Abstract
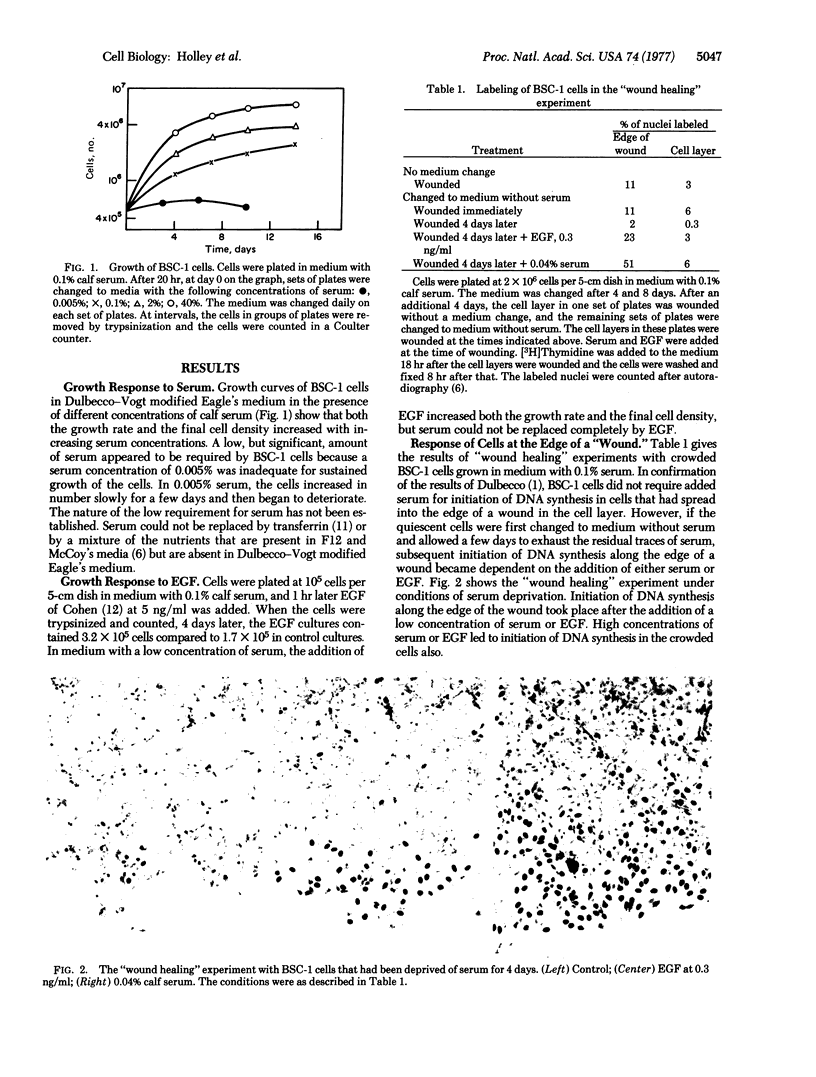
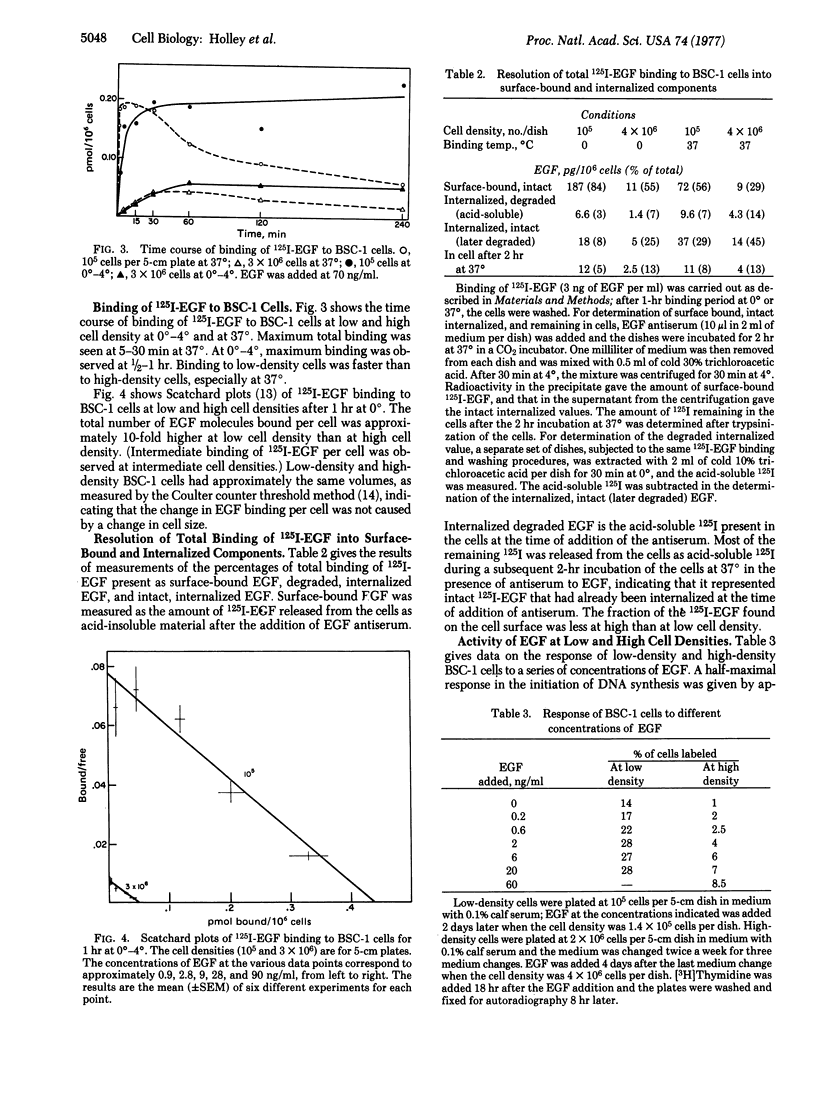
BSC-1 cells grow slowly, to high cell density, in medium with 0.1% calf serum. An increase in the serum concentration increases both the growth rate of the cells and the final cell density. The serum can be replaced to some extent by epidermal growth factor (EGF). Initiation of DNA synthesis in BSC-1 cells that have spread into a "wound" in a crowded cell layer requires the addition of a trace of serum or EGF, if the cells have previously been deprived of serum. The binding of 125I-labeled EGF to low-density and high-density BSC-1 cells has been studied. Binding is faster to low-density cells. Cells at low cell density also bind much more EGF per cell than cells at high cell density. The fraction of bound 125I-labeled EGF that is present on the cell surface as intact EGF is larger at low than at high cell density. The results indicate that the number of available EGF receptors per cell decreases drastically as the cell density increases. It is suggested that a decrease in the number of available EGF receptor sites per cell, and the accompanying decrease in sensitivity of the cells to EGF, contributes to density-dependent regulation of growth of these cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Cohen S., Taylor J. M. Epidermal growth factor: chemical and biological characterization. Recent Prog Horm Res. 1974;30(0):533–550. doi: 10.1016/b978-0-12-571130-2.50017-1. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973 Nov 23;246(5430):197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Hassell J., Engelhardt D. L. Factors regulating the multiplication of animal cells in culture. Exp Cell Res. 1977 Jun;107(1):159–167. doi: 10.1016/0014-4827(77)90397-4. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer T. O. Nature of the iron requirement for Chinese hamster V79 cells in tissue culture medium. Exp Cell Res. 1973 Mar 15;77(1):404–408. doi: 10.1016/0014-4827(73)90594-6. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Perdue J. F. Mitogen receptors in chick embryo fibroblasts. Kinetics, specificity, unmasking, and synthesis of 125I-insulin binding sites. J Biol Chem. 1976 Oct 25;251(20):6445–6455. [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Thomopoulos P., Roth J., Lovelace E., Pastan I. Insulin receptors in normal and transformed fibroblasts: relationship to growth and transformation. Cell. 1976 Jul;8(3):417–423. doi: 10.1016/0092-8674(76)90154-9. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Weinstein I. B. Temperature-sensitive mutants of chemically transformed epithelial cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):214–218. doi: 10.1073/pnas.72.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]




