Abstract
Hypoxia is the most important stimulus leading to upregulation of VEGF in the retina and this is caused by accumulation of hypoxia-inducible factors-1α (HIF-1α) protein. The effects of zeaxanthin, a natural phytochemical, on the VEGF and HIF-1α expression in the primary culture of human retinal pigment epithelial (RPE) cells were studied. An in vitro RPE cell hypoxia model was established by placing cells under 1% oxygen pressure or by adding cobalt chloride (CoCl2) to the culture medium. RPE cells and conditioned media were collected from cultures treated with and without zeaxanthin under normoxic and hypoxic conditions. VEGF and HIF-1α protein and RNA levels were measured by ELISA kits and RT-PCR, respectively. Hypoxia caused a significant increase of VEGF expression and accumulation of HIF-1α in RPE cells. Zeaxanthin at 50–150 μM significantly inhibited the expression of VEGF and accumulation of HIF-1α protein caused by hypoxia but did not affect expression of VEGF and HIF-1α under normoxic conditions. This is the first report on the effect of zeaxanthin on VEGF and HIF-1α levels in cultured RPE cells and suggests that zeaxanthin may have potential value in the prevention and treatment of various retinal diseases associated with vascular leakage and neovascularization.
1. Introduction
Vascular endothelial growth factor (VEGF) is a growth factor that stimulates the proliferation and migration of vascular endothelial cells and increases vascular permeability [1, 2]. VEGF is the most powerful angiogenesis promoter and plays a significant role in the pathogenesis of ocular neovascularization diseases, such as diabetic retinopathy and exudative type of age-related macular degeneration (AMD) [3–9].
The most important pathophysiological stimulus leading to high levels of VEGF expression in the retina is hypoxia [3, 4, 7, 9]. Hypoxia causes the increase of VEGF by the accumulation of a transcription factor, hypoxia-inducible factor-1α (HIF-1α), which promotes the production and secretion of VEGF by various cells including retinal pigment epithelial (RPE) cells [10–26]. Hypoxia is closely related to the development of neovascularization in the eye. Any drug that inhibits this process may have a therapeutic effect on various retinal diseases related to neovascularization.
Zeaxanthin, a natural phytochemical, is a carotenoid pigment of the xanthophyll subclass with a chemical formula C40H56O2. Zeaxanthin is present in various tissues and, in particular, is highly concentrated in the central retina (macula) of the eye [27–32]. Various observational and interventional studies have indicated that zeaxanthin might help reduce the risk of age-related macular degeneration (AMD) [33–41]. In vitro studies have demonstrated that zeaxanthin protected RPE cells and various retinal neurons against oxidative stress [42–46]. Zeaxanthin is an antioxidant and also works as a filter protecting the macula from the blue light [42–47]. Recent studies have shown that in addition to traditional mechanisms, zeaxanthin can influence the viability and function of cells through various signal pathways or transcription factors [46, 48].
It has been reported that zeaxanthin decreased the upregulation of VEGF in the retina in diabetic rats and in apoliprotein deficient mice [49, 50]. Lutein, a carotenoid with similar structure and function as zeaxanthin, decreases high VEGF expression of human RPE cells or mouse macrophages following tumor necrosis factor-α and lipopolysaccharide stimulations, respectively [51]. To the best of our knowledge, the effects of zeaxanthin on VEGF expression in cultured RPE cells have not been previously reported.
The purposes of the present study were to investigate the effects of zeaxanthin on the expression and secretion of VEGF by RPE cells under normoxic and hypoxic conditions and to explore the mechanism of action by measurement of HIF-1α levels in RPE cells under normoxia and hypoxia, with and without zeaxanthin.
2. Materials and Methods
2.1. Cell Culture
A culture of primary human RPE cells was isolated from a donor eye (56 years old) and cultured as previously described [52, 53]. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, GIBCO). Cells were incubated in a humidified 5% CO2 atmosphere at 37°C. After reaching confluence, cells were detached by trypsin-EDTA solution (GIBCO), diluted at 1 : 3-1 : 4, plated for subculture, and passaged routinely at a dilution of 1 : 3-1 : 4 every 5–7 d.
Phase-contrast microscopy revealed pigmentation of RPE cells during the primary culture and the first and second subcultures. Cells displayed characteristic epithelial morphology throughout the culture period. The purity of the cell lines was demonstrated by immunocytochemical methods as previously reported. RPE cells display positive staining of cytokeratin, whereas fibroblasts and melanocytes do not [54].
2.2. Effect of Hypoxia on Secretion of VEGF by RPE Cells
RPE cells were seeded into 24-well plates at a density of 1 × 105 cells per well. After 24 h, the culture medium was withdrawn. The cultures were washed with PBS twice and fresh culture medium was added. In the hypoxia experiment, cells were incubated in a sealed chamber at 37°C for 24 h in a controlled environment of 1% O2 in the presence of 5% CO2 and 94% N2 by using a PROOX 100 System (BioSherix, Redfield, NT). Cells cultured under standard conditions (21% O2, 5% CO2, and 74% N2) served as normoxia control cultures. Conditioned medium was collected 24 hours later and centrifuged at 800 ×g for 5 min, and the supernatants were transferred to vials and stored at −70°C until analysis. All experiments were performed in triplicate.
2.3. Effect of Chemical Hypoxia on Secretion of VEGF by RPE Cells
RPE cells were seeded into 24-well plates as described above. Culture medium was replaced 24 h after seeding and cobalt chloride (CoCl2) (Sigma, St. Louis, MO), an iron analogue, was added into the medium to mimic hypoxic conditions. The CoCl2 concentrations used in the literatures had a wide variation [55–57]. Therefore, the dose effects of CoCl2 were tested over a wide range of CoCl2 (at concentrations of 50, 100, 150, and 200 μM). Cells cultured without CoCl2 served as normal controls. Conditioned medium was collected 24 h later, centrifuged, and stored as described above. All experiments were performed in triplicate.
2.4. Effect of Zeaxanthin on Secretion of VEGF by RPE Cells under Normoxia
RPE cells were seeded into 24-well plates at a density of 1 × 105 cells per well. After 24 h, the culture medium was withdrawn. The cultures were washed with PBS twice and fresh culture medium was added. Zeaxanthin (ZeaVision LLC, Chesterfield, MO) 6.82 mg was dissolved in 200 μL DMSO to make a stock solution of 60 mM. Tested cells were treated by different concentrations of zeaxanthin. The cells in the control group were cultured in medium containing the same levels of DMSO as in the zeaxanthin solution. A separate investigation of the effects of the highest DMSO levels (1 : 400) used in this experiment did not show significant differences in the cell viability between the cells with and without DMSO (data not shown). Conditioned medium was collected 24 h later, centrifuged, and stored as described above. All experiments were performed in triplicate.
2.5. Effect of Zeaxanthin on the Secretion of VEGF by RPE Cells under Hypoxia
RPE cells were seeded into 24-well plates and the culture medium was replaced 24 h later as described above. Zeaxanthin was added to the medium at different concentrations. One hour later, cells were incubated in a controlled environment of 1% O2 as described above. Cells cultured under hypoxia without zeaxanthin were used as positive controls. Cells cultured under normoxic conditions were used as negative controls. Conditioned medium was collected 24 h later, centrifuged, and stored as described above. All experiments were performed in triplicate.
2.6. Effect of Zeaxanthin on the Secretion of VEGF by RPE Cells under Chemical Hypoxia
RPE cells were seeded into 24-well plates and the culture medium was replaced 24 h later as described above. CoCl2 was added to mimic hypoxic conditions. Cells cultured with CoCl2 but without zeaxanthin were used as positive controls. Cells cultured without CoCl2 were used as negative controls. Conditioned medium was collected 24 h later, centrifuged, and stored as described above. All experiments were performed in triplicate.
2.7. Effects of Hypoxia and Zeaxanthin on Cell Viability of RPE Cells
RPE cells were seeded into 96-well plates at a density of 5 × 103 cells per well. Cells were incubated under normoxic or hypoxic conditions (1% O2) or with added CoCl2 at various concentrations. In the study of effects of zeaxanthin on the cell viability, zeaxanthin at 1, 50, 100, and 150 μM was added into the culture medium under normoxic condition. After 24 h, MTT solution (1 mg/mL, 50 μL) was added. After 4 h incubation, the medium and MTT were aspirated and 100 μL of DMSO was added. Optical density of the plates was determined with a microplate reader (Multiskan MCC/340, Fisher Scientific, Pittsburgh, PA, USA) at 540 nm [58]. The optical density in control (normoxic) cells was taken as 100% viability. All tests were performed in three independent experiments.
2.8. Measurement of VEGF Levels
The amount of VEGF protein in the conditioned media was determined using the human VEGF Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Optical density was read using a microplate reader at 450 nm and corrected with 540 nm. The amount of VEGF (pg/mL) was calculated from a standard curve. The sensitivity of the VEGF kits was 5.0 pg/mL.
2.9. RNA Isolation and RT-PCR
RPE cells were seeded into 6-well plates and the culture medium was replaced 24 h later as described above. Zeaxanthin was added to the medium at 150 μM. One hour later, cells were incubated in a controlled environment of 1% O2 as described above. Cells cultured with 1% O2 but without zeaxanthin were used as positive controls. Cells cultured under normoxic conditions were used as negative controls. After 24 h, the culture medium was withdrawn, the cultures were washed with cold PBS, and cells were harvested. After microcentrifuging at 800 ×g for 5 min at 4°C, cell pellets were collected for mRNA extraction. Total RNA was isolated with the RNeasy mini kit (QIAGEN, Valencia, CA), according to the manufacturer's instructions. The SuperScript first-strand synthesis system for RT-PCR kit (Invitrogen, Camarillo, CA) was used to perform cDNA synthesis. The PCR primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were TGAACTGAAAGCTCTCCACC and CTGATGTACCAGTTGGGGAA. VEGF primers were AGGGCAGAACATCACGAAGT and ACGGTCTCGATTGGATGGCA. HIF-1 primers were GAACGTCGAAAAGAAAAGTCTCG and CCTTATCAAGATGCGAACTCACA. All primers were obtained from Invitrogen. The first-strand cDNAs were synthesized from 1.0 μg of total RNA at 50°C for 50 min. PCR amplification was conducted in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) using the following parameters: first denaturation at 94°C for 5 min followed by 35 cycles of reactions of denaturation at 94°C for 30 s, annealing at 58°C for 45 s, and extension at 72°C for 45 s and last extension for 5 min at 72°C. After amplification, samples were run on a 1% agarose gel (Invitrogen) in TBE (0.01 M Tris-borate) and 0.001 M EDTA (Invitrogen) containing 2.0 μg/mL ethidium bromide (Invitrogen). Bands were visualized and photographed on a UV transilluminator (ChemiDoc XRS System, Bio-Rad, Hercules, CA, USA).
2.10. Effect of Hypoxia and Zeaxanthin on HIF-1α Protein Levels in RPE Cells
RPE cells were seeded into 6 cm culture dishes at a density of 2 × 106 cells per well. After 24 h, the culture medium was replaced as described above. One hour later, cells were incubated in a controlled environment of 1% O2 or with added CoCl2 at various concentrations. Cells cultured under normoxic condition and without CoCl2 were served as normal controls. After 24 h, cells were collected as described above and treated with cell lysis buffer (SIGMA) and centrifuged at 2000 ×g for 5 min and the supernatant was collected. Protein levels were measured with BCA Protein Assay Kit (Thermo Scientific, Rockfield, IL). In zeaxanthin studies, zeaxanthin at different concentrations was added to the culture medium; 1 h later, cells were incubated under 1% O2 or added with CoCl2 at 150 μM. Cells cultured under normoxic condition and without CoCl2 served as negative controls. Cells cultured under O2 or with CoCl2 but without zeaxanthin served as positive controls.
2.11. Measurement of HIF-1α Levels in the Cell Extracts
The amount of HIF-1α protein in the cell extracts was determined using the Human/Mouse Intracellular HIF-1α DuoSet IC ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Optical density was read by using a microplate reader at 450 nm and corrected with 540 nm. The amounts of HIF-1α (pg/mL) were calculated from a standard curve and expressed as pg/mg protein.
2.12. Statistical Analysis
Statistical significances of the difference of means throughout this study were calculated using the ANOVA one-way test for comparing data from more than two groups and Student's t-test for comparing data between two groups. A difference at P < 0.05 was considered to be statistically significant.
3. Results
3.1. Effects of Hypoxia and Zeaxanthin on Cell Viability of RPE Cells
RPE cells cultured at 1% O2 or with CoCl2 at 50–200 μM did not significantly affect the cell viability (P > 0.05). Zeaxanthin at 50–150 μM also did not significantly affect RPE cell viability (P > 0.05) (Figure 1).
Figure 1.
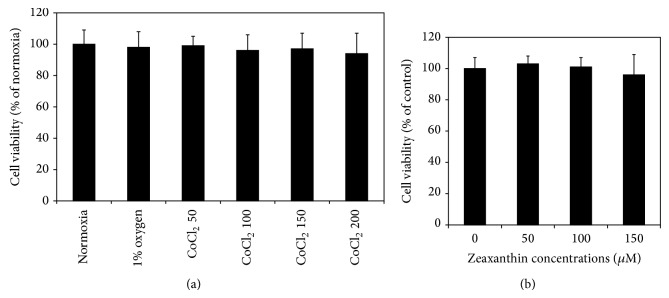
Effects of hypoxia and zeaxanthin on cell viability of retinal pigment epithelial (RPE) cells. RPE cells were seeded into 96-well plates at a density of 5 × 103 cells per well. Cells were incubated under normoxic or hypoxic conditions (1% O2) or cultured with CoCl2 at 50, 100, 150, and 200μM (a). Zeaxanthin at 1, 50, 100, and 150μM was added into the culture medium under normoxic condition (b). After 24 h, cell viability was determined by MTT assay (see Section 2). Hypoxia and zeaxanthin at test concentrations did not affect the cell viability (P > 0.05). Data are expressed as the percentage of optical readings in normoxia (mean ± SD, n = 3).
3.2. Secretion of VEGF by RPE Cells under Normoxia and Hypoxia
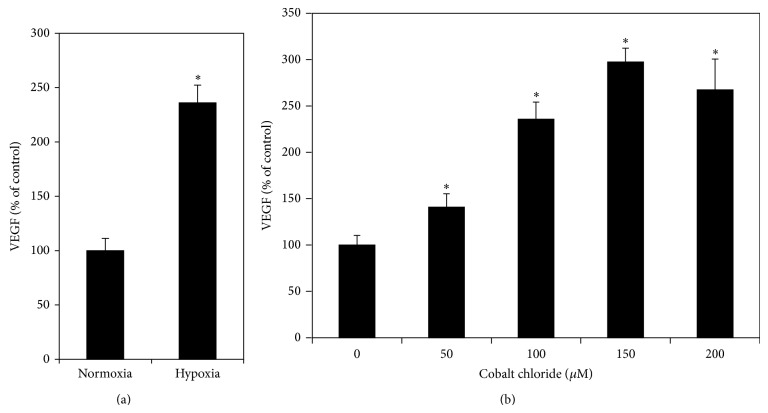
RPE cells had a relatively high constitutive secretion of VEGF. VEGF levels in conditioned culture medium of RPE cells cultured under normoxia were 451 ± 50.8 pg/mL (mean ± SD). Cells cultured under hypoxia (1% O2) significantly increased the VEGF levels in the culture medium 2.36-fold over cells cultured under normoxia (P < 0.05) (Figure 2(a)). CoCl2 at concentrations from 50 to 200 μM caused a dose-dependent increase of VEGF levels (all CoCl2 treated cultures compared to the control, P < 0.05; 50 μM compared to 100 μM and 100 μM compared to 150 μM, P < 0.05; 150 μM compared with 200 μM, P > 0.05) (Figure 2(b)). CoCl2 stimulated effects on secretion of VEGF reached the peak at 150 μM, which induced a 2.97-fold increase in secretion of VEGF as compared with cells cultured under normoxia. Therefore, 150 μM CoCl2 was selected as the concentration of CoCl2 used in the following experiments.
Figure 2.
Effects of hypoxia on secretion of VEGF by RPE cells. RPE cells were seeded into 24-well plates at a density of 1 × 105 cells per well. After 24 h, cells were incubated in a sealed chamber in a controlled environment of 1% O2 (a) or cultured with CoCl2 at 50, 100, 150, and 200μM concentrations (b). Cells cultured under normal oxygen pressure (21% O2, 5% CO2, and 74% N2) were served as the control. Conditioned medium was collected 24 h later; VEGF levels were measured by VEGF ELISA kits and expressed as percentages of the control (mean ± SD, n = 3). Hypoxia significantly increased VEGF secretion by RPE cells. * P < 0.05, compared with the controls.
3.3. Effect of Zeaxanthin on Secretion of VEGF by RPE Cells under Normoxia and Hypoxia
Under normoxic condition, zeaxanthin at 50–100 μM did not influence the secretion of VEGF by RPE cells (P > 0.05) (Figure 3). Cells cultured with zeaxanthin at 150 μM showed a slight decrease of VEGF secretion as compared with cells not cultured with zeaxanthin; however, this difference was not statistically significant (P > 0.05).
Figure 3.
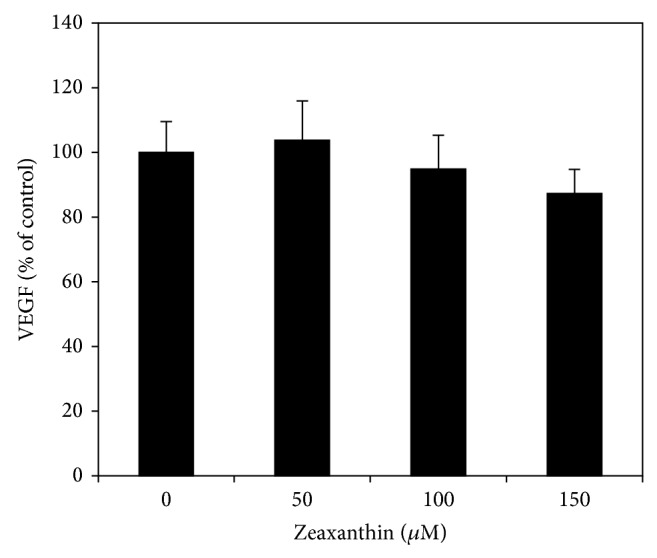
Effect of zeaxanthin on secretion of VEGF by RPE cells under normoxia. RPE cells were seeded into 24-well plates at a density of 1 × 105 cells per well. After 24 h, zeaxanthin at 50, 100, and 150μM was added. Conditioned medium was collected 24 h later; VEGF levels were measured by VEGF ELISA kits and expressed as percentages of the control (mean ± SD, n = 3). Zeaxanthin did not significantly affect the secretion of VEGF in cells cultured under normoxia (P > 0.05).
Under hypoxic condition (1% O2), zeaxanthin at 50–150 μM dose-dependently decreased the secretion of VEGF as compared with cells cultured under hypoxia but without zeaxanthin (compared to hypoxia without zeaxanthin, P > 0.05 at 50 μM and P < 0.05 at 100–150 μM) (50 μM compared to 100 μM, P < 0.05; 100 μM compared to 150 μM, P > 0.05) (Figure 4(a)). Secretion of VEGF in cells cultured with zeaxanthin at 150 μM decreased to levels near those of cells cultured under normoxia (P > 0.05), indicating that zeaxanthin at 150 μM completely blocked hypoxia-induced secretion of VEGF by RPE cells.
Figure 4.
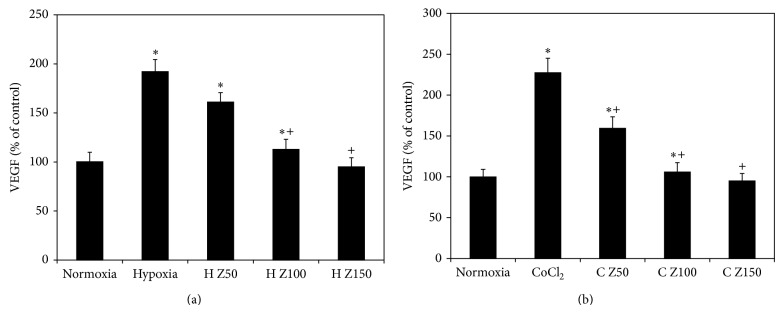
Effects of zeaxanthin on secretion of VEGF by RPE cells under hypoxia. RPE cells were seeded into 24-well plates at a density of 1 × 105 cells per well. After 24 h, zeaxanthin was added at 50, 100, and 150μM concentrations. One hour later, cells were incubated under 1% O2 (a) or cultured with CoCl2 at 150μM (b). Cells cultured under normoxia and without zeaxanthin were served as negative control. Cells cultured under hypoxia and without zeaxanthin were served as positive control. Conditioned medium was collected 24 hours later; VEGF levels were measured by VEGF ELISA kits and expressed as percentages of the negative control (mean ± SD, n = 3). Zeaxanthin significantly inhibited hypoxia-induced secretion of VEGF. * P < 0.05, compared with the negative controls. + P < 0.05, compared with the positive controls.
In the chemical hypoxic condition (150 μM CoCl2), zeaxanthin at 50–150 μM also dose-dependently decreased the secretion of VEGF as compared to cells cultured under chemical hypoxia but without zeaxanthin (P < 0.05 at all concentrations of zeaxanthin) (50 μM compared to 100 μM and 100 μM compared to 150 μM, P < 0.05) (Figure 4(b)). Zeaxanthin at the highest tested concentrations also completely blocked CoCl2-induced secretion of VEGF by RPE cells (P > 0.05, as compared with cells cultured without zeaxanthin).
3.4. Effects of Hypoxia and Zeaxanthin on Expression of VEGF mRNA
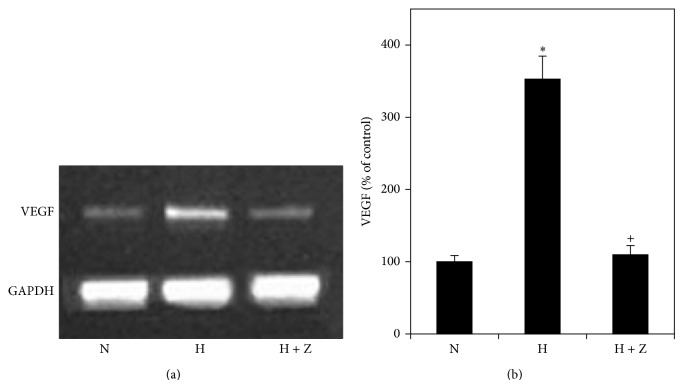
Expression of VEGF mRNA in RPE cells increased significantly in cells cultured with 1% O2 for 24 h (P < 0.05) (Figure 5). Adding of zeaxanthin at 150 μM significantly decreased the expression of VEGF mRNA (P < 0.05) (Figure 5), indicating that zeaxanthin blocked hypoxia-induced expression of VEGF mRNA.
Figure 5.
Effects of hypoxia and zeaxanthin on VEGF mRNA expression of RPE cells. (a) Representative RT-PCR profiles from three experiments. Cells were cultured under hypoxia (1% oxygen) with (H + Z) and without zeaxanthin (H). Cells cultured under normal oxygen condition were used as the control (N). Cells were collected 24 hours later, mRNA was extracted, and RT-PCR analysis was performed as described in Section 2. GAPDH was used as an internal loading control. (b) Quantitative analysis showed that the expression of VEGF mRNA (mean ± SD, n = 3) by cells exposed to hypoxia (1% oxygen) was significantly increased (H) and zeaxanthin significantly inhibited hypoxia-induced expression of VEGF (H + Z). * P < 0.05, compared with the controls.
3.5. Effects of Hypoxia on Intracellular HIF-1α Protein Levels in RPE Cells
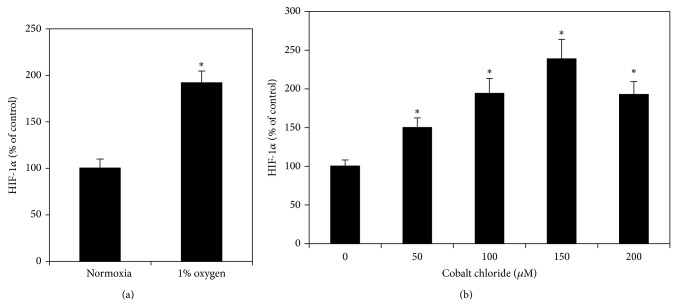
RPE cells cultured under hypoxia (1% O2) produced significantly increased levels of HIF-1α protein to 1.92-fold of cells cultured under normoxia (P < 0.05) (Figure 6(a)). CoCl2 at all concentrations from 50 to 200 μM caused a dose-dependent increase of HIF-1α protein levels in RPE cells (P < 0.05) (all the CoCl2 treated group at different dosages compared to the next group, P < 0.05) (Figure 6(b)). CoCl2 stimulated effects on HIF-1α levels reached the peak at 150 μM, which induced a 2.39-fold increase in HIF-1α levels as compared with cells cultured without CoCl2. Therefore, 150 μM CoCl2 was selected as the concentration of CoCl2 used in the following experiments.
Figure 6.
Effect of hypoxia on HIF-1α protein levels in RPE cells. RPE cells were seeded into 6 cm culture dishes at a density of 2 × 106 cells per well. After 24 h, cells were incubated in a controlled environment of 1% O2 (a) or added with CoCl2 (b) at 50, 100, 150, and 200μM. Cells cultured under normoxic condition and without CoCl2 served as normal controls. Cells were collected 24 h later; HIF-1α protein levels in cell extracts were determined by using the Intracellular HIF-1α ELISA kits and expressed as percentages of the control (mean ± SD, n = 3). Hypoxia significantly increased HIF-1α protein levels in RPE cells. * P < 0.05, compared with the controls.
3.6. Effects of Zeaxanthin on Intracellular HIF-1α Protein Levels in RPE Cells
In cells cultured with 1% O2, zeaxanthin at 50–150 μM caused a dose-dependent decrease of HIF-1α levels as compared with cells cultured under 1% O2 but without zeaxanthin (P < 0.05 at all concentrations of zeaxanthin) (50 μM compared to 100 μM, P < 0.05; 100 μM compared to 150 μM, P > 0.05) (Figure 7(a)). HIF-1α levels in cells cultured with 1% O2 and zeaxanthin at 100–150 μM showed no difference from cells cultured under normoxia (P > 0.05), indicating that zeaxanthin at 100–150 μM could completely block hypoxia-induced intracellular accumulation of HIF-1α protein.
Figure 7.
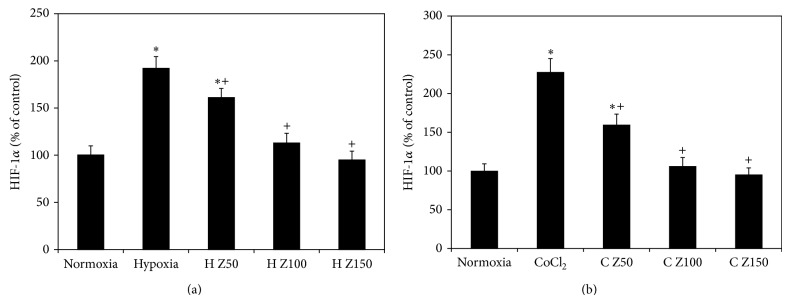
Effect of zeaxanthin on HIF-1α protein levels in RPE cells under hypoxia. RPE cells were seeded into 6 cm culture dishes at a density of 2 × 106 cells per well. After 24 h, zeaxanthin was added at 50, 100, and 150μM concentrations. One hour later, cells were incubated under 1% O2 (a) or cultured with CoCl2 at 150μM (b). Cells cultured under normoxic condition and without zeaxanthin served as negative controls. Cells cultured under hypoxia and without zeaxanthin served as positive control. Cells were collected 24 h later; HIF-1α protein levels in cell extracts were determined by using the Intracellular HIF-1α ELISA kits and expressed as percentages of negative control (mean ± SD, n = 3). Zeaxanthin significantly inhibited hypoxia-induced accumulation of HIF-1α protein. * P < 0.05, compared with the negative controls. + P < 0.05, compared with the positive controls.
In cells cultured with CoCl2, zeaxanthin at 50–150 μM also caused a dose-dependent decrease of HIF-1α (P < 0.05 at all concentrations of zeaxanthin compared with cells cultured with CoCl2 but no zeaxanthin) (50 μM compared to 100 μM, P < 0.05; 100 μM compared to 150 μM, P > 0.05) (Figure 7(b)). Zeaxanthin at 100–150 μM completely blocked CoCl2-induced accumulation of HIF-1α protein (P > 0.05, as compared with cells cultured without CoCl2).
3.7. Effects of Hypoxia and Zeaxanthin on Expression of HIF-1α mRNA
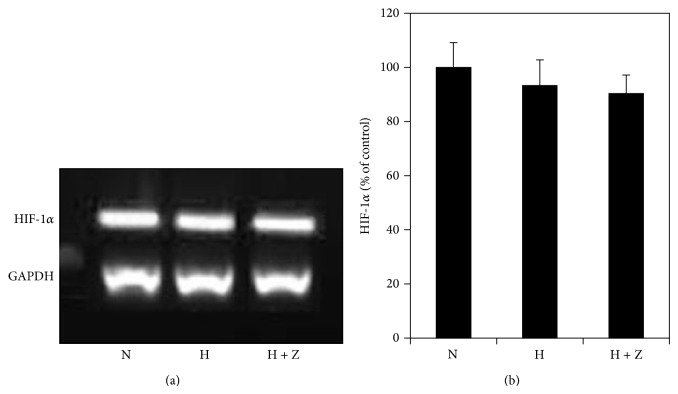
Cells cultured under 1% O2 with and without zeaxanthin did not show any significant effect on the expression of HIF-1α mRNA (P > 0.05) (Figure 8). This indicates that both hypoxia and zeaxanthin have no effect on the expression of HIF-1α. Their effects on the HIF-1α protein levels are mainly via the stabilization and accumulation process of HIF-1α protein. This is consistent with previous reports [17, 18].
Figure 8.
Effects of hypoxia and zeaxanthin on HIF-1α mRNA expression of RPE cells. (a) Representative RT-PCR profiles from three experiments. Cells were cultured under hypoxia (1% oxygen) with (H + Z) and without zeaxanthin (H). Cells cultured under normal oxygen condition were used as the control (N). Cells were collected 24 hours later, mRNA was extracted, and RT-PCR analysis was performed as described in Section 2. GAPDH was used as an internal loading control. (b) Quantitative analysis showed that the expression of HIF-1α mRNA (mean ± SD, n = 3) was not affected by being exposed to hypoxia or zeaxanthin (P > 0.05).
4. Discussion
In vivo and in vitro studies have indicated that the most important pathophysiological stimulus leading to high expression of VEGF in the retina is hypoxia [3, 4, 7, 9]. Hypoxia causes the increase of VEGF by RPE cells mainly through the stabilization of HIF-1α protein [17–22].
HIF-1 is a transcription factor for cellular and tissue adaptation to low oxygen tension and is the most important factor promoting angiogenesis via upregulation of VEGF under hypoxia [23–26]. HIF-1 is a heterodimeric factor consisting of an inducible oxygen-sensitive alpha subunit (HIF-1α) and a constitutive oxygen-insensitive beta subunit (HIF-1β/ARNT). The expression of HIF-1β is not affected by changes of oxygen pressure. Cells continuously synthesize and degrade HIF-1α protein. The protein level of HIF-1α is tightly regulated by cellular oxygen concentration [23–26]. Under normoxic conditions, HIF-α subunits have a very short half-life; the proline residues in the oxygen-dependent degradation domain of HIF-1α are hydroxylated by prolyl hydroxylase. Subsequently, the hydroxylated HIF-1α is recognized by the Von Hippel-Lindau tumor suppressor protein, leading to ubiquitination and degradation of HIF-1α and thereby abolishing HIF-1α protein accumulation. Under hypoxia, the hydroxylation of HIF-1α is impaired, which enhances stabilization and accumulation of HIF-1α protein. Upon HIF-1α protein accumulation, it translocates to the nucleus, stimulates expression of VEGF gene, and leads to angiogenesis [23–26].
In this in vitro study, the hypoxic condition was induced by changing the oxygen pressure in the incubator or by adding CoCl2 [10–22, 55–57]. Under normoxia, HIF-1α is degraded by the hydroxylases. The ferrous ion bound at the active sites is essential for the activity of hydroxylases. Cobalt displaces the single free ferrous at the active site and thus deactivates the hydroxylases [59]. Therefore, cobalt is able to stabilize HIF-1α protein and it has been used widely in experiments for producing chemical hypoxia [55–57, 60].
It has been reported that hypoxia-induced expression of VEGF mRNA and secretion of VEGF by RPE cells could be produced by culturing the cells under low oxygen environment (usually 1% oxygen) [10–16] or by adding CoCl2 (100–200 μM) into the culture medium [55–57]. Hypoxia induces accumulation of HIF-1α protein levels in RPE cells by culturing the cells in hypoxia [17–22] or by using CoCl2 [55, 60].
In the present study, hypoxia significantly induced expression of VEGF and accumulation of HIF-1α protein in cultured RPE cells. These results are consistent with the previous reports [10–22, 55–57].
Zeaxanthin is a carotenoid pigment and belongs to the xanthophyll subclass with a chemical formula C40H56O2. It is found at high levels in various foods (e.g., egg yolk, corn, and many vegetables and fruits). Zeaxanthin is present in the tissues and is highly concentrated in the retina, especially in the macula [27–32].
Epidemiologic studies suggest that insufficient dietary lutein and zeaxanthin intake and lower levels of lutein and zeaxanthin in the retina or serum may be associated with increased risk for AMD [33–36]. Several but not all supplementation studies or clinical trials have shown that supplementation of zeaxanthin with other antioxidants may have a favorable effect on the prevention and treatment of AMD [37–40]. Recently, a meta-analysis study on 6 longitudinal cohort studies indicated that dietary intake of lutein and zeaxanthin was significantly related to a reduction of risk for late AMD but not for early AMD [41].
One in vitro study showed that zeaxanthin significantly reduced lipofuscin formation in photoreceptor outer segment-fed RPE cells [45]. Zeaxanthin reduces photooxidative damage and decreases upregulation of expression of IL-8 (a proinflammation and angiogenic chemokine) in RPE cells caused by A2E and blue light irradiation [43]. Zeaxanthin protects cultured photoreceptors against oxidative stress caused by H2O2 or paraquat [46].
In an experimental animal study, zeaxanthin combined with other antioxidants increased retinal antioxidants activity and slowed down the photoreceptor degeneration in a retinitis pigmentosa model (rd1 mouse) [61].
Zeaxanthin protects the retina against oxidative stresses by two mechanisms: it acts as an antioxidant or as a blue light filter [47]. In addition to these two traditional mechanisms, recent studies found that zeaxanthin and its closely related molecule (lutein) may affect the growth, viability, and functions of various cell types via different signal pathways, transcription factors, growth factors, and cytokines [46, 48].
Experimental animal studies showed that zeaxanthin could decrease the upregulation of VEGF in the retinal-choroid tissues in apolipoprotein deficient mice [50] and prevent diabetes-induced increase of retinal VEGF in diabetic rats [49]. Lutein has been reported to decrease high VEGF expression following tumor necrosis factor-α stimulation of human RPE cells and inhibits lipopolysaccharide-induced VEGF expression in mouse macrophages [51]. A preliminary report suggested that adding oral zeaxanthin treatment (20 mg/day) to an aggressive treatment regimen (bevacizumab, steroid, and PDT therapy) for choroidal neovascularization (CNV) improved therapeutic efficiency and reduced the number of PDT therapies. The progression to CNV in the fellow eye in zeaxanthin treated patients was reduced to 50% of patients without zeaxanthin [62]. However, the effects of zeaxanthin on VEGF expression in cultured RPE cells have not been previously reported.
In the present study, zeaxanthin did not cause a significant change in constitutive VEGF secretion by cultured human RPE cells. Zeaxanthin significantly reduced hypoxia-induced expression of VEGF mRNA and secretion of VEGF by RPE cells in a dose-dependent manner. Zeaxanthin at a higher concentration could completely block hypoxia-induced expression and secretion of VEGF by RPE cells. Zeaxanthin also inhibited hypoxia-induced intracellular accumulation of HIF-1α, which is the main transcription factor involved in hypoxia-induced expression of VEGF.
5. Conclusions
In the present study, zeaxanthin blocked hypoxia-induced VEGF secretion in cultured RPE cells but not constitutive secretion of VEGF. Therefore, the possible detrimental effects caused by complete local blocking of VEGF might be avoided through the use of high-dose zeaxanthin supplementation [63–66]. Additionally, zeaxanthin, by inhibition of hypoxia-induced accumulation of HIF-α1 protein, may have a broader effect on the control of angiogenesis caused by factors other than VEGF [26, 67]. Zeaxanthin taken orally could be used as an adjunct to intravitreal anti-VEGF therapy enabling a decrease in the frequency of injections with reduced risk of local side effects [68–70]. Therefore, zeaxanthin might be a promising agent to be explored for the prevention and treatment for a variety of retinal diseases associated with revascularization.
Acknowledgments
The authors thank Dr. Dennis L. Gierhart for his helpful comments and providing pure zeaxanthin. This work was supported by Bendheim Family Foundation, the Pathology Research Fund of New York Eye and Ear Infirmary, and an unrestricted grant from the Dennis L. Gierhart Charitable Gift Fund.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Loureiro R. M. B., D'Amore P. A. Transcriptional regulation of vascular endothelial growth factor in cancer. Cytokine & Growth Factor Reviews. 2005;16(1):77–89. doi: 10.1016/j.cytogfr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Xie K., Wei D., Shi Q., Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine and Growth Factor Reviews. 2004;15(5):297–324. doi: 10.1016/j.cytogfr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Pournaras C. J., Miller J. W., Gragoudas E. S., et al. Systemic hyperoxia decreases vascular endothelial growth factor gene expression in ischemic primate retina. Archives of Ophthalmology. 1997;115(12):1553–1558. doi: 10.1001/archopht.1997.01100160723009. [DOI] [PubMed] [Google Scholar]
- 4.Witmer A. N., Vrensen G. F. J. M., Van Noorden C. J. F., Schlingemann R. O. Vascular endothelial growth factors and angiogenesis in eye disease. Progress in Retinal and Eye Research. 2003;22(1):1–29. doi: 10.1016/S1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 5.Arjamaa O., Nikinmaa M., Salminen A., Kaarniranta K. Regulatory role of HIF-1α in the pathogenesis of age-related macular degeneration (AMD) Ageing Research Reviews. 2009;8(4):349–358. doi: 10.1016/j.arr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Penn J. S., Madan A., Caldwell R. B., Bartoli M., Caldwell R. W., Hartnett M. E. Vascular endothelial growth factor in eye disease. Progress in Retinal and Eye Research. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller J. W., Adamis A. P., Shima D. T., et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. The American Journal of Pathology. 1994;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto N., Tobe T., Hackett S. F., et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. The American Journal of Pathology. 1997;151(1):281–291. [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce I. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. H. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Geisen P., Wittchen E. S., et al. The role of RPE cell-associated VEGF189 in choroidal endothelial cell transmigration across the RPE. Investigative Ophthalmology and Visual Science. 2011;52(1):570–578. doi: 10.1167/iovs.10-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bento C. F., Fernandes R., Matafome P., Sena C., Seia R., Pereira P. Methylglyoxal-induced imbalance in the ratio of vascular endothelial growth factor to angiopoietin 2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Experimental Physiology. 2010;95(9):955–970. doi: 10.1113/expphysiol.2010.053561. [DOI] [PubMed] [Google Scholar]
- 12.Wu W.-C., Kao Y.-H., Hu P.-S., Chen J.-H. Geldanamycin, a HSP90 inhibitor, attenuates the hypoxia-induced vascular endothelial growth factor expression in retinal pigment epithelium cells in vitro. Experimental Eye Research. 2007;85(5):721–731. doi: 10.1016/j.exer.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Geisen P., McColm J. R., King B. M., Hartnett M. E. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Current Eye Research. 2006;31(9):739–748. doi: 10.1080/02713680600837408. [DOI] [PubMed] [Google Scholar]
- 14.Ghiso N., Rohan R. M., Amano S., Garland R., Adamis A. P. Suppression of hypoxia-associated vascular endothelial growth factor gene expression by nitric oxide via cGMP. Investigative Ophthalmology & Visual Science. 1999;40(6):1033–1039. [PubMed] [Google Scholar]
- 15.Blaauwgeers H. G. T., Holtkamp G. M., Rutten H., et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris: evidence for a trophic paracrine relation. The American Journal of Pathology. 1999;155(2):421–428. doi: 10.1016/s0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullberg K. B., Olholm J., Paulsen S. K., et al. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro . European Journal of Pharmaceutical Sciences. 2013;49(2):251–257. doi: 10.1016/j.ejps.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Forooghian F., Razavi R., Timms L. Hypoxia-inducible factor expression in human RPE cells. British Journal of Ophthalmology. 2007;91(10):1406–1410. doi: 10.1136/bjo.2007.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C. H., Li C. H., Liao P. L., et al. Silibinin inhibits VEGF secretion and age-related macular degeneration in a hypoxia-dependent manner through the PI-3 kinase/Akt/mTOR pathway. British Journal of Pharmacology. 2013;168(4):920–931. doi: 10.1111/j.1476-5381.2012.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P., Zhang X., Hao X., et al. Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefe's Archive for Clinical and Experimental Ophthalmology. 2009;247(5):633–639. doi: 10.1007/s00417-008-1031-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J., Wang Y.-S., Zhang J., et al. Focal adhesion kinase signaling pathway participates in the formation of choroidal neovascularization and regulates the proliferation and migration of choroidal microvascular endothelial cells by acting through HIF-1 and VEGF expression in RPE cells. Experimental Eye Research. 2009;88(5):910–918. doi: 10.1016/j.exer.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Xiao Q., Zeng S., Lv M., Ling S. Small hairpin loop RNA targeting HIF-1α down-regulates VEGF and up-regulates PEDF in human retinal pigment epithelial cells under hypoxic condition. Journal of Huazhong University of Science and Technology—Medical Science. 2008;28(4):460–464. doi: 10.1007/s11596-008-0419-8. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Q., Zeng S., Ling S., Lv M. Up-regulation of HIF-1α and VEGF expression by elevated glucose concentration and hypoxia in cultured human retinal pigment epithelial cells. Journal of Huazhong University of Science and Technology. 2006;26(4):463–465. doi: 10.1007/s11596-006-0422-x. [DOI] [PubMed] [Google Scholar]
- 23.Chen M.-C., Lee C.-F., Huang W.-H., Chou T.-C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells. Biochemical Pharmacology. 2013;85(9):1278–1287. doi: 10.1016/j.bcp.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Imtiyaz H. Z., Simon M. C. Hypoxia-inducible factors as essential regulators of inflammation. Current Topics in Microbiology and Immunology. 2010;345(1):105–120. doi: 10.1007/82-2010-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brihimi-Horn M. C., Pouysségur J. HIF at a glance. Journal of Cell Science. 2009;122(8):1055–1057. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- 26.Weidemann A., Johnson R. S. Biology of HIF-1α . Cell Death & Differentiation. 2008;15(4):621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 27.Sommerburg O., Keunen J. E. E., Bird A. C., van Kuijk F. J. G. M. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. British Journal of Ophthalmology. 1998;82(8):907–910. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vishwanathan R., Neuringer M., Max Snodderly D., Schalch W., Johnson E. J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutritional Neuroscience. 2013;16(1):21–29. doi: 10.1179/1476830512Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson E. J., Neuringer M., Russell R. M., Schalch W., Snodderly D. M. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investigative Ophthalmology and Visual Science. 2005;46(2):692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein P. S., Khachik F., Carvalho L. S., Muir G. J., Zhao D.-Y., Katz N. B. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Experimental Eye Research. 2001;72(3):215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 31.Snodderly D. M., Handelman G. J., Adler A. J. Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys. Investigative Ophthalmology & Visual Science. 1991;32(2):268–279. [PubMed] [Google Scholar]
- 32.Ahmed S. S., Lott M. N., Marcus D. M. The macular xanthophylls. Survey of Ophthalmology. 2005;50(2):183–193. doi: 10.1016/j.survophthal.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Seddon J. M., Ajani U. A., Sperduto R. D., et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. The Journal of the American Medical Association. 1994;272(18):1413–1420. doi: 10.1001/jama.272.18.1413. [DOI] [PubMed] [Google Scholar]
- 34.Tan J. S. L., Wang J. J., Flood V., Rochtchina E., Smith W., Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115(2):334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 35.SanGiovanni J. P., Chew E. Y., Clemons T. E., et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report No. 22. Archives of Ophthalmology. 2007;125(9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 36.Michikawa T., Ishida S., Nishiwaki Y., et al. Serum antioxidants and age-related macular degeneration among older Japanese. Asia Pacific Journal of Clinical Nutrition. 2009;18(1):1–7. [PubMed] [Google Scholar]
- 37.Beatty S., Chakravarthy U., Nolan J. M., et al. Secondary outcomes in a clinical trial of Carotenoids with Coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120(3):600–606. doi: 10.1016/j.ophtha.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 38.Ho L., van Leeuwen R., Witteman J. C. M., et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and ω-3 fatty acids: the Rotterdam Study. Archives of Ophthalmology. 2011;129(6):758–766. doi: 10.1001/archophthalmol.2011.141. [DOI] [PubMed] [Google Scholar]
- 39.Barker F. M., II Dietary supplementation: effects on visual performance and occurrence of AMD and cataracts. Current Medical Research and Opinion. 2010;26(8):2011–2023. doi: 10.1185/03007995.2010.494549. [DOI] [PubMed] [Google Scholar]
- 40.The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. The Journal of the American Medical Association. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 41.Ma L., Dou H.-L., Wu Y.-Q., et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. British Journal of Nutrition. 2012;107(3):350–359. doi: 10.1017/s0007114511004260. [DOI] [PubMed] [Google Scholar]
- 42.Bian Q., Gao S., Zhou J., et al. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radical Biology and Medicine. 2012;53(6):1298–1307. doi: 10.1016/j.freeradbiomed.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhosale P., Serban B., Da Y. Z., Bernstein P. S. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica . Biochemistry. 2007;46(31):9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S. R., Nakanishi K., Itagaki Y., Sparrow J. R. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Experimental Eye Research. 2006;82(5):828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson S. E. G., Sundelin S. P., Wihlmark U., Brunk U. T. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Documenta Ophthalmologica. 2003;106(1):13–16. doi: 10.1023/a:1022419606629. [DOI] [PubMed] [Google Scholar]
- 46.Chucair A. J., Rotstein N. P., SanGiovanni J. P., During A., Chew E. Y., Politi L. E. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Investigative Ophthalmology and Visual Science. 2007;48(11):5168–5177. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]
- 47.Loane E., Nolan J. M., O'Donovan O., Bhosale P., Bernstein P. S., Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Survey of Ophthalmology. 2008;53(1):68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T., Shnimizu M., Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17(3):3202–3242. doi: 10.3390/molecules17033202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowluru R. A., Menon B., Gierhart D. L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investigative Ophthalmology & Visual Science. 2008;49(4):1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 50.Fernández-Robredo P., Recalde S., Arnáiz G., et al. Effect of zeaxanthin and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression in apolipoprotein-e deficient mice. Current Eye Research. 2009;34(7):543–552. doi: 10.1080/02713680902963142. [DOI] [PubMed] [Google Scholar]
- 51.Kijlstra A., Tian Y., Kelly E. R., Berendschot T. T. J. M. Lutein: More than just a filter for blue light. Progress in Retinal and Eye Research. 2012;31(4):303–315. doi: 10.1016/j.preteyeres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Hu D.-N., Savage H. E., Roberts J. E. Uveal melanocytes, ocular pigment epithelium, and Müller cells in culture: in vitro toxicology. International Journal of Toxicology. 2002;21(6):465–472. doi: 10.1080/10915810290169891. [DOI] [PubMed] [Google Scholar]
- 53.Wu W.-C., Hu D.-N., Gao H.-X., et al. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Molecular Vision. 2010;16:1864–1873. [PMC free article] [PubMed] [Google Scholar]
- 54.Hu D.-N., McCormick S. A., Ritch R., Pelton-Henrion K. Studies of human uveal melanocytes in vitro: isolation, purification and cultivation of human uveal melanocytes. Investigative Ophthalmology & Visual Science. 1993;34(7):2210–2219. [PubMed] [Google Scholar]
- 55.Dong X., Wang Y.-S., Dou G.-R., et al. Influence of dll4 via hif-1α-vegf signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0018481.e18481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notari L., Miller A., Martínez A., et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Investigative Ophthalmology and Visual Science. 2005;46(8):2736–2747. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 57.Wang B., Zou Y., Yuan Z.-L., Xiao J.-G. Genistein suppressed upregulation of vascular endothelial growth factor expression by cobalt chloride and hypoxia in rabbit retinal pigment epithelium cells. Journal of Ocular Pharmacology and Therapeutics. 2003;19(5):457–464. doi: 10.1089/108076803322473015. [DOI] [PubMed] [Google Scholar]
- 58.Cui Z., Song E., Hu D.-N., Chen M., Rosen R., McCormick S. A. Butein induces apoptosis in human uveal melanoma cells through mitochondrial apoptosis pathway. Current Eye Research. 2012;37(8):730–739. doi: 10.3109/02713683.2012.671436. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z., Yan J., Chang Y., ShiDu Yan S., Shi H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Current Medicinal Chemistry. 2011;18(28):4335–4343. doi: 10.2174/092986711797200426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh J.-H., Togloom A., Kim S.-W., Huh K. Effects of ginkgo biloba extract on cultured human retinal pigment epithelial cells under chemical hypoxia. Current Eye Research. 2013;38(10):1072–1082. doi: 10.3109/02713683.2013.804093. [DOI] [PubMed] [Google Scholar]
- 61.Sanz M. M., Johnson L. E., Ahuja S., Ekström P. A. R., Romero J., van Veen T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience. 2007;145(3):1120–1129. doi: 10.1016/j.neuroscience.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 62.Olk R. J., Peralta E., Sayegh S., Gierhart D. L. Does Oral zeaxanthin improve the anatomic and visual outcome of triple therapy for subfoveal choroidal CNV in age related macular degeneration (ARMD). Proceedings of the 44th Retina Society Meeting; 2011; Rome, Italy. [Google Scholar]
- 63.Manousaridis K., Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? British Journal of Ophthalmology. 2012;96(2):179–184. doi: 10.1136/bjophthalmol-2011-301087. [DOI] [PubMed] [Google Scholar]
- 64.Carneiro Â. M., Costa R., Falcão M. S., et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmologica. 2012;90(1):e25–e30. doi: 10.1111/j.1755-3768.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- 65.Semeraro F., Morescalchi F., Parmeggiani F., Arcidiacono B., Costagliola C. Systemic adverse drug reactions secondary to anti-VEGF intravitreal injection in patients with neovascular age-related macular degeneration. Current Vascular Pharmacology. 2011;9(5):629–646. doi: 10.2174/157016111796642670. [DOI] [PubMed] [Google Scholar]
- 66.Christoforidis J., Ricketts R., Pratt C., et al. The effect of intravitreal anti-VEGF agents on peripheral wound healing in a rabbit model. Clinical Ophthalmology. 2012;6(1):61–69. doi: 10.2147/OPTH.S28275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rezvani H. R., Ali N., Nissen L. J., et al. HIF-1α in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. Journal of Investigative Dermatology. 2011;131(9):1793–1805. doi: 10.1038/jid.2011.141. [DOI] [PubMed] [Google Scholar]
- 68.Nuzzi R., Tridico F. Local and systemic complications after intravitreal administration of anti-vascular endothelial growth factor agents in the treatment of different ocular diseases: a five-year retrospective study. Seminars in Ophthalmology. 2013 doi: 10.3109/08820538.2013.835833. [DOI] [PubMed] [Google Scholar]
- 69.Day S., Acquah K., Mruthyunjaya P., Grossman D. S., Lee P. P., Sloan F. A. Ocular complications after antivascular endothelial growth factor therapy in medicare patients with age-related macular degeneration. The American Journal of Ophthalmology. 2011;152(2):266–272. doi: 10.1016/j.ajo.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi D. Y., Ortube M. C., McCannel C. A., et al. Sustained elevated intraocular pressures after intravitreal injection of bevacizumab, ranibizumab, and pegaptanib. Retina. 2011;31(6):1028–1035. doi: 10.1097/IAE.0b013e318217ffde. [DOI] [PubMed] [Google Scholar]