Abstract
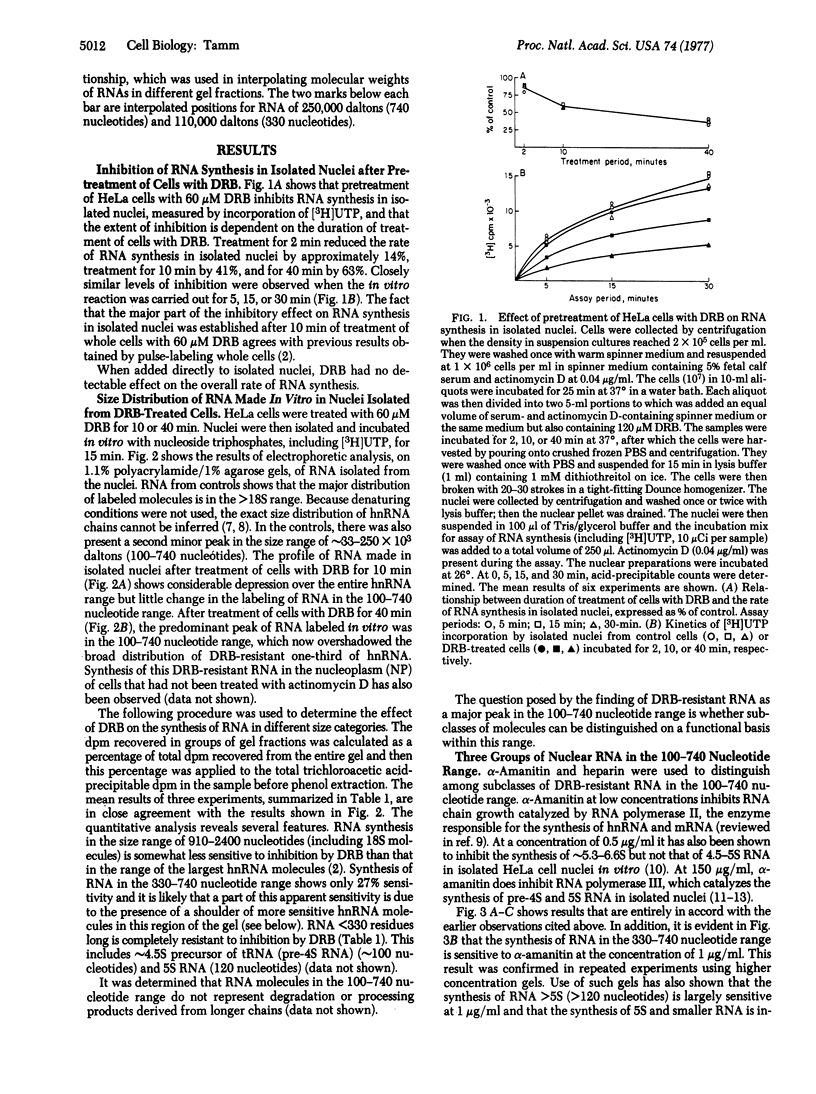
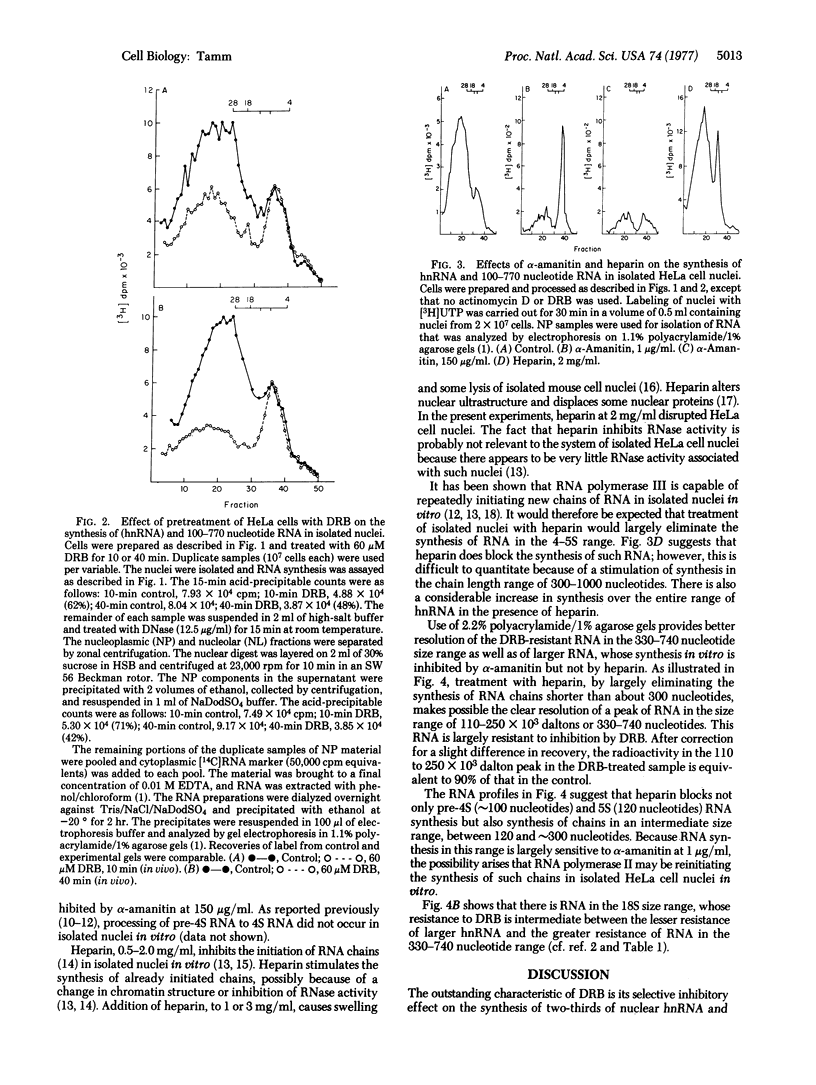
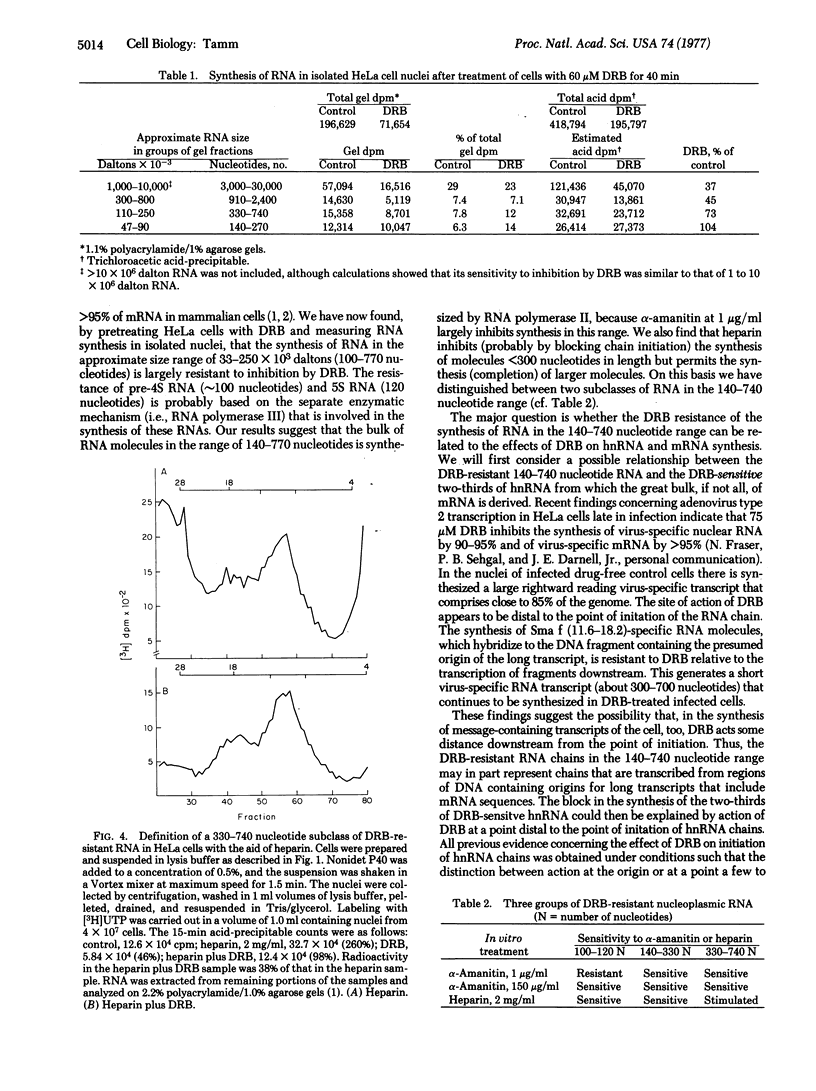
RNA synthesis in isolated HeLa cell nuclei prepared from cells pretreated with 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) is inhibited in a time-dependent manner. After 40-min pretreatment of cells with 60 μM DRB in the presence of actinomycin D (0.04 μg/ml), the rate of RNA synthesis in isolated nuclei, measured by [3H]UTP incorporation, is decreased by 63%. The DRB-resistant one-third of heterogeneous nuclear is distributed over the entire size range of heterogeneous nuclear RNA with some enrichment in the 18S range, as was observed earlier by pulse-labeling whole cells. A subclass of nucleoplasmic RNA molecules is defined in the approximate size range 110 to 250 × 103 daltons (330-740 nucleotides). By using heparin (2 mg/ml) to block the synthesis of smaller RNA, a peak in the chain-length range 330-740 nucleotides can be clearly resolved on 2.2% polyacrylamide/1% agarose gels in nuclei from control and DRB-treated cells. The synthesis of these molecules is largely (∼90%) resistant to DRB but sensitive to α-amanitin at 1 μg/ml. The in vitro synthesis of molecules in the 140-330 residue range is also sensitive to α-amanitin at 1 μg/ml, and it is not at all affected by pretreatment of cells with DRB. Although the synthesis of the RNA in both the 330-740 and the 140-330 residue size ranges appears to be catalyzed by RNA polymerase II, the results with heparin suggest that there may be reinitiation of molecules in the 140-330 size range but not in the 330-740 range in vitro. The synthesis of 4.5S RNA (∼100 nucleotides) and 5S RNA (120 nucleotides) is unaffected by pretreatment of cells with DRB and, as previously reported, is catalyzed by RNA polymerase III, with reinitiation occurring in vitro. Addition of DRB directly to isolated HeLa cell nuclei in vitro has no detectable effect on the overall rate of RNA synthesis.
Keywords: nuclear RNA-synthesizing system; HeLa cells; 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole; α-amanitin; heparin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook R. T., Aikawa M. The effects of heparin on endogenous DNA polymerase activity of rat liver nuclei and chromatin fractions. Exp Cell Res. 1973 Apr;78(2):257–270. doi: 10.1016/0014-4827(73)90068-2. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Modified 5'-termini in small nuclear RNAs of mouse myeloma cells. Mol Biol Rep. 1975 Dec;2(4):287–294. doi: 10.1007/BF00357015. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Transcription of high-molecular-weight RNA from hen-oviduct chromatin by bacterial and endogenous form-B RNA polymerases. Eur J Biochem. 1973 Nov 1;39(1):49–61. doi: 10.1111/j.1432-1033.1973.tb03102.x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Egyhazi E. Initiation inhibition and reinitiation of the synthesis of heterogenous nuclear RNA in living cells. Nature. 1976 Jul 22;262(5566):319–321. doi: 10.1038/262319a0. [DOI] [PubMed] [Google Scholar]
- Egyházi E., Daneholt B., Edström J. E., Lambert B., Ringborg U. Intracellular distribution of low molecular weight RNA in Chironomus tentans. J Cell Biol. 1971 Jan;48(1):120–127. doi: 10.1083/jcb.48.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyházi E. Inhibition of Balbiani ring RNA synthesis at the initiation level. Proc Natl Acad Sci U S A. 1975 Mar;72(3):947–950. doi: 10.1073/pnas.72.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Ferencz A., Seifart K. H. Comparative effect of heparin on RNA synthesis of isolated rat-liver nucleoli and purified RNA polymerase A. Eur J Biochem. 1975 May 6;53(2):605–613. doi: 10.1111/j.1432-1033.1975.tb04104.x. [DOI] [PubMed] [Google Scholar]
- Frederiksen S., Hellung-Larsen P. Precursors to small molecular weight RNA components. FEBS Lett. 1975 Oct 15;58(1):374–378. doi: 10.1016/0014-5793(75)80301-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Williams J. G., Penman S. Message and non-message sequences adjacent to poly(A) in steady state heterogeneous nuclear RNA of HeLa cells. Cell. 1976 Mar;7(3):429–437. doi: 10.1016/0092-8674(76)90173-2. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, White E. L., Benjamin R., Huang R. C. Low molecular weight RNA species from chromatin. Biochemistry. 1975 Aug 12;14(16):3715–3724. doi: 10.1021/bi00687a031. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Ringborg U., Rydlander L. Nucleolar-derived ribonucleic acid in chromosomes, nuclear sap, and cytoplasm of Chironomus tentans salivary gland cells. J Cell Biol. 1971 Nov;51(21):355–368. doi: 10.1083/jcb.51.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro-Choi T. S., Choi Y. C., Henning D., McCloskey J., Busch H. Nucleotide sequence of U-2 ribonucleic acid. The sequence of the 5'-terminal oligonucleotide. J Biol Chem. 1975 May 25;250(10):3921–3928. [PubMed] [Google Scholar]
- Sehgal P. B., Darnell J. E., Jr, Tamm I. The inhibition by DRB (5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole) of hnRNA and mRNA production in HeLa cells. Cell. 1976 Nov;9(3):473–480. doi: 10.1016/0092-8674(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Derman E., Molloy G. R., Tamm I., Darnell J. E. 5,6-Dichloro-1-Beta-D-ribofuranosylbenzimidazole inhibits initiation of nuclear heterogeneous RNA chains in HeLa cells. Science. 1976 Oct 22;194(4263):431–433. doi: 10.1126/science.982026. [DOI] [PubMed] [Google Scholar]
- Sklar V. E., Roeder R. G. Transcription of specific genes in isolated nuclei by exogenous RNA polymerases. Cell. 1977 Mar;10(3):405–414. doi: 10.1016/0092-8674(77)90028-9. [DOI] [PubMed] [Google Scholar]
- Spohr G., Mirault M. E., Imaizumi T., Scherrer K. Molecular-weight determination of animal-cell RNA by electrophoresis in formamide under fully denaturing conditions on exponential polyacrylamide gels. Eur J Biochem. 1976 Feb 16;62(2):313–322. doi: 10.1111/j.1432-1033.1976.tb10163.x. [DOI] [PubMed] [Google Scholar]
- Tamm I., Hand R., Caliguiri L. A. Action of dichlorobenzimidazole riboside on RNA synthesis in L-929 and HeLa cells. J Cell Biol. 1976 May;69(2):229–240. doi: 10.1083/jcb.69.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy A., Seifart K. H. Transcription of specific genes in isolated nuclei from HeLa cells in vitro. Eur J Biochem. 1976 Feb 16;62(2):353–363. doi: 10.1111/j.1432-1033.1976.tb10167.x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Brendler T. G., Raskas H. J., Roeder R. G. Low molecular weight viral RNAs transcribed by RNA polymerase III during adenovirus 2 infection. Cell. 1976 Apr;7(4):557–566. doi: 10.1016/0092-8674(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Penman S. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell. 1976 May;8(1):19–31. doi: 10.1016/0092-8674(76)90181-1. [DOI] [PubMed] [Google Scholar]


