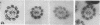Abstract
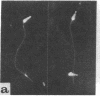
The location of dynein, the main flagellar ATPase, within the sea urchin sperm axoneme was investigated by the use of immunofluorescence and immunoelectron microscopy, employing an antiserum against a tryptic fragment of dynein 1 (Fragment 1A) purified from sea urchin sperm flagella. The axonemes were found to be stained with the antiserum when examined by an indirect immunofluorescence technique. Immunoelectron microscopy with the antiserum and a ferritin-conjugated IgG fraction of goat antiserum to rabbit IgG revealed that, among the structures within the axoneme, only the outer arms were labeled with ferritin particles. With either the normal serum or antiserum absorbed with Fragment 1A, there were no ferritin particles within the axonemes. When the outer arms were extracted with 0.5 M NaCl, leaving the inner arms intact, again no ferritin dots were detected. Furthermore, it was found that the outer arm on the no. 5 doublet microtubule, which connects with the extra arm projection backward from the no. 6 doublet, had no attached ferritin particles. From these observations, it can be concluded that the outer arm consists of dynein (at least dynein 1) and that Fragment 1A, containing the active site for ATPase activity of dynein 1, is located at the distal end of the outer arms. The significance of the present findings is considered in connection with flagellar movement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AFZELIUS B. Electron microscopy of the sperm tail; results obtained with a new fixative. J Biophys Biochem Cytol. 1959 Mar 25;5(2):269–278. doi: 10.1083/jcb.5.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C. J. Flagellar movement: a sliding filament model. Science. 1972 Nov 3;178(4060):455–462. doi: 10.1126/science.178.4060.455. [DOI] [PubMed] [Google Scholar]
- DAEMS W. T., PERSIJN J. P., TATES A. D. FINE-STRUCTURAL LOCALIZATION OF ATPASE ACTIVITY IN MATURE SPERM OF DROSOPHILA MELANOGASTER. Exp Cell Res. 1963 Oct;32:163–167. doi: 10.1016/0014-4827(63)90080-6. [DOI] [PubMed] [Google Scholar]
- GIBBONS I. R. STUDIES ON THE PROTEIN COMPONENTS OF CILIA FROM TETRAHYMENA PYRIFORMIS. Proc Natl Acad Sci U S A. 1963 Nov;50:1002–1010. doi: 10.1073/pnas.50.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. The effect of partial extraction of dynein arms on the movement of reactivated sea-urchin sperm. J Cell Sci. 1973 Sep;13(2):337–357. doi: 10.1242/jcs.13.2.337. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Ogawa K., Gibbons I. R. The effect of antidynein 1 serum on the movement of reactivated sea urchin sperm. J Cell Biol. 1976 Dec;71(3):823–831. doi: 10.1083/jcb.71.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid H. L., Jr, Gibbons B. H., Gibbons I. R. The salt-extractable fraction of dynein from sea urchin sperm flagella: an analysis by gel electrophoresis and by adenosine triphosphatase activity. J Supramol Struct. 1973;1(6):461–470. doi: 10.1002/jss.400010603. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Shimizu T. Electrophoretic studies on dyneins from Tetrahymena cilia. J Biochem. 1974 Nov;76(5):991–999. [PubMed] [Google Scholar]
- Ogawa K., Asai D. J., Brokaw C. J. Properties of an antiserum against native dynein 1 from sea urchin sperm flagella. J Cell Biol. 1977 Apr;73(1):182–192. doi: 10.1083/jcb.73.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Gibbons I. R. Dynein 2. A new adenosine triphosphatase from sea urchin sperm flagella. J Biol Chem. 1976 Sep 25;251(18):5793–5801. [PubMed] [Google Scholar]
- Ogawa K., Mori H. Preparation of antiserum against a tryptic fragment (fragment A) of dynein and an immunological approach to the subunit composition of dynein. J Biol Chem. 1975 Aug 25;250(16):6476–6483. [PubMed] [Google Scholar]
- Ogawa K. Studies on flagellar ATPase from sea urchin spermatozoa. II. Effect of trypsin digestion on the enzyme. Biochim Biophys Acta. 1973 Feb 15;293(2):514–525. doi: 10.1016/0005-2744(73)90358-6. [DOI] [PubMed] [Google Scholar]
- Ogawa K. Studies on flagellar ATPase from sea urchin spermatozoa. II. Effect of trypsin digestion on the enzyme. Biochim Biophys Acta. 1973 Feb 15;293(2):514–525. doi: 10.1016/0005-2744(73)90358-6. [DOI] [PubMed] [Google Scholar]
- Okuno M., Ogawa K., Mohri H. Inhibition of movement and ATP-ase activity of demembranated sea urchin spermatozoa by anti-dynein antiserum. Biochem Biophys Res Commun. 1976 Feb 9;68(3):901–906. doi: 10.1016/0006-291x(76)91230-4. [DOI] [PubMed] [Google Scholar]
- SRI RAM J., TAWDE S. S., PIERCE G. B., Jr, MIDGLEY A. R., Jr Preparation of antibody-ferritin conjugates for immunoelectron microscopy. J Cell Biol. 1963 Jun;17:673–675. doi: 10.1083/jcb.17.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T., Hasegawa S., Mohri H. The bound nucleotides of the isolated microtubules of sea-urchin sperm flagella and their possible role in flagellar movement. Exp Cell Res. 1968 Sep;52(1):86–100. doi: 10.1016/0014-4827(68)90549-1. [DOI] [PubMed] [Google Scholar]