
- Laurila J, Ahola V, Lehtinen A, Joutsjoki T, Hannukkala A, Rahkonen A, Pirhonen M. Characterization of Dickeya strains isolated from potato and river water samples in Finland. European Journal of Plant Pathology. 2008;122:213–225. [Google Scholar]
- Laurila J, Hannukkala A, Nykyri J, Pasanen M, Hélias V, Garlant L, Pirhonen M. Symptoms and yield reduction caused by Dickeya spp. strains isolated from potato and river water in Finland. European Journal of Plant Pathology. 2010;126:249–262. [Google Scholar]
- Lee YA, Yu CP. A differential medium for the isolation and rapid identification of a plant soft rot pathogen, Erwinia chrysanthemi. Journal of Microbiological Methods. 2005;64:200–206. doi: 10.1016/j.mimet.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Lee YA, Chen KP, Hsu YW. Characterization of Erwinia chrysanthemi, the soft-rot pathogen of white-flowered calla lily, based on pathogenicity and PCR-RFLP and PFGE analyses. Plant Pathology. 2006;55:530–536. [Google Scholar]
- Li X, Nie J, Ward LJ, Nickerson J, De Boer SH. Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection and identification of Pectobacterium atrosepticum. Canadian Journal of Plant Pathology. 2011;33:447–457. [Google Scholar]
- Lopez MM, Gorris MT, Llop P, Cubero J, Vicedo B, Cambra M. Selective enrichment improves isolation, serological and molecular detection of plant pathogenic bacteria. In: Dehne HW, editor. Diagnosis and Identification of Plant Pathogens. The Netherlands: Kluwer Academic Publishers; 1997. pp. 117–121. [Google Scholar]
- Lui LH, Vikram A, Abu-Nada Y, Kushalappa AC, Raghavan GSV, Al-Mughrabi K. Volatile metabolic profiling for discrimination of potato tubers inoculated with dry and soft rot pathogens. American Journal of Potato Research. 2005;82:1–8. [Google Scholar]
- Mäki-Valkama T, Karjalainen R. Differentiation of Erwinia carotovora subsp. atroseptica and carotovora by RAPD-PCR. Annals of Applied Biology. 1994;125:301–309. [Google Scholar]
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan S, Wydra K, Linde M, Debener T. The use of two complementary DNA assays, AFLP and MLSA, for epidemic and phylogenetic studies of pectolytic enterobacterial strains with focus on the heterogeneous species Pectobacterium carotovorum. Plant Pathology. 2012;61:498–508. [Google Scholar]
- Nassar A, Bertheau Y, Dervin C, Narcy JP, Lemattre M. Ribotyping of Erwinia chrysanthemi strains in relation to their pathogenic and geographic distribution. Applied and Environmental Microbiology. 1994;60:3781–3789. doi: 10.1128/aem.60.10.3781-3789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar A, Darrasse A, Lemattre M, Kotoujansky A, Dervin C, Vedel R, Bertheau Y. Characterization of Erwinia chrysanthemi by pectinolytic isozyme polymorphism and restriction fragment length polymorphism analysis of PCR-amplified fragments of pel genes. Applied and Environmental Microbiology. 1996;62:2228–2235. doi: 10.1128/aem.62.7.2228-2235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngadze E, Brady CL, Coutinho T, Van Der Waals JE. Pectinolytic bacteria associated with potato soft rot and blackleg in South Africa and Zimbabwe. European Journal of Plant Pathology. 2012;134:533–549. [Google Scholar]
- Nykyri J, Niemi O, Koskinen P. Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathogens. 2012;8:e1003013. doi: 10.1371/journal.ppat.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio-Bielsa A, Cambra MA, López MM. Characterisation of potato isolates of Dickeya chrysanthemi in Spain by a microtitre system for biovar determination. Annals of Applied Biology. 2006;148:157–164. [Google Scholar]
- Parent JG, Lacroix M, Page D, Vezina L. Identification of Erwinia carotovora from soft rot diseased plants by random amplified polymorphic DNA (RAPD) analysis. Plant Disease. 1996;80:494–499. [Google Scholar]
- Park DS, Shim JK, Kim JS, Kim BY, Kang MJ, Seol YJ, Hahn JH, Sherstha R, Lim CK, Go SJ, Kim HG. PCR-based sensitive and specific detection of Pectobacterium atrosepticum using primers based on Rhs family gene sequences. Plant Pathology. 2006;55:625–629. [Google Scholar]
- Pérombelon MCM. The extent and survival of contamination of potato stocks in Scotland by Erwinia caratovora var. carotovora and Erwinia carotovora var. atroseptica. Annals of Applied Biology. 1972;71:111–117. [Google Scholar]
- Pérombelon MCM. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathology. 2002;51:1–12. [Google Scholar]
- Pérombelon MCM, Burnett EM. Two modified crystal violet pectate (CVP) media for the detection, isolation and enumeration of soft rot Erwinia. Potato Research. 1991;34:79–85. [Google Scholar]
- Pérombelon MCM, Hyman LJ. Survival of soft rot coliforms, Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica in soil in Scotland. Journal of Applied Bacteriology. 1989;66:95–106. [Google Scholar]
- Pérombelon MCM, Hyman LJ. Serological methods to quantify potato seed contamination by Erwinia carotovora subsp. atroseptica. EPPO Bulletin. 1995;25:195–202. [Google Scholar]
- Pérombelon MCM, Kelman A. Ecology of the soft rot Erwinias. Annual Review of Phytopathology. 1980;18:361–387. [Google Scholar]
- Pérombelon MCM, Van Der Wolf JM. 2002. Erwinia carotovora atroseptica Pectobacterium carotovorum atrosepticum Scottish Crop Research Institute Annual Report10 Methods for the detection and quantification of subsp. subsp. on potatoes: a laboratory manual.
- Peters J, Sledz W, Bergervoet JHW, Van Der Wolf JM. An enrichment microsphere immunoassay for the detection of Pectobacterium atrosepticum and Dickeya dianthicola in potato tuber extracts. European Journal of Plant Pathology. 2007;117:97–107. [Google Scholar]
- Phillips JA, Kelman A. Direct fluorescent antibody stain procedure applied to insect transmission of Erwinia carotovora. Phytopathology. 1982;72:898–901. [Google Scholar]
- Pickup RW. Development of molecular methods for the detection of specific bacteria in the environment. Journal of General Microbiology. 1991;137:1009–1019. [Google Scholar]
- Pierce L. Selective medium for isolation of pectolytic Erwinia sp. Plant Disease. 1992;76:382–384. [Google Scholar]
- Pirhonen M, Palva ET. Occurrence of bacteriophage T4 receptor in Erwinia carotovora. Molecular Genetics and Genomics. 1988;214:170–172. [Google Scholar]
- Pitman AR, Wright PJ, Galbraith MD, Harrow SA. Biochemical and genetic diversity of pectolytic enterobacteria causing soft rot disease of potatoes in New Zealand. Australasian Plant Pathology. 2008;37:559–568. [Google Scholar]
- Pitman AR, Harrow SA, Visnovsky SB. Genetic characterisation of Pectobacterium wasabiae causing soft rot disease of potato in New Zealand. European Journal of Plant Pathology. 2010;126:423–435. [Google Scholar]
- Pritchard L, Humphris S, Saddler GS, Parkinson NM, Bertrand V, Elphinstone JG, Toth IK. Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathology. 2012;62:587–596. [Google Scholar]
- Quinn CE, Sells IA, Graham DC. Soft rot Erwinia bacteria in the atmospheric bacterial aerosol. Journal of Applied Microbiology. 1980;49:175–181. [Google Scholar]
- Samson R, Nassan-Agha N. 1978. Erwinia chrysanthemi Proceedings of the 4th International Conference on Plant-Pathogenic Bacteria Biovars and serovars among 129 strains of, 547–553.
- Samson R, Legendre JB, Christen R, Fischer-Le SauxM, Achouak W, Gardan L. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology. 2005;55:1415–1427. doi: 10.1099/ijs.0.02791-0. [DOI] [PubMed] [Google Scholar]
- Shepard JF, Claflin LE. Critical analyses of the principles of seed potato certification. Annual Reviews of Phytopathology. 1975;13:271–293. [Google Scholar]
- Sławiak M, Van Beckhoven JRCM, Speksnijder AGCL, Czajkowski R, Grabe G, Van Der Wolf JM. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. European Journal of Plant Pathology. 2009;125:245–261. [Google Scholar]
- Sławiak M, Van Doorn R, Szemes M, Speksnijder AGCL, Waleron M, van der Wolf JM, Łojkowska E, Schoen CD. Multiplex detection and identification of bacterial pathogens causing potato blackleg and soft rot in Europe, using padlock probes. Annals of Applied Biology. 2013;163:378–393. [Google Scholar]
- Smid EJ, Jansen AH, Gorris LG. Detection of Erwinia carotovora subsp. atroseptica and Erwinia chrysanthemi in potato tubers using polymerase chain reaction. Plant Pathology. 1995;44:1058–1069. [Google Scholar]
- Smit ML, Giesendorf BJ, Vet JM, Trijbels FJM, Blom HJ. Semiautomated DNA mutation analysis using a robotic workstation and molecular beacons. Clinical Chemistry. 2001;47:739–744. [PubMed] [Google Scholar]
- Starr MP, Cosens G, Knackmuss HJ. Formation of the blue pigment indigoidine by phytopathogenic Erwinia. Applied Microbiology. 1966;14:870–872. doi: 10.1128/am.14.6.870-872.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takle G, Toth I, Brurberg M. Evaluation of reference genes for real-time RT-PCR expression studies in the plant pathogen Pectobacterium atrosepticum. BMC Plant Biology. 2007;7:50. doi: 10.1186/1471-2229-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth IK, Bertheau Y, Hyman LJ, Laplaze L, López MM, McNicol J, Niepold F, Persson P, Salmond GPC, Sletten A, van der Wolf JM, Pérombelon MCM. Evaluation of phenotypic and molecular typing techniques for determining diversity in Erwinia carotovora subsp. atroseptica. Journal of Applied Microbiology. 1999;87:770–781. doi: 10.1046/j.1365-2672.1999.00929.x. [DOI] [PubMed] [Google Scholar]
- Toth IK, Avrova AO, Hyman LJ. Rapid identification and differentiation of the soft rot Erwinias by 16S–23S intergenic transcribed spacer-PCR and restriction fragment length polymorphism analyses. Applied and Environmental Microbiology. 2001;67:4070–4076. doi: 10.1128/AEM.67.9.4070-4076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth IK, Bell KS, Holeva MC, Birch PRJ. Soft rot erwiniae: from genes to genomes. Molecular Plant Pathology. 2003;4:17–30. doi: 10.1046/j.1364-3703.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, Tsror (Lahkim) L, Elphinstone JG. Dickeya species: an emerging problem for potato production in Europe. Plant Pathology. 2011;60:385–399. [Google Scholar]
- Tsror (Lahkim) L, Erlich O, Lebiush S, Hazarovsku M, Zig U, Slawiak M, Grabe G, van der Wolf JM, van der Haar JJ. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. European Journal of Plant Pathology. 2009;123:311–320. [Google Scholar]
- Tsror (Lahkim) L, Erlich O, Hazanovsky M, Ben DanielB, Zig U, Lebiush S. Detection of Dickeya spp. latent infection in potato seed tubers using PCR or ELISA and correlation with disease incidence in commercial field crops under hot-climate conditions. Plant Pathology. 2012;61:161–168. [Google Scholar]
- Tsror (Lahkim) L, Ben-Daniel B, Chalupowicz L, van der Wolf J, Lebiush S, Erlich O, Dror O, Barel V, Nijhuis E, Manulis-Sasson S. Characterization of Dickeya strains isolated from potato grown under hot-climate conditions. Plant Pathology. 2013;62:1097–1105. [Google Scholar]
- van Vaerenbergh J, Baeyen S, De Vos P, Maes M. Sequence diversity in the Dickeya fliC gene: phylogeny of the Dickeya genus and TaqMan® PCR for ‘D. solani’, new biovar 3 variant on potato in Europe. PLoS ONE. 2012;7:e35738. doi: 10.1371/journal.pone.0035738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varns JL, Glynn MT. Detection of disease in stored potatoes by volatile monitoring. American Potato Journal. 1979;56:185–197. [Google Scholar]
- Versalovic J, Schneider M, De Bruijn FJ, Lupski JR. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods in Molecular and Cellular Biology. 1994;5:25–40. [Google Scholar]
- Waleron M, Waleron K, Podhajska AJ, Łojkowska E. Genotyping of bacteria belonging to the former Erwinia genus by PCR-RFLP analysis of a recA gene treatment. Microbiology. 2002;148:583–595. doi: 10.1099/00221287-148-2-583. [DOI] [PubMed] [Google Scholar]
- Waleron M, Waleron K, Lojkowska E. Occurrence of Pectobacterium wasabiae in potato field samples. European Journal of Plant Pathology. 2013;137:149–158. [Google Scholar]
- Waleron M, Waleron K, Lojkowska E. Characterization of Pectobacterium carotovorum subsp. odoriferum causing soft rot of stored vegetables. European Journal of Plant Pathology. 2014;139:457–469. [Google Scholar]
- Willerslev E, Cooper A. Review paper. Ancient DNA. Proceedings of the Royal Society B: Biological Sciences. 2005;272:3–16. doi: 10.1098/rspb.2004.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wolf JM, Gussenhoven GC. Reaction of saprophytic bacteria from potato peel extracts and plant pathogenic bacteria in ELISA with antisera to Erwinia chrysanthemi (serogroup O1Ha) Netherlands Journal of Plant Pathology. 1992;98:33–44. [Google Scholar]
- van der Wolf JM, Hyman LJ, Jones DAC, Grevesse C, Van Backhoven JRCM, Van Vuurde JWL, Pérombelon MCM. Immunomagnetic separation of Erwinia carotovora subsp. atroseptica from potato peel extracts to improve detection sensitivity on a crystal violet pectate medium or by PCR. Journal of Applied Microbiology. 1996a;80:487–495. doi: 10.1111/j.1365-2672.1996.tb03247.x. [DOI] [PubMed] [Google Scholar]
- van der Wolf JM, Kastelein P, Van Beckhoven JRCM, Van Den Brink M, De Vries PM. Verification of immunofluorescence colony-staining of Erwinia carotovora subsp. atroseptica by reisolation and immunodiffusion or PCR. EPPO Bulletin. 1996b;26:707–715. [Google Scholar]
- van der Wolf JM, Nijhuis EH, Kowalewska MJ, Saddler GS, Parkinson N, Elphinstone JG, Pritchard L, Toth IK, Lojkowska E, Potrykus M, Waleron M, de Vos P, Cleenwerck I, Pirhonen M, Garlant L, Hélias V, Pothier JF, Pflüger V, Duffy B, Tsror L, Manulis S. Dickeya solani sp. nov., a pectinolytic plant pathogenic bacterium isolated from potato (Solanum tuberosum. International Journal of Systematic and Evolutionary Microbiology. 2013;64:768–774. doi: 10.1099/ijs.0.052944-0. DOI: 10.1099/ijs.0.052944-0. [DOI] [PubMed] [Google Scholar]
- Yishay M, Burdman S, Valverde A, Luzzatto T, Ophir R, Yedidia I. Differential pathogenicity and genetic diversity among Pectobacterium carotovorum ssp. carotovorum isolates from monocot and dicot hosts support early genomic divergence within this taxon. Environmental Microbiology. 2008;10:2746–2759. doi: 10.1111/j.1462-2920.2008.01694.x. [DOI] [PubMed] [Google Scholar]
- Young JM, Park DC. Relationships of plant pathogenic enterobacteria based on partial atpDcarA, and recA as individual and concatenated nucleotide and peptide sequences. Systematic and Applied Microbiology. 2007;30:343–354. doi: 10.1016/j.syapm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Zeigler DR. Gene sequences useful for predicting relatedness of whole genomes in bacteria. International Journal of Systematic and Evolutionary Microbiology. 2003;53:1893–1900. doi: 10.1099/ijs.0.02713-0. [DOI] [PubMed] [Google Scholar]


