Abstract
An applicator designed for rectal delivery of microbicides was tested for acceptability by 95 young men who have sex with men, who self-administered 4mL of placebo gel prior to receptive anal intercourse over 90 days. Subsequently, 24 of the participants self-administered rectally 4mL of tenofovir or placebo gel over 7 days using a vaginal applicator, and compared both applicators on a Likert scale of 1–10, with 10 the highest rating. Participants reported high likelihood to use either applicator in the future (mean scores 9.3 and 8.8 respectively, p= ns). Those who tested both liked the vaginal applicator significantly more than the rectal applicator (7.8 vs. 5.2, p=0.003). Improvements in portability, conspicuousness, aesthetics, tip comfort, product assembly and packaging were suggested for both. This rectal-specific applicator was not superior to a vaginal applicator. While likelihood of future use is reportedly high, factors that decrease acceptability may erode product use over time in clinical trials. Further attention is needed to develop user-friendly, quick-acting rectal microbicide delivery systems.
Keywords: HIV prevention, young MSM, rectal microbicides, applicators, delivery systems, acceptability, USA, Puerto Rico
Introduction
Men and women who practice anal intercourse often use lubricants to facilitate penetration (1–3). If an affordable and acceptable lubricant included anti-HIV agent(s), it could decrease HIV transmission risk during receptive anal intercourse (RAI). Over the past decade, research efforts have been focused on developing a rectal microbicide gel (RM).
Lubricants are most frequently applied during sex on the penis or on the anus with fingers. in volumes ranging approximately from 1 – 4 tablespoons. (1) To deliver a microbicide on the vulnerable rectal mucosa, studies to-date have used applicators. Unfortunately, RM applicators have encountered sub-optimal acceptability. For example, in the first human trial of a potential RM, Gross et al. (4) used a nonoxynol-9 (N9) contraceptive gel delivered with an applicator consisting of a plastic pouch with a long stem ending in a snap-off tip. In this trial of 35 male couples, 68% of receptive participants reported applicator-related discomfort. Also, 54% of all participants stated they would not use the gel, and applicator features figured prominently among dislikes noted in open-ended comments. Some receptive partners reported rectal distension that they attributed to release of air with the gel; others disliked the sharp edges at the tip of some applicators after removal of the twist-tab opener, the applicator’s large size which made it not easily portable, and the applicator’s appearance that was judged “tampon like.”
In a subsequent RM trial (5) the study gel was delivered with a vaginal applicator consisting of a barrel with plunger and a cap. (Fig 1). An evaluation of male and female user acceptability of the product (6) highlighted that although 58% of the participants (N=36) rated the applicator in the upper third of a 10-point scale, several problems related with it were identified: that it hurt upon insertion; it was difficult to handle; it had no tactile indicators to signal when the barrel was sufficiently inserted in the rectum, no indicator (like a “click”) that the gel was ejected, and no grips to facilitate product delivery; and it came inside an overwrap that for some required a sharp object to open. One of the participants said that applicator-related problems resulted in using less than the specified amount of gel on two occasions. There were no differences in acceptability ratings by gender.
Figure 1.
Vaginal Microbicide Applicator
Nevertheless, when an applicator-based delivery system for RM (the same one used in Anton et al. 2011 trial) was compared to enema or suppository, Pines et al. (7) found that the applicator filled with lubricant received the highest overall acceptability score and was used more frequently than the other products by those who had RAI in the prior two weeks. This study involved 117 HIV-uninfected males and females (79% and 21% respectively) who engaged in RAI and participated in a 6-week randomized crossover acceptability trial. However, Pines et al. (7) caution that the magnitude of differences between complete product acceptability scores differed by age, gender, and data collection mode (computer assisted self-interviews --CASI-- and a telephone audio CASI --T-ACASI); furthermore, significant differences disappeared with age among males and diminished with age among females. Furthermore, patterns in complete product acceptability scores were not consistent across data collection modes.
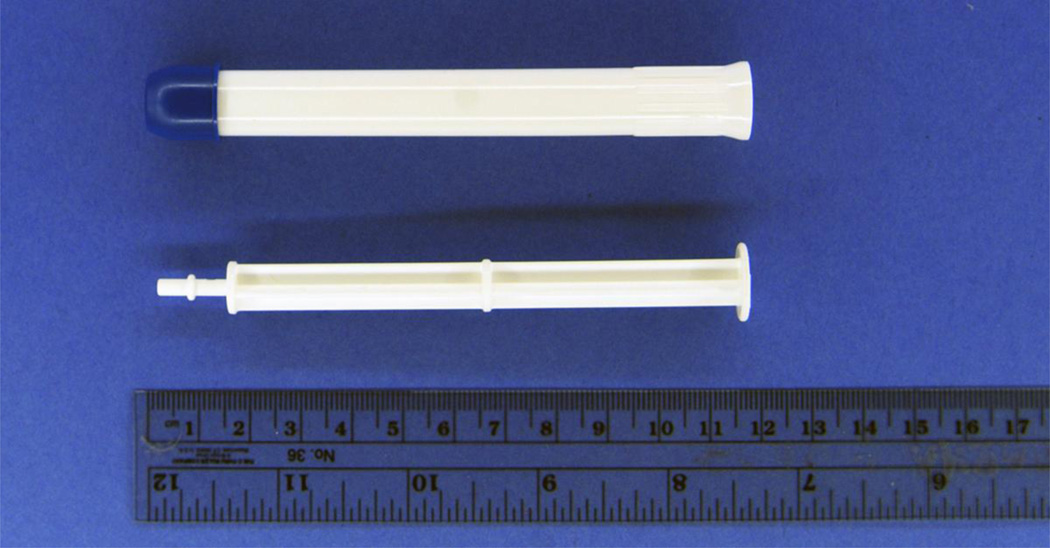
Given that problems with the applicator may negatively affect adherence to product use and product acceptability, which are critical features in early clinical trials, a rectal-specific applicator was designed for rectal delivery of a microbicide or placebo gel during RM trials. This work, conducted under an American Foundation for AIDS Research grant (BLINDED) sought to develop an inexpensive rectal applicator that could be used across rectal microbicide trials to ensure ease of use, comfort, and effective delivery of microbicide gel with a wide range of dose volumes for both women and men who have RAI. (Institutions BLINDED) developed specifications for the rectal applicator based on input from experts working in the field of rectal microbicides. The rectal applicator was designed according to product specifications through an iterative process with HTI Plastics (Lincoln, NE), the manufacturer of the vaginal applicator commonly used in vaginal microbicide trials. The rectal applicator used the Fleet Comfort Tip™ that has been commercialized for rectal administration of enemas for decades. Further compatibility and stability testing was conducted with four rectal placebo formulations by (Institution BLINDED) which led to further product improvements. The rectal applicator design was completed, including manufacture of products, in August 2008 (Fig 2).
Figure 2.
Rectal Microbicide Applicator
This manuscript describes a study of this rectal applicator in which participants were asked to provide feedback on features and use of the rectal applicator. A subset of participants was asked to compare the acceptability and use of the rectal applicator with an applicator designed for vaginal use.
Methods
In 2009, we were awarded funds to study microbicide safety and acceptability in young men (BLINDED). The study received IRB approval of all participating institutions. It included a “run-in” period in which participants applied a rectal placebo gel using the rectal-specific applicator, followed by a safety trial in which participants applied tenofovir gel using the vaginal applicator for rectal delivery of the gel. A vaginal rather than a rectal applicator was used in the safety trial because the former is the only applicator for which the stability and compatibility of tenofovir 1% gel has been established. A subset of participants had the chance to compare both applicators.
The study took place at three sites: (Institutions BLINDED). Study candidates were recruited between December 2010 and June 2012 from clinics, bars, clubs, newspaper advertisements and social networks.
Recruitment materials indicated that the investigators were looking for young men who have sex with men (YMSM), ages 18–30, for a study about their sexual health and their feelings about inserting rectally a placebo gel resembling a microbicide gel currently under development prior to receptive anal sex.
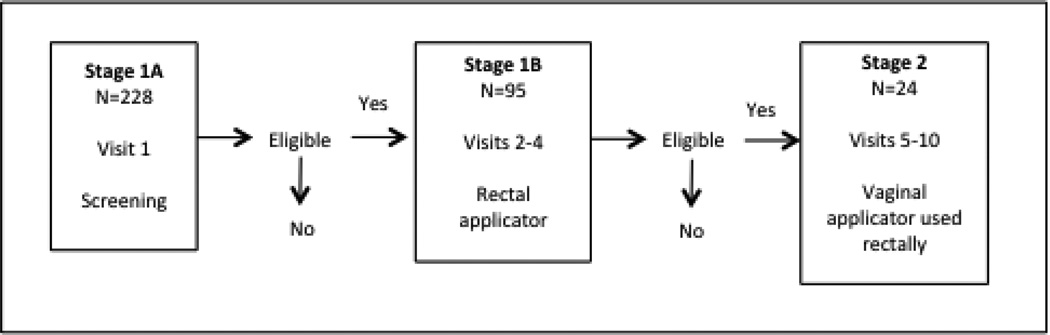
Stage 1A – Screening
Participants underwent a pre-eligibility screen by phone or in person to determine age, same sex behavior, and presumed negative HIV status. Those who passed pre-screening were invited to the clinic for in-person screening (Visit 1) to enroll in Stage 1A. (See Fig 3 and Table I). Target sample size for each stage was determined by the primary objective of each stage. The primary objective of Stage 1A was to determine the prevalence of STIs and anal and rectal pathologies that may facilitate HIV infection. Eligibility criteria included being sexually active (operationalized as at least one RAI episode in the prior month) and engaging in some potential risk behavior. So as to cast a wide net for the epidemiological objective of Stage 1A (i.e., prevalence of HIV/STIs in rectum), risk behavior for Stage 1A was operationalized as at least one episode of condomless RAI in the prior twelve months. After informed consent procedures, participants answered a medical history and received a physical exam including a digital rectal exam and anoscopy. Specimens were collected to test for HIV and STIs. In addition, participants completed a Web-based CASI that included demographic questions among other topics. HIV counseling and condoms were provided.
Figure 3.
Study Stagej and Applicator Use
Table I.
Study Procedures by Visit
| Stage | Visit | Study Procedures | Applicator Use |
|---|---|---|---|
| 1A | 1 | Screening/Enrollment in Stage 1A | None |
| 1B | 2 | Screening/Enrollment in Stage 1B | |
| 6 weeks outpatient use of HEC placebo gel with RAI | Rectal Applicator | ||
| 3 | Mid-trial 1B Follow-up | ||
| 6 weeks outpatient use of HEC placebo gel with RAI | |||
| 4 | End-trial 1B Follow-up | None | |
| 2 | 5 | Screening for Stage 2 | |
| 6 | Enrollment in Stage 2 | ||
| 7 | Single in-clinic dose | Vaginal Applicator for Rectal Use | |
| 8 | In-clinic dose Follow-up Phone Call | None | |
| 9 | Safety Clearance for 1 week outpatient use of study gel | ||
| 1 week outpatient use of study gel daily | Vaginal Applicator for Rectal Use | ||
| 10 | Outpatient Use Follow-up | None |
Stage 1B – Three-month non-clinic placebo use
Participants returned to the clinic within 28 days (Visit 2) and were informed of test results. From those who received medical clearance, we selected for enrollment in Stage 1B those fulfilling the more stringent eligibility criterion of having had condomless RAI within the prior three months. The primary behavioral objective of Stage 1B was to determine acceptability of and use-adherence to a placebo gel delivered rectally with a rectal delivery device. Including only participants who acknowledged condomless RAI in the past three months allowed us to focus on product use among those with more recent potential risk who may benefit the most from rectal microbicides. After undergoing a new informed consent process and update of medical history, participants received 20 rectal applicators filled with hydroxyethylcellulose (HEC) placebo gel (CONRAD, Arlington, VA) and instructions to insert the entire content of one rectal applicator rectally within 90 minutes prior to each RAI episode. Participants were asked to call an interactive voice response system at least once a week to report sexual activity and product use.
Six weeks after Visit 2, participants returned for the Mid-trial Follow-up Visit (Visit 3) at which the medical history was reviewed and updated; any reported adverse event was further explored; a physical exam including digital rectal exam and anoscopy was performed; samples were collected for STI and HIV testing if clinically indicated; used and unused applicators were collected, counted and recorded; and 20 new rectal applicators containing HEC were dispensed. HIV counseling and condoms were provided.
Six weeks after Visit 3, participants returned for the Final Follow-up Visit of Stage 1B (Visit 4). All procedures of Visit 3 were repeated but no rectal applicators were dispensed at this time. Additionally, participants completed a new Web-based CASI that included questions on gel and applicator use. They also underwent a Web-video semi-structured interview conducted from our offices in (Location BLINDED). This interview modality was found to be effective and acceptable to participants in a previous study of microbicide acceptability. (8)
Stage 2 – One-week non-clinic tenofovir/placebo use
A sub-sample of participants who had completed Stage 1 and were available and willing to participate were invited to enroll in Stage 2, which was a Phase 1 safety and acceptability randomized trial of tenofovir 1% gel. The behavioral objective of Stage 2 was to determine acceptability of and use-adherence to a placebo gel or tenofovir 1% gel delivered rectally with a vaginal delivery device. Each participant underwent consent process plus a new physical exam including a digital rectal exam and anoscopy. Specimens were collected to test for HIV and STIs. Each participant was randomized to one of two blinded study regimens: reduced glycerin tenofovir 1% gel (CONRAD, Arlington, VA) or HEC placebo gel (CONRAD, Arlington, VA) to be applied rectally. Participants returned to the clinic between 7 and 28 days later. Those with medical clearance applied one dose of study product under direct observation. They returned 7 to 21 days later to receive safety clearance and were given 8 vaginal applicators filled with 4 mL of the assigned study gel. They were instructed to insert the entire contents of one applicator rectally daily at night before bed or the longest period of rest, over 7 consecutive days. One extra dose was dispensed should an applicator become unusable for any reason. Following one week of daily gel use, participants returned for the final clinic visit, in which they completed a Web-based Product Acceptability Questionnaire and a video teleconference to further discuss opinions on product and applicator, among other topics. A physical exam and specimen collection took place. In addition, participants brought the used and unused applicators back to the clinic to be counted and the count recorded.
Compensation
Participants received $50 for each study visit (4 visits for Stage 1AB and 6 visits for Stage 2), an additional $50 for each visit that included biopsies, and $50 for each video teleconference interview completed. They also received $1 per applicator returned at visits 2, 3 and 10 plus maximum incentives of $60 in Stage 1B and $40 in Stage 2 for reporting their product use consistently by phone during the trial. The maximum a participant could make adhering to all visits and procedures from the first to the last visit of all stages of the study was $898.
Instruments
Structured and semi-structured data were collected on demographics and rectal and vaginal applicator evaluations via a Web-based CASI. Web-video in-depth interviews were conducted using an open-ended, semi-structured interview guide to collect qualitative data on participants’ assessments of the application process and the rectal and vaginal applicators.
Demographics
Demographic information included age, education (1=less than 8th grade, 2=partial high school, 3=high school graduate/GED, 4=partial college, 5=college graduate, 6=partial graduate school, 7=graduate school degree), annual income, work/student status (full or part time), race/ethnicity (White, Latino, African American, Asian, American Indian, or other), and sexual identity (“Do you consider yourself… gay/homosexual, bisexual, straight/heterosexual, other”)
Rectal Applicator Evaluation
Quantitative questions via CASI focused on applicator use (where participants applied the gel, in what position, how long before sex, and whether there were any problems applying the gel), attitudes about the applicator (how much they liked the gel applicator, how portable it was, and how likely they were to use it if it contained a microbicide that provided protection against HIV), and attitudes about the application process (how much they liked the process of applying the gel; the ease of gel use instructions, insertion of the applicator inside the rectum, and gel delivery; usefulness of finger grips; the level of comfort in using the applicator; the level of confidence of having inserted the correct amount of gel into the rectum). Wherever applicable, possible responses were restricted to a 10-point Likert scale (1=Disliked very much/Very difficult, 10=Liked very much/Very easy). Questions without a 10-point Likert scale, such as where gel was applied and in what position, included relevant response choices (i.e., bathroom, bedroom, other specify; or kneeling, laying on side, standing, etc.) Finally, participants were asked for recommendations through qualitative, open-ended questions via CASI that focused on whether they would change anything about the applicator, its tip, and how the product is packaged; we also asked whether the applicators should be disposable or refillable and why. These questions were presented to participants at the end of Stage 1B.
Also, at the end of Stage 1B participants underwent a qualitative in-depth interview via video teleconference using a semi-structured interview guide. Questions on rectal applicator acceptability included how the participant felt about the applicator, what it was like to use the applicator, any problems with applicators, what happened to used applicators, and where the applicators were stored.
Vaginal Applicator Evaluation
Quantitative and qualitative questions via CASI on the vaginal applicator were similar to those on the rectal applicator and used the same Likert scale or similar response options. These questions were presented only to participants who completed Stage 2, at the end of the stage.
Also, at the end of Stage 2 participants underwent a qualitative in-depth interview via video teleconference using a semi-structured interview guide. Questions on the vaginal applicator were similar to those asked during Stage 1B, but participants in Stage 2 were also asked to compare the two applicators and which they preferred.
Data Analysis
Quantitative Data
Descriptive statistics (mean, SD) were calculated for demographic, applicator count, and applicator variables. The men who continued into Stage 2 were compared to those who did not continue beyond Stage 1B using t-tests for continuous variables and chi-square tests for dichotomous variables. For dichotomous/categorical applicator use variables, binomial tests were conducted to test whether the proportion ascribing to a particular method differed for the men who used both applicators. For continuous attitude and process variables, paired t-tests were used to test whether ratings significantly differed for the 2 applicators. Comparisons of scores between rectal and vaginal applicators were restricted to the participants who used both applicators.
Qualitative Data
Compiled responses to open-ended CASI items were summarized based on themes that emerged, and participant comments were selected that exemplified these themes. Given the qualitative nature of the data, no attempts were made to tally frequency of responses.
Web-video interviews were audio-recorded, transcribed, and checked for accuracy. A codebook that incorporated categories and themes from the interview guide was developed to analyze transcriptions (9). The codebook included definitions, inclusion and exclusion criteria, and examples of passages for inclusion. To validate and finalize the codebook, three researchers coded an initial set of three transcripts independently and then compared the codes to assess concordance. Any discrepancies were discussed until consensus was reached. The codebook was modified where necessary, and researchers coded the remaining transcripts independently using QSR NVivo 8.0 software for qualitative data analysis, (10) coding every fifth transcript in pairs to ensure intercoder agreement. Comparisons yielded over 90% agreement consistently. Coding reports used for this study were “experience using the applicator” and “comparison between two applicators.” Coding reports were analyzed using content analysis, (11) then summarized and discussed by team members.
Results
Demographics
Two hundred twenty-eight YMSM were enrolled and completed Stage 1A. The initial 124 who both fulfilled eligibility criteria for Stage 1B and were available for a 3-month trial were enrolled in Stage 1B. Thirteen participants withdrew from the study either because they moved to another state, had family problems, lacked time for study participation, or other reasons; three of them completed early termination procedures. Furthermore, 16 participants were lost to follow up despite repeated attempts to contact them. The final sample for Stage 1B consisted of 95 participants who completed all procedures and provided feedback on applicator use. We compared the 95 participants who completed 1B to the 29 participants who did not on demographics and sexual risk behavior. The only significant difference found was that those who completed 1B had more education (M=4.55, SD=1.13) than those who did not (M=3.86, SD=1.06; t = −2.90, df = 122, p = .004).
Table II presents the demographic characteristics of these 95 men. On average, they were 23.2 years of age (SD 3.2), had completed some college education; and were working, studying, or both. Sixty-four percent were racial/ethnic minorities and 86% identified as gay.
Table II.
Demographic characteristics of Stage 1B and Stage 2 samples
| Stage 1B (N=95) | Stage 2 (N=24) | |||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Age | 23.2 (3.2) | 18–30 | 23.6 (3.4) | 19–30 |
| Educationa | 4.6 (1.1) | 2–7 | 4.6 (1.2) | 3–7 |
| Annual income | $15,260 (16,163) | 0–68,000 | $17,525 (17,961) | 0–58,000 |
| N | % | N | % | |
| Currently working full- or part-timeb | 60 | 63% | 14 | 58% |
| Currently in school full- or part-timeb | 47 | 50% | 13 | 54% |
| Race/Ethnicity | ||||
| White/European American | 34 | 36% | 10 | 42% |
| Latino/Hispanic | 46 | 48% | 9 | 38% |
| Black/African American | 9 | 10% | 2 | 8% |
| Mixed/Other | 6 | 6% | 3 | 12% |
| Sexual identity self-label | ||||
| Gay/Homosexual | 81 | 86% | 21 | 88% |
| Bisexual | 13 | 14% | 3 | 12% |
Measured on a 7-point scale (4=partial college).
23% of Stage 1B and 17% of Stage 2 participants were both working and in school.
NOTE: The 24 participants who continued into Stage 2 did not statistically differ from the 71 who did not on any of these demographic variables.
Table II also presents the demographic characteristics of the 24 participants who went on to enroll and complete Stage 2. They did not statistically differ from the 71 participants in Stage 1B who did not participate in Stage 2.
Applicator use
Stage 1B, rectal applicator
The 95 participants who completed the 3-month trial received 40 applicators each. Sixty-nine participants (73%) returned all 40 applicators, and 24 (25%) made only partial returns, ranging from 19–39 applicators (mean = 29.54, SD = 9.36). Two participants (2%) did not return any applicators. There were site differences in the percent of participants who returned all applicators: (Site 1 BLINDED) 83%, (Site 2 BLINDED) 75%, and (Site 3 BLINDED) 54%; at (X2=6.88 (2); p=.032). Based on returned applicator counts, there was a median of 12 used rectal applicators returned. If any applicators were not returned, participants were asked how many unreturned applicators were used vs. unused. Approximately 2/3 of the unreturned applicators were reported as unused. Additional information on product adherence and applicator use is reported elsewhere. (Reference BLINDED)
Stage 2, vaginal applicator used rectally
The 24 participants who enrolled in Stage 2 received 8 applicators each (7 to be used plus a spare one). All 24 returned all 8 applicators. Based on returned applicator counts, there was a median of 7 used vaginal applicators returned. Two participants (8%) returned 6 used applicators (i.e., they had used one applicator less than required), one participant (4%) returned 8 used applicators (one more than required); all others (88%) returned 7 used applicators.
Table III shows participants reports of applicator use (regardless of whether applicators were returned). Most participants used the applicators in their bedrooms or bathrooms, laying on their side or standing. Not shown in Table III, 64 (67%) participants in Stage 1B used applicators immediately before sex, while 13 (14%) used it within 30 minutes, 10 (11%) between 30 and 60 minutes, and 4 (4%) more than 60 minutes before sex (information was missing on the remaining 4%). (Participants in Stage 2 were asked to use the applicators daily regardless of sexual intercourse.) Table III also shows that 18 (20%) of the participants in Stage 1B who used the rectal applicator and 4 (17%) of those in Stage 2 who used the vaginal applicator rectally reported having problems with the tip (discussed in more detail in the qualitative data). There were no significant differences in the proportion of participants reporting each mode of application or experiencing problems with the tip among the 24 who used both applicators.
Table III.
Applicator use
| Stage 1B (N=95) | Stage 2 (N=24)* | |
|---|---|---|
| Where were you when you applied the gel? | N (%) | N (%) |
| Bedroom | 60 (63%) | 18 (75%) |
| Bathroom | 24 (25%) | 5 (21%) |
| Other | 8 (8%) | 1 (4%) |
| Refuse to Answer | 3 (3%) | 0 (0%) |
| Position when you applied the gel (check all that apply) | ||
| Laying on side | 35 (37%) | 9 (38%) |
| Standing | 29 (31%) | 8 (33%) |
| Kneeling | 21 (22%) | 3 (13%) |
| Squatting | 17 (18%) | 4 (17%) |
| Other (laying on back, one leg up) | 12 (13%) | 5 (21%) |
| Refuse to Answer | 3 (3%) | 0 (0%) |
| Problems with tip | 18 (20%) | 4 (17%) |
Note: There were no significant differences in the proportion of participants reporting each mode of application or experiencing problems with the tip among the 24 who used both applicators.
Applicator evaluation
Stage 1B, rectal applicator
Table IV summarizes the recommendations participants wrote in response to CASI open-ended items on how to improve the rectal applicator. Many participants commented that a smaller, easy-to-carry applicator would be preferable. Some men preferred a different color for the applicator and its tip, although color preferences varied. There were a number of suggestions on how to improve the tip of the applicator to decrease discomfort during insertion, such as making it softer, rounder, smoother, of a different size, and having it more lubricated; it was also suggested that it have lateral orifices (not just one orifice on the front) to better lubricate the rectum.
Table IV.
Examples of recommendations for rectal applicator improvement from Participants in Stage 1B1
| Size/Portability | Applicator not practical for carrying/ Make it thinner, maybe just a tube/ Easier to fit inside your jeans…like a condom or traveling size lube…you want something that is easy to take with you when you are out and about…you don’t want a product that is to be used at home only because it is too huge to carry around/ Make the plastic soft so a person can put it in his jeans pocket and won’t look like he is carrying a…/ The current applicator is so large it’s embarrassing to bring with me. I wouldn’t carry it around unless I know I will have sex with someone |
| Color | Change color to a brownish/ I would change the color of the applicator tip [cap] so that it wasn’t orange (it looks too much like a douche). I would make it a more neutral color so it would be more discreet in a bag/ Perhaps in a dark color/ What if it were black or red and a little sleeker. |
| Applicator Tip | For some reason, the tip causes pain during insertion [and] removal/ If the tip itself was lubricated [it is]/ It has too much point/ Maybe smoother…generally it’s ok but sometimes it felt like it was stabbing me/ The Fleet applicator isn’t the most comfortable; in this case something bigger would actually feel better/ Flexible would be better/ The nozzle has to be changed, to be able to apply gel to the sides, not only in the front direction |
| Looks/design | Make it sexier –and streamline the design to resemble a sex toy or prostate stimulator/ Make it in a fun, curvaceous container/ Make it so I could feel it up my rectum/ I am blessed to not be diabetic, but this feels as though I have one of those mechanisms in my pocket/ Make the applicator look more modern, it looks too much like Elmer’s glue/ The enema shape of the applicator is kind of a turn off. The shape of an enema draws up thoughts of constipation/ Publish specifications for the applicator and let the medical or sex toy industry come up with a design and worry more about the gel itself/ If you made the bottle clear with loud colors and made it so you can squeeze out certain amounts whenever you want that would be more attractive and effective/ Get rid of the plunger |
| Refillable? | Refillable is great for me, so I don’t have to have a million applicators. BUT I don’t know how guys would feel about using the same applicator over and over again…/ Definitely a refillable & reusable product would be ideal to make it a bit more environmentally friendly…/ With the whole tip getting messy from your bum (remnants of feces), I would recommend one time use only/ Refillable for use with a lover-disposable for use with one-night stands. |
| Process | The application process either more discrete or have it become a natural part of the sexual experience/ I ended up at times applying product on my fingers and then use it as any regular gel to apply on condom and on the front and walls of the rectum/ |
| Product Packaging | Packaging needs to be more easily accessible. I had to use a sharp object every time/ One time broke the applicator while trying to open package/ Especially if you have lube on your hands. It is not pleasant to struggle with it/ I would change the product package to a more vibrant color |
| No applicator | Prefer no applicator and to use like lube/ I think the best solution would be to concentrate the gel enough so that only a small amount is required. Then it could be sold in a tube just like any other lubricant and people could apply it themselves/ What about making it in the form of a repository? [suppository?] That takes away the worry of carrying it around after use/ I love the gel but hate the applicator |
Quotes are write-in responses from participants on CASI questionnaire at follow-up
In terms of the appearance and design, participants stressed that instead of being so medical or experimental looking --which evoked ideas of constipation and disease-- it should be sexy, fun, modern, stylish, and in varieties to appeal to different tastes or age groups. Suggestions were given to make it in only one piece and eliminate the plunger. The possibility of the applicator being refillable drew both favorable (more portable, environmentally friendly) and unfavorable (messy) opinions, with some support for having both refillable and disposable options available. Concerning mode of use, one participant reported delivering the product on his fingers and then applying it with fingers rather than applicator, and there was a complaint about the applicator being slippery. Some expressed that carrying the applicator could be embarrassing and requested a more discreet applicator. The applicator packaging was criticized as being unappealing and difficult to open, sometimes requiring a sharp object. Finally, there were calls to eliminate the applicator altogether and deliver the microbicide in the form of a concentrated gel or suppository.
During the semi-structured interviews conducted by videoconference, a few additional comments and suggestions were made. Participants reported that the rectal applicator was easy to use, that the grips on the side made it easy to control, and that, for some, practice made use of the applicator easier and quicker. Some complained that air was coming out of the tip, and that there were too many steps involved in using the applicator (unwrapping, assembling, inserting, delivering, disposing of packaging). At times, the plunger fell off.
Stage 2, vaginal applicator used rectally
Table V summarizes the recommendations participants wrote in response to semi-structured items in the CASI evaluation on how to improve the vaginal applicator for rectal use. Given that Stage 2 had a quarter of the participants in Stage 1B, there were fewer recommendations. Yet, they grouped around the same topics. Although the vaginal applicator is smaller than the rectal applicator, participants wanted it to be smaller yet. Some liked the color but others said it reminded them of a tampon and should be different. It was recommended that the tip be smaller, smoother, thinner, although some wanted it wider. The vaginal applicator tip was not pre-lubricated, and some people called for it to be lubricated. Unlike the rectal applicator, the vaginal applicator did not have finger grips, which was seen as a drawback. The plunger at times fell out. Other times, it offered too little resistance when pressed with the risk of delivering the gel before full insertion in the rectum. In some cases, less than the full dose was delivered. There were calls for a “fun and enjoyable” applicator, “maybe in the shape of a penis,” and with improved wrapping. Finally, there were also suggestions for a delivery system without an applicator.
Table V.
Examples of recommendations for vaginal applicator improvement for its rectal use from Participants in Stage 21
| Size/Portability | Make it smaller/ More portable |
|---|---|
| Color | I like the color/ Perhaps because of the color, the applicator reminded me of a tampon; the applicator could be a more distinguishable color. |
| Applicator Tip | It’s sharp, though I’m aware the tip is designed for women/ [Make] pre-lubricated applicator tip/ Tip was wider and needed more lube. |
| Looks/design | Make it in fun colors with pictures/ A place for the pointer and middle finger to have to hold the applicator when administering the gel/ The two pieces should just be connected so I don’t lose the plunge stick/ Ideally a green product for the environment; maybe in the shape of a penis would make it fun and enjoyable. |
| Process | Make the applicator less sensitive; if I was not careful, the gel would have leaked before it was inside the rectum/ Sometimes, when I finished applying the gel, there was some gel left in the tip of the applicator, so I think the right amount of gel was not being applied the way it should. |
| Product Packaging | Maybe use a small hard case that’s more thin and less “prescription drug” looking/ Can be a tight wrap around he applicator itself. |
| No applicator | Make it like individual lubricants that come in pouches |
Quotes are write-in responses from participants on CASI questionnaire at follow-up
During the semi-structured interviews conducted by videoconference, a few additional comments and suggestions were made about the vaginal applicator as well. Most participants found that adding lubrication to the tip of the applicator prior to insertion made the process go more smoothly. A few who did not use lubricant found the tip to feel sharp. Almost all participants felt they were certain that they had inserted the entire contents of the applicator into the rectum. However, one complained about the gel leaking out during application, while a few others said it took one or two tries to become used to the process and to be able to insert the gel without expelling it before the applicator was fully inserted.
Attitudes about applicator and application process, and comparison between applicators
Table VI presents the participants’ structured evaluation of both applicators and the application process. Participants in Stage 1B (N=95) both liked the rectal applicator mildly (score 5.8, SD 3.2) and were mildly enthusiastic about its portability (5.5, SD 3.0). Not shown in the table, participants felt that the applicator was easy to use (easy to press the plunger: 8.6, SD 2.2; finger grips useful 8.8, SD 1.9). When the analysis was restricted to the 24 participants who used the rectal and the vaginal applicator, it showed that these participants liked the vaginal applicator more (7.8, SD 3.0) than the rectal applicator (5.2, SD 3.1; p=.003) and felt it was more portable (rectal, 5.3, SD 3.4; vaginal 7.6, SD 2.4; p<.001). Yet, there were no differences concerning likelihood of using either applicator in the future, which was high for both (rectal 9.3, SD 1.6; vaginal 8.8, SC 2.2).
Table VI.
Attitudes about applicator and application processa
| Stage 1B (N=95) |
Stage 1Bb (N=24) |
Stage 2 (N=24) |
||
|---|---|---|---|---|
| Applicator | Mean (SD) | Mean (SD) | Mean (SD) | p- valuea, c |
| Liked the gel applicator | 5.8 (3.2) | 5.2 (3.1) | 7.8 (3.0) | .003 |
| Portability | 5.5 (3.0) | 5.3 (3.4) | 7.6(2.4) | <.001 |
| Likely to use in the future | 8.8 (2.2) | 9.3 (1.6) | 8.8 (2.2) | .455 |
| Application Process | Mean (SD) | Mean (SD) | Mean (SD) | |
| Overall process of applying gel | 6.2 (2.8) | 6.3 (2.5) | 8.3 (1.5) | .003 |
| How easy were the instructions | 9.0 (1.7) | 9.0 (2.0) | 9.5 (1.3) | .229 |
| How easy to insert applicator | 8.1 (2.2) | 8.3 (2.3) | 8.2 (2.1) | .759 |
| How easy to deliver gel | 8.9 (1.6) | 9.0 (1.4) | 9.3 (0.9) | .479 |
| Comfort level using applicator | 7.3 (2.7) | 7.0 (3.0) | 8.4 (2.0) | .052 |
| How confident inserted correct amount | 8.1 (2.2) | 7.9 (2.2) | 9.3 (1.4) | .011 |
Measured on 10-point Likert scale from (1=Disliked very much/Very difficult, 10=Liked very much/Very easy)
These are the 24 participants in Stage 1B who also enrolled in Stage 2
p-values refer to the comparison of N=24 in Stage 1B to N=24 in Stage 2
Concerning the process of using the applicators, also summarized in Table VI, the 95 participants in Stage 1B gave overall a mildly positive evaluation of the rectal applicator process (6.2, SD 2.8) despite finding the use instructions easy to understand (9.0, SD 1.7), the applicator easy to insert (8.1, SD 2.2), the gel easy to deliver with the applicator (8.9, SD 1.6), and expressing confidence in having inserted the correct amount of gel (8.1, SD 2.2). The 24 participants who used both applicators gave the vaginal applicator similar or better scores in all these categories (see Table VI), with significant differences observed in the scores given to overall process of applying the gel (rectal 6.3, SD 2.5; vaginal 8.3, SD 1.5, p=.003) and confidence in inserting the full amount (rectal 7.9, SD 2.2; vaginal 9.3, SD 1.4, p=.011).
Discussion
Complaints about the characteristics of applicators used in prior microbicide studies to deliver gel intrarectally led us to develop a rectal-specific applicator for use in clinical trials. Its evaluation showed that there are many problems yet to be resolved. Furthermore, a small subsample of participants who used rectally both a rectal-specific applicator and a vaginal one preferred the vaginal applicator.
Nevertheless, the evaluation of both applicators showed persistent, unsolved problems. Paramount among them is the issue of portability, which is key for a product that is generally used immediately before sexual intercourse, wherever it happens. Furthermore, users want a product that is discreet, not medical looking, aesthetically appealing, with an attractive, easy to open wrap. None of the applicators used in RM trials thus far meet these expectations. Additionally, the tip of the applicator continues to be problematic, whether it is a stem with a snap off tip (4), a rounded vaginal applicator tip (5,6,12), or the Fleet Comfort Tip® used in our study that is the same that has been marketed with enema products for several decades. Both applicators used in our study required assembly (to insert the plunger in the barrel); participants reported that no assembly was preferable.
Interestingly, despite complaints about both applicators used in this study, participants expressed high likelihood to use them in the future if they delivered an efficacious product. Yet, expressions of likelihood of use may be strongly influenced by rational thinking (e.g., if this product confers protection, I will use it), particularly if not associated with other variables (e.g., emotions; considerations of pleasure and trust) that may erode the determination to protect oneself using the product in the long run. (13) Therefore, likelihood of use assertions in isolation of counterbalancing factors should be taken with caution. Furthermore, considering that we had carefully selected our participants to include individuals reporting inconsistent condom use, it is possible that they had a heightened awareness of engaging in risk behavior and therefore were more hopeful about a gel product that would interfere with sexual satisfaction less than condoms. This may have increased their ratings of likelihood to use a protective gel despite some discomfort with the applicator.
Could a RM be delivered without the need for an applicator? In a prior study (14) in which participants compared a gel delivered rectally with a syringe versus a rectal suppository, 75% of participants preferred the gel vs. 25% who liked the suppository more. However, the volume of both the gel and suppository used in that study were large (35 mL of gel and 8 g of suppository) because at the time of the study design it was assumed that such large volumes were necessary to coat the rectal mucosa. Current microbicide candidates incorporate antiretrovirals (ARV), and use much lower volumes (e.g., 4 mL). (12) In another previously mentioned study (7) in which men and women compared three potential RM delivery systems including a 1.4 g anorectal suppository (TucksTM)), the applicator-based system was preferred. Yet, both Carballo-Diéguez (14) and Pines (7) studies used preexisting suppositories that had not been developed specifically for RM delivery with input from potential users not only about the suppository’s physical characteristics but also on packaging and marketing. Given the results of the present study concerning the evaluation of currently available applicators for rectal delivery, it seems worthwhile to re-examine the possibility of developing suppositories specifically for the delivery of a RM with attention to different behavioral aspects of their future use.
Furthermore, thus far it was assumed that since many people who engage in rectal intercourse use lubricants, a microbicidal gel could solve lubrication and protection problems at the same time. (1,3) Yet, rectal lubricants are applied on the anus with the fingers or on the partner’s penis, and the volume used is enough to facilitate penetration but not too much to decrease sensation. By contrast, until now, a microbicide gel needs to be delivered intrarectally and its dose needs to be precise and not subject to users’ preferences. If a microbicide could be applied on the anus and spread by the penis during penetration, then indeed anal lubricant use and microbicide use would be quite similar. However, as long as a rectal specific applicator is needed to deliver the RM, lubricant and RM use are different behaviors. Furthermore, not everybody who has rectal intercourse uses lubricants (some prefer saliva or no lubrication at all), and those who use lubricants want to have choices (water based, oil based, or silicone). (3) It is unlikely that a microbicide gel could be efficiently produced in such variety of formulations.
Ultimately, no applicator appears to be the best choice. This means that the topical microbicide needs to be formulated in a way that can be transported inconspicuously “in the pocket of one’s jeans” without being damaged in the process, be applied with no applicator or need for extra lubricant, be quick-acting (i.e., become effective almost instantly given that short time elapses between product application and intercourse), and be packaged in a way that is easy to open even with slippery fingers (which is also a persistent complaint about the packaging of condoms).
Phase III effectiveness trials of vaginal microbicides have been highly affected by poor adherence to product use. (15,16) Before embarking on a Phase III rectal microbicide trial, it is advisable to pay increased attention to microbicide formulation, delivery system, packaging, and the aesthetics of it all. Workgroups are needed involving chemists, engineers, physicians, behavioral scientists, marketing experts, and end users among others. Thus far, we have used a sequential development process for microbicides, assuming that we could first show its efficacy and acceptability and worry later about promotion and marketing issues. The experience acquired in recent trials indicates that while a small number of participants in brief trials may tolerate suboptimal product presentations, when thousands of people are expected to try products in Phase III trials of long duration all efforts should be made to make the delivery system user friendly, attractive and desirable. This in turn would also benefit subsequent Phase I and II trials of other products.
In addition, due to funding constraints, behavioral research in the areas of adherence and acceptability of microbicides and microbicide applicators is often carried out in the context of clinical trials for product safety and efficacy. This limits the freedom to include randomization to different behavioral conditions or other study designs that could more effectively remove confounds. Yet, within these constraints, we are able to point out numerous behavioral issues that are key to future product use, which constitutes a significant contribution to science.
Limitations of the study
This study has several limitations. The actual comparison between rectal and vaginal applicators was limited to the 24 men who actually had a chance to use both products. We were unable to vary the sequence of applicator use (all participants used the rectal applicator first) because, by design, the safety stage of the trial followed from the initial placebo run-in stage, and we did not have authorization to package tenofovir in the rectal applicator. This sequence may have led to a practice effect, with participants being more experienced with rectal delivery of a gel by the time they used the vaginal applicator, which may have influenced their evaluation. In addition, more recent use of the vaginal applicator may have affected applicator preference. Also, the time frames (90 days vs. 7 days), frequency of use (within 90 minutes prior to RAI vs daily) and sexual contingency (prior to RAI vs. no RAI) of both applicators varied by study design; this may have affected the evaluation of the applicators in ways that we could not control in this study. Furthermore, our participants were YMSM. Older participants or women who engage in anal intercourse may have different preferences.
Nevertheless, within these limitations, our study sheds light on the importance of the delivery method for rectal microbicides and offers specific suggestions for future development. A delivery system perceived as enhancing sexual pleasure and satisfaction while providing disease prevention will have a higher likelihood of consistent use than a delivery system that only provides disease protection or, worse, interferes with sexual pleasure. Our results point out both the need to improve delivery systems to make them user friendly and to make products sexy, appealing and desirable to the target populations.
Acknowledgements
We would like to thank the panel of experts that helped us during the original development of our rectal applicator. They are Drs. Peter Anton, Pamina Gorbach, Craig Hendrix, Ken Mayer, Ian McGowan, and Tom Moench, as well as activists in this field, Ms. Anna Forbes and Mr. Jim Pickett. We would also like to thank Jessica Cohen from PATH and Dr. Ana Ventuneac (formerly from HIV Center for Clinical and Behavioral Studies) for their collaboration in early stages of this study and all study volunteers without whom this project would not have been feasible. This research was sponsored by the US National Institutes of Health (NIH), including NICHD and NIMH, under R01 HD59533 (McGowan and Carballo-Diéguez, Co-PIs) and co-sponsored by CONRAD. Rectal applicator development was sponsored by the Foundation for AIDS Research (amfAR Research Grant # 106765-41-RGMC; Carballo-Diéguez, PI). Additional support came from the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30-MH43520; Principal Investigator: Robert Remien). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
References
- 1.Carballo-Diéguez A, Stein Z, Sáez H, Dolezal C, Nieves-Rosa L, Díaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000 Jul;90(7):1117–1121. doi: 10.2105/ajph.90.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbach PM, Weiss RE, Fuchs E, Jeffries RA, Hezerah M, Brown S, et al. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sex Transm Dis. 2012 Jan;39(1):59–64. doi: 10.1097/OLQ.0b013e318235502b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javanbakht M, Murphy R, Gorbach P, LeBlanc M-A, Pickett J. Preference and practices relating to lubricant use during anal intercourse: implications for rectal microbicides. Sex Health. 2010 Jun;7(2):193–198. doi: 10.1071/SH09062. [DOI] [PubMed] [Google Scholar]
- 4.Gross M, Celum CL, Tabet SR, Kelly CW, Coletti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999 Nov;26(10):572–578. doi: 10.1097/00007435-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Anton PA, Saunders T, Elliott J, Khanukhova E, Dennis R, Adler A, et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PloS One. 2011;6(9):e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventuneac A, Carballo-Diéguez A, McGowan I, Dennis R, Adler A, Khanukhova E, et al. Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav. 2010 Jun;14(3):618–628. doi: 10.1007/s10461-009-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pines HA, Gorbach PM, Weiss RE, Hess K, Murphy R, Saunders T, et al. Acceptability of potential rectal microbicide delivery systems for HIV prevention: a randomized crossover trial. AIDS Behav. 2013 Mar;17(3):1002–1015. doi: 10.1007/s10461-012-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mabragaña M, Carballo-Diéguez A, Giguere R. Young women’s experience with using videoconferencing for the assessment of sexual behavior and microbicide use. Telemed J E-Health Off J Am Telemed Assoc. 2013 Nov;19(11):866–871. doi: 10.1089/tmj.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacQueen KM, McLellan E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. Cult Anthropol Methods. 1998;10(2):31–36. [Google Scholar]
- 10.NVivo qualitative data analysis software. Version 8. QSR International Pty Ltd.; 2008. [Google Scholar]
- 11.Patton MQ. Qualitative Research and Evaluation Methods. 3rd Edition. Thousand Oaks: Sage Publications, Inc.; 2002. [Google Scholar]
- 12.McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L, Dai JY, et al. A Phase 1 Randomized, Double Blind, Placebo Controlled Rectal Safety and Acceptability Study of Tenofovir 1% Gel (MTN-007) PloS One. 2013;8(4):e60147. doi: 10.1371/journal.pone.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfister HR, Böhm G. The multiplicity of emotions: A framework of emotional functions in decision making. Judgm Decis Mak. 2008 Jan;3(1):5–17. [Google Scholar]
- 14.Carballo-Diéguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008 Nov;84(6):483–487. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s Experiences with Oral and Vaginal Pre-Exposure Prophylaxis: The VOICE-C Qualitative Study in Johannesburg, South Africa. PLoS ONE. 2014 Feb 21;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003) Atlanta, GA: 2013. [Google Scholar]