Abstract
BACKGROUND AND PURPOSE
Natural scenes like forests and flowers evoke neurophysiological responses that can suppress anxiety and relieve stress. We examined whether images of natural objects can elicit neural responses similar to those evoked by real objects by comparing the activation of the prefrontal cortex during presentation of real foliage plants with a projected image of the same foliage plants.
METHODS
Oxy-hemoglobin concentrations in the prefrontal cortex were measured using time-resolved near-infrared spectroscopy while the subjects viewed the real plants or a projected image of the same plants.
RESULTS
Compared with a projected image of foliage plants, viewing the actual foliage plants significantly increased oxy-hemoglobin concentrations in the prefrontal cortex. However, using the modified semantic differential method, subjective emotional response ratings (“comfortable vs. uncomfortable” and “relaxed vs. awakening”) were similar for both stimuli.
CONCLUSIONS
The frontal cortex responded differently to presentation of actual plants compared with images of these plants even when the subjective emotional response was similar. These results may help explain the physical and mental health benefits of urban, domestic, and workplace foliage.
Keywords: Time-resolved near-infrared spectroscopy, prefrontal cortex, foliage plants, stress response, semantic differential method
Introduction
Human evolution is an adaptation to nature; therefore, natural scenes have significant effects on brain activity and physiological responses, including stress responses.1 However, because of progression in society over the past few centuries, humans have experience stressful situations due to urbanization and artificial environment.2–5 To date, we have accumulated large amounts of data on the physiological relaxation effect derived from various aspects of nature.6–15 In contrast, recent developments in image projection technology have enabled us to visualize images, such as natural environments with greater clarity. Accordingly, familiar and convenient visual stimulation derived from nature is expected. However, it remains unknown whether the physiological effects of stimulation due to foliage plant images are comparable to those due to real foliage plants. We examined potential differences in neurophysiological responses to natural and projected scenes by measuring activation of the prefrontal cortex in response to presentation of foliage plants and projected images of the same plants.
Materials and Methods
Eighteen female university students (21.6 ± 1.5 years old) participated in this experiment. They were sufficiently informed about the aim and the procedure of this experiment, and all subjects provided their written informed consent. This study was performed according to the regulations of the Ethics Committee of the Center for Environment, Health, and Field Sciences, Chiba University, Japan. Physiological measurements were performed in an artificial climate chamber maintained at 25 °C with 50% relative humidity and 300 lux illumination. For foliage plants, we used three dracaena plants (Dracaena deremensis). Subjects viewed the three plants, sitting side-by-side on the floor or a high-resolution image of the three plants projected at the same size, luminosity, and position on a TV screen (58V type, TH-58PZ800 by Panasonic). Figure 1 shows the study protocol, Figure 2 the scene at rest (control, a box), and Figure 3 the scene during visual stimulation (the plants). The subjects viewed the stimuli in the following order: an actual cardboard box for 30 seconds (Fig 2, left), actual dracaena plants for 3 minutes (Fig 3, left), a projected image of the cardboard box for 30 seconds (Fig 2, right), and finally, the projected image of the dracaena plants for 3 minutes (Fig 3, right). The images were then presented in a different order (Fig 1, lower). While viewing the plants and images, oxy-hemoglobin (oxy-Hb) concentrations in the prefrontal cortex were measured using near-infrared time resolved spectroscopy using the TRS-20 system (Hamamatsu Photonics K.K.).16,17 The oxy-Hb concentrations in the left and right prefrontal cortex were measured at 1 Hz for the last 10 seconds of box presentation (real or image, termed the pre-measurement condition) and during the entire 3-minutes presentation of the plant or plant image (postmeasurement condition). Data were transformed by linear interpolation because the 1 Hz sampling rate was only approximate. We used the oxy-Hb concentration difference between the pre- and postmeasurements for analysis. We excluded approximately 30 seconds of data acquired while the assessor removed the box from the experimental scene, because it would cause cortical activation that was unrelated to object perception. In addition to the neurophysiological measurements, subjects gave a subjective evaluation of the emotional impact of the image and plant using the modified semantic differential (SD) method.18 The SD method uses three pairs of adjectives on 13 scales, including “comfortable–uncomfortable,” “relaxed–awakening,” and “natural–artificial.” This SD rating test was performed after presentation of the visual stimuli (Fig 1).
Fig 1.
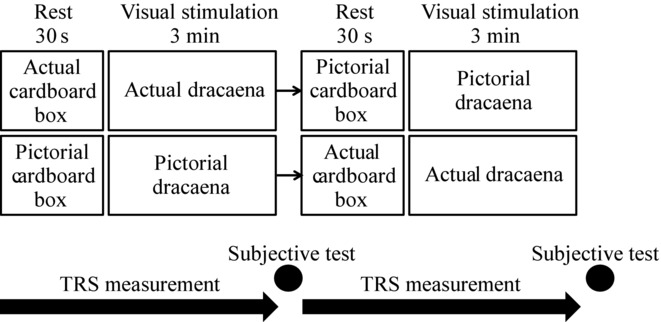
Study protocol.
Fig 2.

The scene at rest.
Fig 3.

The scene during visual stimulation.
Statistical Package for Social Sciences software (v21.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Neurophysiological responses to the plants and images were compared by two-way analysis of variance (ANOVA) for repeated measures, followed by analysis of simple main effects and multiple comparisons with the Holm correction. The Wilcoxon signed-rank test was applied to analyze differences in psychological indices between the two visual stimuli (plants or image).
Results and Discussion
We measured oxy-Hb concentrations in the prefrontal cortex of healthy women while they viewed real dracaena plants or a projected image of the same plants (Fig 4). Two-way ANOVA revealed a significant effect of the visual stimulus (real vs. image) [F (1,15) = 6.292, P < .05], a significant effect of viewing time [F (2,30) = 15.805, P < .01], and a significant stimulus × time interaction in the left prefrontal cortex [F (2,30) = 6.424, P < .01). Similarly, there was a significant effect of visual stimulus [F (1,15) = 6.299, P < .05], time [F (2,30) = 11.913, P < .01], and stimulus × time interaction [F (2,30) = 6.945, P < .01] in the right prefrontal cortex. In the left prefrontal cortex, simple main effects analysis revealed that the average oxy-Hb concentrations were significantly higher while viewing the actual dracaena plants compared with the projected image over the 1–2 minutes interval [F (1,15) = 7.919, P < .05] and over the 2−3 minutes interval [F (1,15) = 7.269, P < .05]. Moreover, multiple comparison tests indicated significantly higher mean oxy-Hb concentrations during the 1−2 minutes and the 2−3 minutes intervals compared with the 0−1 minutes interval for the real dracaena plants (P < .01 for both intervals), but not for the projected image. The oxy-Hb concentration changes were similar in the right prefrontal cortex, with significantly higher concentrations in response to the real dracaena plants compared with the image [1−2 minutes interval: F (1,15) = 6.047, P < 0.05; 2−3 minutes interval: F (1,15) = 6.884, P < .05] and compared with the 0−1 minutes interval while viewing the actual plants (1−2 minutes: P < .01; 2−3 minutes: P < .05). Despite the distinct cortical responses, the subjective feelings elicited by the two stimuli were similar (Fig 5). With both the real plants and image inducing slightly “comfortable” and “relaxed” feelings. The actual dracaena plants were rated as significantly more “natural,” as expected (P < .01).
Fig 4.
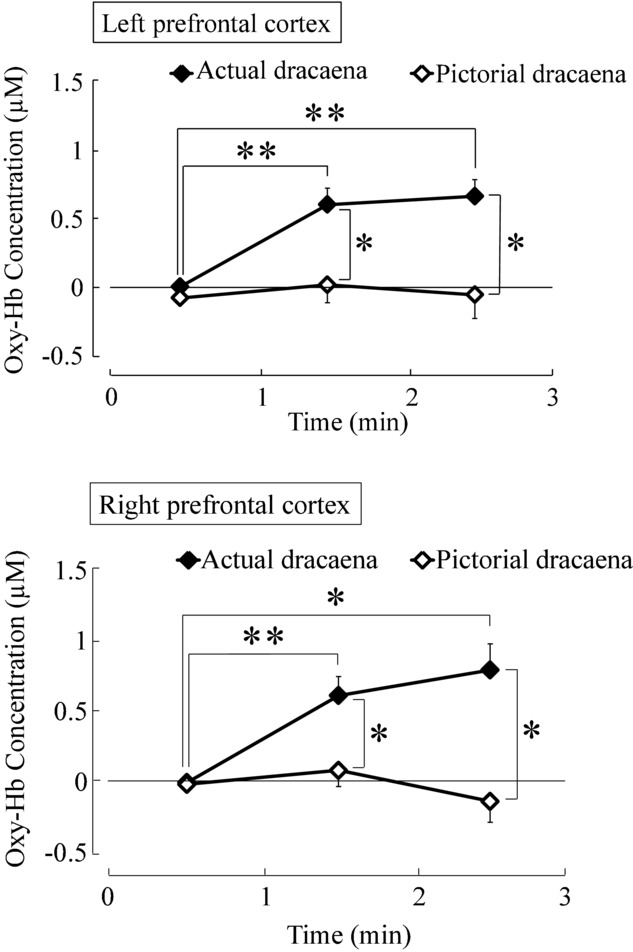
Time-dependent oxy-Hb concentration changes in the prefrontal cortex while viewing dracaena plants or a projected image of the plants. The oxy-Hb concentration shown is the difference between the pre- and postmeasurement conditions. Data are expressed as mean ± SE; n = 16. *P < .05, **P < .01 by two-way analysis of variance (ANOVA) for repeated measures with post-hoc Holm correction.
Fig 5.
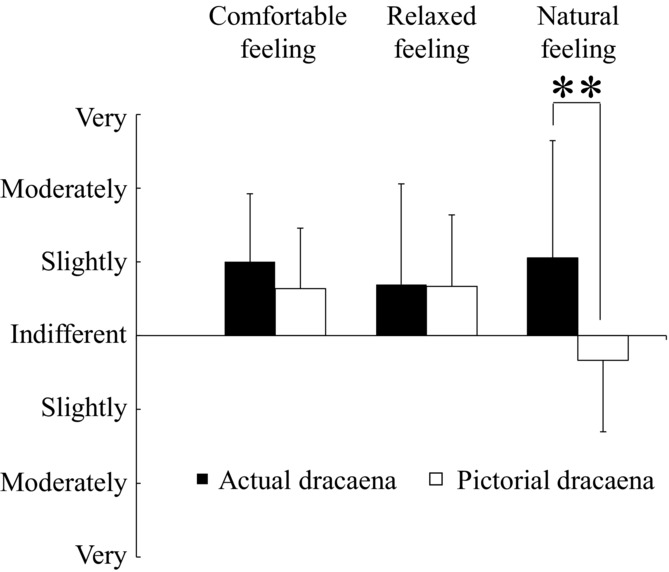
Subjective feeling measured by the modified semantic differential (SD) questionnaire after viewing real dracaena plants or an image of the plants. Data are expressed as mean ± SD; n = 18. **P < .01 by Wilcoxon signed-rank test.
We report the first demonstration of a differential cortical response to actual foliage plants compared with an image of the same plants. Viewing actual foliage plants significantly increased oxy-Hb concentrations in the prefrontal cortex, whereas the projected image did not. Surprisingly, both the real plants and the image induced feelings of comfort and relaxation as measured by the modified SD method. Given the role of the prefrontal cortex in emotional regulation, the actual plants may have psychological benefits not replicated by the image. These results underscore the possible benefits of plants for stress relief in homes and offices. Future studies will examine if this prefrontal activation is dependent on plant volume, number, or species. Perhaps certain plants are more “anxiolytic” than others.
References
- 1.Miyazaki Y, Park BJ, Lee J. Nature therapy. In: Osaki M, Braimoh AK, Nakagami K, editors. Designing Our Future: Local Perspectives on Bioproduction, Ecosystems and Humanity. United Nations, New York: United Nations University; 2011. pp. 407–412. [Google Scholar]
- 2.Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 3.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 4.Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr Bull. 2005;31:795–799. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 6.Tsunetsugu Y, Lee J, Park BJ, et al. Physiological and psychological effects of viewing urban forest landscapes assessed by multiple measurement. Landscape Urban Plan. 2013;113:90–93. [Google Scholar]
- 7.Park BJ, Lee J, Miyazaki Y, et al. Effect of the forest environment on physiological relaxation-the results of field tests at 35 sites throughout Japan. In: Li Q, editor. Forest Medicine. United Nations, New York: Nova Science Publishers; 2011. pp. 55–65. [Google Scholar]
- 8.Lee J, Park BJ, Tsunetsugu Y, et al. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health. 2011;125:93–100. doi: 10.1016/j.puhe.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Tsunetsugu Y, Park BJ, Miyazaki Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ Health Prev Med. 2010;15:27–37. doi: 10.1007/s12199-009-0091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park BJ, Kasetani T, Morikawa T, et al. Physiological effects of forest recreation in a young conifer forest in Hinokage Town, Japan. Silva Fenn. 2009;43:291–301. [Google Scholar]
- 11.Lee J, Park BJ, Tsunetsugu Y, et al. Restorative effects of viewing real forest landscapes, based on a comparison with urban landscapes. Scand J Forest Res. 2009;24:227–234. [Google Scholar]
- 12.Park BJ, Tsunetsugu Y, Ishii H, et al. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in a mixed forest in Shinano Town, Japan. Scandi J Forest Res. 2008;23:278–283. [Google Scholar]
- 13.Li Q, Morimoto K, Kobayashi M, et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmacol. 2008;21:117–127. doi: 10.1177/039463200802100113. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Morimoto K, Kobayashi M, et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J Biol Regul Homeost Agents. 2008;22:45–55. [PubMed] [Google Scholar]
- 15.Li Q, Morimoto K, Nakadai A, et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmacol. 2007;20:3–8. doi: 10.1177/03946320070200S202. [DOI] [PubMed] [Google Scholar]
- 16.Ohmae E, Oda M, Suzuki T, et al. Clinical evaluation of time-resolved spectroscopy by measuring cerebral hemodynamics during cardiopulmonary bypass surgery. J Biomed Optics. 2007;12:062112. doi: 10.1117/1.2804931. [DOI] [PubMed] [Google Scholar]
- 17.Ohmae E, Ouchi Y, Oda M, et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: correlation with simultaneous positron emission tomography measurements. NeuroImage. 2006;29:697–705. doi: 10.1016/j.neuroimage.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Osgood CE, Suci GJ, Tannenbaum P. The Measurement of Meaning. Chicago, IL: Univ Illinois Press; 1957. [Google Scholar]


