Abstract
Introduction
Our objective was to find out if there are antibodies binding to homocitrulline-containing type I and II collagen carboxyterminal telopeptides in sera of patients with rheumatoid arthritis (RA), and if these antibodies cross-react with citrulline and homocitrulline in the same peptide sequence.
Methods
A total of 72 RA and 72 control sera were analyzed for binding using enzyme-linked immunosorbent assay to citrulline- or homocitrulline-containing type I and II collagen carboxyterminal telopeptides, as well as to cyclic citrullinated peptide (CCP) and to mutated citrullinated vimentin (MCV). Specificities of the antibodies were tested using inhibition-ELISA.
Results
Of the RA sera, 39 (54%) and 41 (57%) were positive for binding to CCP and MCV, respectively. Further, 34 (47%) and 30 (42%) of the patients had specific antibodies binding to and being inhibited by citrulline-containing type I collagen telopeptides and by citrulline-containing type II collagen carboxyterminal telopeptides, respectively. The corresponding figures regarding homocitrulline-containing type I and homocitrulline-containing type II collagen telopeptides were 16 (22%) and 14 (19%). Most of the patients, who were seropositive for citrullinated peptides, showed binding in multiple assays. A total of 10 (14%) RA patients were positive for all the tested peptide pairs, while 28 (39%) of them had antibodies that contained overlapping specifities between citrulline and homocitrulline in the same peptide sequence.
Conclusions
Antibodies to both citrulline and homocitrulline containing type I and II collagen telopeptides can be found in sera of RA patients. These antibodies are not constant from one RA patient to another, but contain separate or overlapping specificities within the same peptide sequence varying between individuals. Our results suggest some relationship between citrulline and homocitrulline-recognizing antibodies, since homocitrulline antibodies exist mainly in individuals seropositive to anti-CCP and anti-MCV.
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-014-0515-z) contains supplementary material, which is available to authorized users.
Introduction
The search for citrullinated antigens in patients with rheumatoid arthritis (RA) has been in focus during recent years. The identification of the original immunogen for their production could be the key to the prevention of the immune response and the consequent cascade of the inflammatory process leading to tissue damage and loss of function. Antibodies against citrullinated proteins are a frequent finding in RA patients and may precede the onset of clinical symptoms by several years [1-3]. We have previously shown the presence of antibodies binding to citrullinated type I and II collagen telopeptides in sera of RA patients [4]. Several tests for antibodies binding to citrullinated peptides have been developed and some are in clinical use; however, they do not present the original antigen but rather rely on the cross-reaction of anti-citrullinated protein antibodies (ACPA). The presence of ACPA in the sera, measured by the anti-cyclic citrullinated protein (anti-CCP) method, has been included as an important component of the American College of Rheumatology – European League against Rheumatism (ACR – EULAR) 2010 classification criteria for RA [5]. Anti-CCP, however, measures the binding of the sera to a mixture of several citrullinated peptides. These peptides have been chosen to give an optimal distinction between controls and patients with RA [6]. Nevertheless, the presence of ACPA in RA patient sera has been connected to poor prognosis and erosive course of the disease.
Lately, also, the contingent effect of carbamylation on the pathogenetic process of RA has become of interest. Carbamylation through myeloperoxidase is part of a natural defense mechanism and takes place in inflammatory conditions (reviewed in [7]), and has been proposed as a link between smoking, uremia, inflammation and atherogenesis [8]. The product of lysine carbamylation is homocitrulline, a structural homolog of citrulline. Recently antibodies against both citrullinated and carbamylated antigens were found to be present simultaneously in sera of RA patients [9]. We have shown that a low amount of homocitrulline can be present in RA tissues simultaneously with citrulline [10]. An interesting coexistence of citrullination and myeloperoxidase activity is found at least in neutrophil extracellular traps (NETs). NETs are also present in periodontitis [11] and by containing citrullinated proteins they may be involved in ACPA formation and trigger subsequent development of RA [12,13].
Altough ACPAs arise in RA patients years before the onset of clinical disease, the origin of these antibodies is not known. The RA patients’ antibody repertoire has been reported to go through epitope spreading between citrullinated antigens [3,14,15]. The specificity of these antibodies for citrulline-containing epitopes is certain. However, the antibodies have been found to be of low avidity [16]. We have demonstrated that antibodies against citrullinated proteins can be developed by immunizing rabbits either with citrullinated or carbamylated (homocitrulline-containing) antigens [17]. Also, antibodies binding to homocitrulline-containing fibrinogen have been detected in RA patients. Furthermore, these were found to cross-react with similar citrulline-containing sequences [18]. In this study, we shed light on the RA patients’ antibody repertoire binding to citrulline- and homocitrulline-containing peptides related to type I and type II collagen carboxyterminal telopeptides. The objective of this study is to define the binding properties of the antibodies binding to citrullinated proteins, not to present another method for RA diagnosis.
Methods
Ethical considerations
The study was conducted according to the requirements of the Declaration of Helsinki and was approved by the ethics committee of the Central Hospital of Jyväskylä. All patients gave verbal informed consent before inclusion in the study. In the 1980s, when the study was designed and conducted, no Good Clinical Practice instructions existed. Verbal consent in clinical studies was common praxis. The study design was accepted by the ethics committee of the hospital.
Subjects and controls
A total of 72 RA patients diagnosed in the years 1987 and 1988 in the Jyväskylä Central Hospital according to the Ropes criteria (Ropes 1959), whose sera were available were included in the present study [19]. At the baseline, the patients were characterized as suffering either from polyarticular (n = 53), oligoarticular (n = 8), palindromic (n = 5) or polymyalgic (n = 6) disease. The cohort included 43 women, 24- to 75-years old (mean 49.1 years) and 29 men, 22- to 72-years old (mean 53.5 years). The erosion status was assessed from 24 to 74 months after the diagnosis according to the method of Larsen et al. [20]. Sera from 72 age and sex matched laboratory staff, non-RA hip surgery patients and medical students were used as controls. Positivity in anti-CCP or in anti-mutated citrullinated vimentin (MCV) assays was used as an excluding factor for the control population. The control sera were collected during 2010 to 2013. All sera were kept at −20°C. Preliminary testing of 38 hemodialysis patients was also performed to exclude the possible effect of high serum urea levels in antibody development (data not shown).
Collagen telopeptide assays
All sera were tested by enzyme-linked immunosorbent assay (ELISA) for binding of immunoglobulin G (IgG) to citrullinated and homocitrullinated type I and II collagen carboxyterminal telopeptides under standard conditions, and the binding was inhibited by addition of soluble antigens [21] to a final concentration of 100 μg/ml. The two biotinylated peptides corresponding to the carboxyterminal telopeptide of the α1 chain of human type I collagen (NeoMPS, Strasbourg, France) represent amino acids 1193 to 1218, and the two biotinylated peptides corresponding to the α 1 chain of human type II collagen represent amino acids 1217 to 1241, counted from the amino-terminus in the pro α 1 chains of type I or type II procollagen, respectively. The synthetic peptides that were used as inhibitors in the ELISA represent the carboxyteminal ends of these parent peptides. The peptide sequences are given in Table 1. In each serum, the difference between bindings with and without inhibition was calculated for all peptide pairs. The patient results were divided into positive and negative using cut-off values defined as mean + 2 standard deviations (SD) of the control group (Table 2).
Table 1.
Synthetic peptides (SP) used in this study
| Type I collagen | Sequence | Abbreviation | |
| SP112 | biotin | SAG FDF SFL PQP PQE KAH DGG RYY Cit A | CitI |
| SP132 | biotin | SAG FDF SFL PQP PQE KAH DGG RYY Hcit A | HcitI |
| SP66 | -- --- --- --- --E KAH DGG RYY Cit A | CitI | |
| SP135 | -- --- --- --- --E KAH DGG RYY Hcit A | HcitI | |
| Type II collagen | |||
| SP86 | biotin | GID MSA FAG LGP REK GPD PLQ YM Cit A | CitII |
| SP109 | biotin | GID MSA FAG LGP REK GPD PLQ YM Hcit A | HcitII |
| SP41 | --- --- --- --- -EK GPD PLQ YM Cit A | CitII | |
| SP136 | --- --- --- --- -EK GPD PLQ YM Hcit A | HcitII | |
The peptides represent different parts of the carboxy-terminal telopeptide of the α1 chain of human type I or II collagen, each containing citrulline (Cit) or homocitrulline (Hcit) as indicated (for other amino acids, the one-letter symbols are used).
Table 2.
Sensitivity and specificity of citrulline and homocitrulline inhibition-ELISA assays for separating RA patients from healthy controls
| Test | Sensitivity | Specificity |
|---|---|---|
| Anti-CCP | 0.54 | 1.00a |
| Anti-MCV | 0.57 | 1.00a |
| CitI | 0.47 | 0.96 |
| HcitI | 0.22 | 0.96 |
| CitII | 0.41 | 0.94 |
| HcitII | 0.19 | 0.96 |
aPositivity in CCP or MCV was considered as excluding factor in controls. Anti-CCP, anti-cyclic citrullinated protein; anti-MCV, anti-mutated citrullinated vimentin; Cit, citrulline; Hcit, homocitrulline.
Anti-CCP and anti-MCV assays
Anti-CCP (Immunoscan RA (Mark 2) from Euro Diagnostica, Malmö, Sweden) and anti-MCV (ORG 546, from ORGANTEC Diagnostica GmbH, Mainz, Germany) assays were performed according to the manufacturers’ instructions. All control and patient sera were tested in duplicate.
Statistical methods
All 72 control sera included were negative for anti-CCP (<25 U/ml) and anti-MCV (<20 U/ml). Logistic regression analysis was used for evaluation of the association of each test or test type (citrulline or homocitrulline tests) with bone erosions in RA patients (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY, USA). Differences were considered significant at P <0.01.
Results
Patients with RA were found to have antibodies that bind to citrulline and homocitrulline-containing sequences related to type I and II collagen telopeptides (Figures 1 and 2). These antibodies were rare in healthy individuals (4% to 5% of the controls) and in hemodialysis patients (data not shown). The differences between patients and controls were statistically significant for all assays (Table 3).
Figure 1.
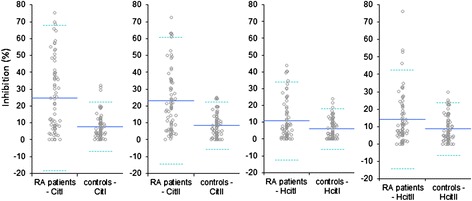
Illustration of the specific binding (inhibition-%) in RA patients (n = 72) and in controls (n = 72). Mean is shown as a solid line and ± 2SD is shown by dotted lines. For abbreviations, see Table 1. RA, rheumatoid arthritis; SD, standard deviation.
Figure 2.
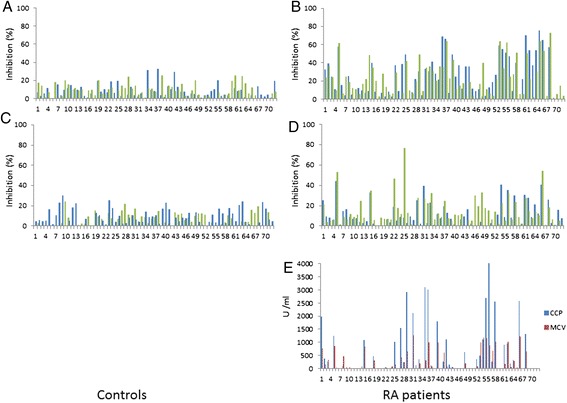
Overview of the antibody specifities in controls (panels A and C) and RA patients (panels B and D) and RA patients’ CCP and MCV results. In panels A and B, antibodies specific to CitI are depicted in dark columns/blue and those specific for HcitI in light columns/green. In panels C and D, antibodies to CitII are presented in dark columns/blue and those to HcitII in light column/green. The values shown are percent of inhibition by respective soluble peptides to binding to the similar immobilized peptide. Panel E shows the binding of RA patient sera to the antigens of the CCP and MCV assays, shown as arbitrary units defined by each manufacturer. CCP, cyclic citrullinated protein; Cit, citrulline; Hcit, homocitrulline; MCV, mutated citrullinated vimentin; RA, rheumatoid arthritis.
Table 3.
Logistic regression analysis of the association of different antibody detection methods to erosions in RA patients
| Test | OR | 95% CI | P |
|---|---|---|---|
| Anti-CCP | 8.455 | 2.82 to 25.34 | <0.001 |
| Anti-MCV | 15.583 | 4.76 to 51.05 | <0.001 |
| RF | 7.506 | 2.54 to 22.20 | <0.001 |
| CitI | 2.815 | 1.03 to 7.66 | 0.043 |
| CitII | 3.632 | 1.27 to 10.37 | 0.016 |
| CitI or CitII | 3.600 | 1.32 to 9.83 | 0.012 |
| HcitI | 2.400 | 0.69 to 8.40 | 0.171 |
| HcitII | 5.200 | 1.06 to 25.41 | 0.042 |
| HcitI or Hcit II | 3.692 | 1.08 to 12.61 | 0.037 |
CCP, cyclic citrullinated protein; CI, confidence interval; Cit, citrulline; Hcit, homocitrulline; MCV, mutated citrullinated vimentin; OR, odds ratio; RA, rheumatoid arthritis; RF, rheumatoid factor.
We measured specific antibodies to citrullinated type I collagen-related peptides using inhibition ELISA, where similar peptides were used both for binding the serum antibodies and in smaller, soluble form also to inhibit the binding. We found CitI specific antibodies in 34 (47%) of the 72 patient sera, while 16 (22%), 30 (42%) and 14 (19%) of the sera contained antibodies specific for HcitI, CitII and HcitII sequences, respectively. Furthermore, a total of 39 (54%) and 41 (57%) of the patient sera bound the respective antigens in direct ELISA for anti-CCP and anti-MCV. Total ELISA data can be found in Additional file 1.
Most of the sera that bound citrullinated peptides were reactive in multiple assays. A total of 10 (14%) sera contained specific antibodies to all four peptide pairs. Moreover, 28 (39%) of the patients had antibodies with overlapping specificities, so that the binding could be inhibited by peptides with different ureido group-containing amino acids. Of these, 16 (22%) sera showed such binding to citrulline-containing peptide that could be inhibited by homocitrulline-containing peptide. For another 16 (22%) sera the binding to homocitrulline-containing peptide could be inhibited by the corresponding citrulline-containing peptide. In only four (6%) of the cases were the antibodies cross-reactive and could be inhibited simultaneously in both directions.
We could analyze sera taken at two different time points from 19 patients (data not shown). There was only slight variation in the titers of antibodies to specific collagen telopeptide sequences between the two samples. We also preliminarily tested sera from 38 hemodialysis patients for binding to citrulline- and homocitrulline-containing peptides to exclude a possible effect of high serum urea levels and found the results comparable to those of healthy controls.
Logistic regression analysis
The association of the results of each inhibition-ELISA to erosive disease was assessed using logistic regression analysis (Table 3). MCV had the strongest association to the development of erosions with an odds ratio (OR) of 15.6. CCP also was well associated to erosions, with an OR of 8, but it gave no additional value when combined with MCV. As expected, rheumatoid factor (RF) was also well associated with erosions with an OR of 7. The presence of antibodies to the collagen-related antigens CitI (OR 2.8; P = 0.043), CitII (OR 3.6; P = 0.016) and HcitII (OR 5.2; P = 0.042) reached close to statistically significant association with erosive RA, whereas for antibodies against HcitI (OR 2.4; P = 0.171) the association did not reach statistical significance. When the presence of joint erosions was checked against the presence of antibodies to either CitI or CitII in patient sera, the OR was 3.6 (P = 0.012), comparable to that of the combined homocitrulline tests; if a patient serum contained either HcitI or HcitII antibodies, the OR for erosions was 3.7 (P = 0.037).
Discussion
We have previously obtained results in rabbit experiments indicating that antibodies against citrullinated antigens can also be induced with homocitrulline-containing immunogens [17] and that homocitrulline can be present in human RA tissue together with citrulline [10]. In this study, we wanted to define properties of RA antibodies that bind to citrulline- and homocitrulline-containing epitopes.
The antibodies binding to citrullinated and carbamylated proteins are induced even years prior to clinical RA onset [2,22]. The development is characterized by epitope spreading between antigens and the fine-tuning of the specificity of these antibodies [3,14]. Furthermore, in established disease the antibody repertoire remains fairly stabile [23]. The same phenomenon was also seen regarding antibodies to citrullinated and homocitrulline-containing collagen telopeptides.
We found that RA patients had specific antibodies to the CitI, CitII, HcitI and HcitII antigens. The antibodies binding to citrulline- or homocitrulline-containing telopeptides could be detected separately or simultaneously in the same serum. Evidently the antibodies present in sera of RA patients were able to discern between citrulline and homocitrulline in the same peptide-sequence, although there were also some overlapping specificities. The overlapping specificities were shown as the sera were able to bind to a citrullinated antigen, but could be inhibited by a soluble homocitrullinated peptide or vice versa. This phenomenon was usually detected only in one direction in a particular serum sample, but in two of the sera there was a cross-reaction in both directions (Additional file 1, inhibition table of all patients).
As we analyzed the two different serum samples of the same patient, the presence of antibodies to homocitrulline-containing antigens was found to be quite a constant phenomenon, in line with that of ACPAs. Once anti-homocitrulline antibodies appear in the sera they remain detectable irrespective of the disease status. Between the two samples the specificities were mainly widening but some fluctuation was seen in a few patients. A weakness of our study is that all samples had been collected in early RA (within a year from diagnosis) and no definite conclusions about long term variation between antibody titer can be drawn. In any case, it is worth noticing that the level of antibodies against homocitrulline-containing antigens in the 38 hemodialysis patient sera was comparable with that of healthy controls (data not shown).
Shi and others have previously found 45% of RA patients to be positive for IgG antibodies that recognize carbamylated protein [24]. The antigen used in this study was carbamylated fetal calf serum, a mixture of several carbamylated proteins. In the same study [24] anti-carbamylated protein antibodies were found to be related to erosive disease in an anti-CCP negative subgroup of RA patients. In our study, in two of the 42 erosive RA patients, homocitrulline binding antibodies were detected alone. All other patients were also positive for citrulline binding antibodies and/or for RF. Recently, also, in a large cohort study [25] antibodies binding to carbamylated proteins were mainly detected in ACPA-positive RA patients. In the present study, we found that anti-MCV had the strongest association with the development of erosive disease, followed by CCP and RF. Our study agrees with the results obtained by Mathsson and coworkers that the anti-MCV test is better than the anti-CCP-test to predict erosive disease [26]. In our study, combining these two tests did not give any additional value for predicting erosions. The results are in line with those obtained by van der Linden et al. [27].
The structural homology of two amino acids is a challenge for the immune system and a predisposing factor for development of overlapping specificities. In our study, the homocitrulline-containing sequences have no match in the human body, but the results show us properties of the binding antibodies. It is most probable that the level of cross-reacting antibodies is dependent on sequence homology of the neighboring amino acids in the immunogen and by analyzing with unnatural sequences it is not possible to directly identify the real target of these antibodies.
Our study does not reveal the original immunogen for anti-citrulline or anti-homocitrulline antibodies. The homocitrulline-containing type I and II collagen carboxyterminal telopeptides used in this study cannot be original immunogens, because the sequences we used do not exist in the body. However, the fact that homocitrulline-binding antibodies mainly exist in individuals showing binding in anti-CCP and anti-MCV assays suggests that some relationship between anti-homocitrulline and anti-citrulline antibodies exist.
At least one situation is known where citrullination and carbamylation are present together. NETs contain both hypercitrullinated histones and myeloperoxidase. In addition, in smokers the thiocynate released from tobacco smoke favors carbamylation. Thus, NETs in the oral cavity or in synovial tissue may be the place for the coexistence citrullination and carbamylation where the antibodies with overlapping specificities could also have a role in the development of chronic inflammation.
Conclusions
Antibodies to both citrulline- and homocitrulline-containing sequences related to type I and II collagen telopeptides can be found in sera of RA patients. These antibodies are not constant from one RA patient to another, but contain separate or overlapping specificities within the same peptide sequence varying between individuals. Our results suggest a relationship between anti-citrulline and anti-homocitrulline antibodies, since anti-homocitrulline antibodies were found to exist mainly in individuals seropositive to anti-CCP and anti-MCV.
Acknowledgements
The authors gratefully acknowledge Risto Bloigu for his statistical expertise, the National Doctoral Programme of Musculoskeletal Disorders and Biomaterials for funding ST, and the Academy of Finland for supporting, in part, the research of JR and MKK.
Abbreviations
- ACPA
anti-citrullinated protein antibodies
- CCP
cyclic citrullinated protein
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
- MCV
mutated citrullinated vimentin
- NETs
neutrophil extracellular traps
- RA
rheumatoid arthritis
- RF
rheumatoid factor
Additional file
Patient data. This file contains RA patients’ primary Anti-CCP and Anti-MCV-ELISA results and inhibition results of the collagen telopeptide assays.
Footnotes
Competing interests
PH has received honoraria and consulting fees from Abbott, Astra-Zeneca, BMS, GSK, MSD Finland, Mundipharma, Pfizer, Roche and UCB Pharma. All other authors declare that they have no competing interests.
Authors’ contributions
ST, PH, MKK, LR and JR participated in the study design, analysis and interpretation of the data and revision of the manuscript. PH collected the samples and analyzed the clinical parameters. ST carried out the immunoassays and statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Sanna Turunen, Email: sanna.turunen@oulu.fi.
Pekka Hannonen, Email: pekka.hannonen@ksshp.fi.
Marja-Kaisa Koivula, Email: marja-kaisa.koivula@oulu.fi.
Leila Risteli, Email: leila.risteli@oulu.fi.
Juha Risteli, Email: juha.risteli@oulu.fi.
References
- 1.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 2.Koivula MK, Heliovaara M, Ramberg J, Knekt P, Rissanen H, Palosuo T, et al. Autoantibodies binding to citrullinated telopeptide of type II collagen and to cyclic citrullinated peptides predict synergistically the development of seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:1450–1455. doi: 10.1136/ard.2006.062919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Stadt LA, de Koning MH, van de Stadt RJ, Wolbink G, Dijkmans BA, Hamann D, et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63:3226–3233. doi: 10.1002/art.30537. [DOI] [PubMed] [Google Scholar]
- 4.Koivula MK, Aman S, Karjalainen A, Hakala M, Risteli J. Are there autoantibodies reacting against citrullinated peptides derived from type I and type II collagens in patients with rheumatoid arthritis? Ann Rheum Dis. 2005;64:1443–1450. doi: 10.1136/ard.2004.031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 6.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Willemze A, Janssen GM, van Veelen PA, Drijfhout JW, Cerami A, et al. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the ‘AMC-Senshu’ method. Ann Rheum Dis. 2013;72:148–150. doi: 10.1136/annrheumdis-2012-201559. [DOI] [PubMed] [Google Scholar]
- 10.Turunen S, Koivula MK, Melkko J, Alasaarela E, Lehenkari P, Risteli J. Different amounts of protein-bound citrulline and homocitrulline in foot joint tissues of a patient with anti-citrullinated protein antibody positive erosive rheumatoid arthritis. J Transl Med. 2013;11:224. doi: 10.1186/1479-5876-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res. 2009;44:664–672. doi: 10.1111/j.1600-0765.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 12.Nesse W, Westra J, van der Wal JE, Abbas F, Nicholas AP, Vissink A, et al. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J Clin Periodontol. 2012;39:599–607. doi: 10.1111/j.1600-051X.2012.01885.x. [DOI] [PubMed] [Google Scholar]
- 13.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69:1554–1561. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 15.Ioan-Facsinay A, El-Bannoudi H, Scherer HU, van der Woude D, Menard HA, Lora M, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70:188–193. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- 16.Suwannalai P, Scherer HU, van der Woude D, Ioan-Facsinay A, der Zijde CM, van Tol MJ, et al. Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens. Ann Rheum Dis. 2011;70:373–379. doi: 10.1136/ard.2010.135509. [DOI] [PubMed] [Google Scholar]
- 17.Turunen S, Koivula MK, Risteli L, Risteli J. Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits. Arthritis Rheum. 2010;62:3345–3352. doi: 10.1002/art.27644. [DOI] [PubMed] [Google Scholar]
- 18.Scinocca M, Bell DA, Racape M, Joseph R, Shaw G, McCormick JK, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol. 2014;41:270–279. doi: 10.3899/jrheum.130742. [DOI] [PubMed] [Google Scholar]
- 19.Hannonen P, Mottonen T, Hakola M, Oka M. Sulfasalazine in early rheumatoid arthritis. A 48-week double-blind, prospective, placebo-controlled study. Arthritis Rheum. 1993;36:1501–1509. doi: 10.1002/art.1780361104. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde DM, van Riel PL, Nuver-Zwart IH, Gribnau FW, vad de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989;1:1036–1038. doi: 10.1016/S0140-6736(89)92442-2. [DOI] [PubMed] [Google Scholar]
- 21.Koivula MK, Aman S, Alasaarela E, Karjalainen A, Hakala M, Risteli J. Inhibitory characteristics of citrullinated telopeptides of type I and II collagens for autoantibody binding in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45:1364–1369. doi: 10.1093/rheumatology/kel113. [DOI] [PubMed] [Google Scholar]
- 22.Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Toes RE, Trouw LA, et al. Anti carbamylated protein antibodies (anti-CarP) are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2012;65:911–915. doi: 10.1002/art.37830. [DOI] [PubMed] [Google Scholar]
- 23.van der Woude D, Syversen SW, van der Voort EI, Verpoort KN, Goll GL, van der Linden MP, et al. The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1110–1116. doi: 10.1136/ard.2009.116384. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Trouw LA, van Wesemael TJ, Shi J, Bengtsson C, Kallberg H, et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis. 2014;73:1761–1768. doi: 10.1136/annrheumdis-2013-205109. [DOI] [PubMed] [Google Scholar]
- 26.Mathsson L, Mullazehi M, Wick MC, Sjoberg O, van Vollenhoven R, Klareskog L, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36–45. doi: 10.1002/art.23188. [DOI] [PubMed] [Google Scholar]
- 27.van der Linden MP, van der Woude D, Ioan-Facsinay A, Levarht EW, Stoeken-Rijsbergen G, Huizinga TW, et al. Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum. 2009;60:2232–2241. doi: 10.1002/art.24716. [DOI] [PubMed] [Google Scholar]


