Abstract
Wyosine and its derivatives are highly modified, acid labile tricyclic bases found at position 37 of tRNAPhe in archaea and eukarya. The formation of the common 4-demethylwyosine structural feature entails condensation of pyruvate and N-methylguanosine catalyzed by TYW1. This review will focus on the mechanism of this complex radical mediated transformation.
Introduction
To date, 92 modifications have been noted in tRNAs from all kingdoms of life and are found distributed throughout the tRNA molecule [1]. Modifications of tRNA have been shown to play a role in both structural stability and in providing enhanced molecular recognition [2]. The modifications can range in complexity from simple methyl groups that are installed in one step transformations to hypermodified bases such as queuosine and wyosine that require multiple steps of unprecedented enzymatic transformations [3,4].
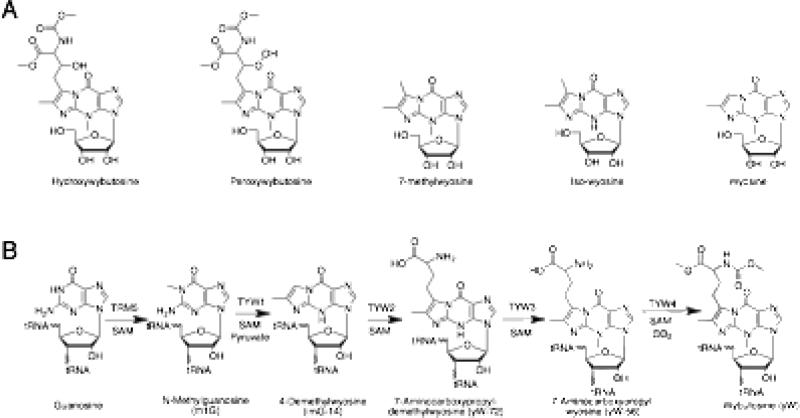
Wyosine and its derivativescontain a characteristic tricyclic core (see Figure 1A for examples) [5]. Wybutosine (yW) was first identified as a component of baker's yeast tRNA in 1968 [6], but its derivatives have been subsequently described in other eukaroytes [7,8]. Bioinformatic analysis of archaeal genomic sequences suggest that archaea may contain various related analogs, including unique derivatives such as isowyosine and 7-methylwyosine [9]. In some cases these predictions have been confirmed by analysis of archaeal unfractionated tRNA preparations [10-13].
Figure 1.
Wyosine analogs and their biosynthesis. The figure shows structures of representative wybutosine analogs (A) and the biosynthetic pathway to wybutosine from GTP (B).
yW is introduced into position 37 (adjacent to the anticodon) of archaeal and eukaryal tRNA by a five step post-transcriptional modification of the genetically encoded guanine base [14] (Figure 1B). The first step in the biosynthetic pathway is methylation of N-1 at G37 to form N-methylguanosine (m1G) [4]. Next TYW1 modifies m1G to form 4-demethylwyosine (imG-14) [4,15], which is further modified by TYW2 via the addition of an α-amino-α-carboxypropyl moiety derived from S-adenosyl-L-methionine (SAM) to form yW-72 [4,16]. SAM-dependent methylation by TYW3 forms yW-56, followed by methylation and carboxymethylation catalyzed by TYW4 using SAM and CO2 to form yW [4,17]. SAM is utilized by every enzyme in the pathway with TRM5, TYW3, and TYW4 using it as the source of a methyl group, while TYW2 uses it as the source of a α-amino-α-carboxypropyl group. TYW1, however, utilizes SAM as an essential cofactor to initiate a complex radical mediated transformation. This review will focus on recent insights on the reaction catalyzed by this TYW1 where recent advances in in vitro reconstitution of its activity have made mechanistic studies possible [18,19].
TYW1
TYW1 was first identified by comparative genomics analysis of S. cerevisiae. Knockout and complementation studies established that the protein encoded by YPL207w catalyzes the conversion of m1G to imG-14 [4,15]. Additional reverse genetics approaches further confirmed the role for TYW1 in this reaction [4]. The requirement for pyruvate as a co-substrate for TYW1 was shown recently [18].
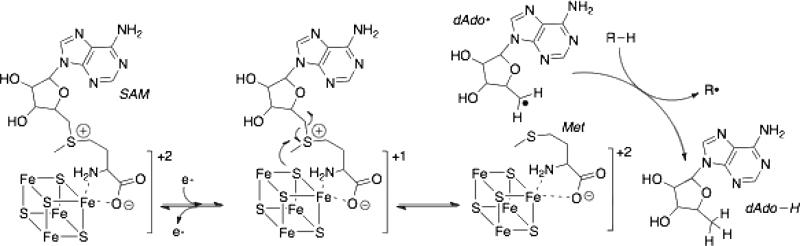
TYW1 is a member of the radical SAM superfamily, which catalyze reductive cleavage of SAM to generate a 5□-deoxyadenosyl radical (5□-dAd0•) to initiate radical mediated transformations [20]. Most but not all of these proteins have a CxxxCxxC motif [21]. Three of the irons of a [4Fe-4S] cluster are ligated to the conserved Cys thiolates whereas the fourth is ligated to the α -amino and α -carboxylate of SAM, as shown in Figure 2 [22]. To date >45,000 sequences in genome databases have been identified that have the radical SAM consensus sequence [23]. The role of the cluster in nearly all cases is reductive cleavage of SAM to generate a 5□-dAdo•, which carries out an H-atom abstraction from either a small molecule or a protein substrate to initiate catalysis [20,24].
Figure 2.
Reductive cleavage of SAM by radical SAM enzymes. 5□-Deoxyadenosyl radical generated from reductive cleavage of SAM abstracts a hydrogen atom to initiate chemistry.
Intriguingly, TYW1 is a member of a growing group of radical SAM enzymes that have an auxiliary [4Fe-4S] cluster in the active site [19,25-27]. In some cases the role of the second cluster is understood. For example, the second cluster in biotin synthase and lipoic acid synthase donate the sulfur atoms that are required in catalysis [28,29]. In MoaA the second cluster appears to be involved in positioning the purine moiety of the substrate [30,31]. In other enzymes, a role for this cluster in electron transfer to or from the substrate has been proposed [32,33]. In TYW1 these residues are located in the presumed active site across from the Cys residues that constitute the radical SAM motif. The structures do not show clear and unambiguous density for either of the metalloclusters [25,26]. In vivo complementation studies, however, suggest that both sets of Cys residues are essential for activity [25].
Recent spectroscopic studies have provided evidence for the presence of a second FeS cluster using the Pyrococcus abyssi homolog of TYW1. The UV-visible spectrum of oxidized TYW1 displays a broad shoulder at 410 nm, which is generally consistent with the presence of [4Fe-4S] clusters. Mössbauer spectroscopy reveals a population that is sensitive to the presence of pyruvate, suggesting that it interacts with one of the clusters. EPR spectroscopic measurements under reducing conditions are consistent with the presence of two [4Fe-4S] clusters. The signal for one of the clusters undergoes a change in the presence of SAM [19]. The signal obtained under these conditions is qualitatively similar to the [4Fe-4S]+ observed with lysine 2,3-aminomutase complexed with SAM or S-adenosylhomocysteine [34]. In the presence of pyruvate and SAM, the signal due to the second cluster disappears [19]. The rationale for the conversion of the cluster from a EPR active to silent form is not transparent. We note in passing that the spectroscopic data suggest a change in the environment of the cluster in the presence of pyruvate but that there is no evidence yet for direct coordination.
While the essential role of TYW1 in forming yW was established early and several structures of the protein had been available, in vitro reconstitution of its activity had eluded the field until recently [4,15,25,26]. The imidazoline ring of yW is built on the m1G backbone of tRNA, suggesting that a 2-carbon donor molecule was involved in the reaction. However, activity of the protein could only be reconstituted in vitro with the 3-carbon pyruvate. Stable isotope labeling studies have established that the C2 and C3 carbon atoms of pyruvate are retained whereas the C1 carbon is lost. These findings place substantial constraints on the mechanism by which the imidazoline ring is formed [18].
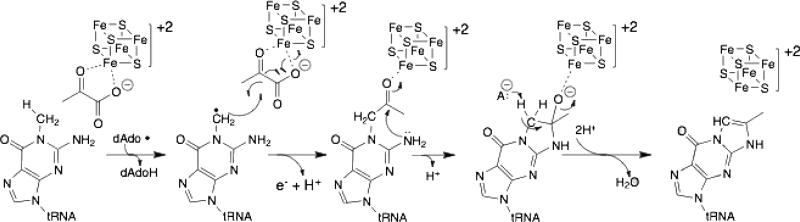
Two mechanistic proposals that take into account the current understanding of the reaction catalyzed by TYW1 are shown in Figures 3 and 4. The first mechanism invokes covalent catalysis involving a catalytically essential conserved Lys residue in the active site of the protein (Figure 3) [25,26]. Pyruvate is proposed to be immobilized in the active site by a Schiff base linkage to this conserved Lys [18]. H-atom abstraction from m1G by 5□-dAdo• leads to formation of a substrate-based radical intermediate, which subsequently combines with the pyruvate. The auxiliary cluster is proposed to play a role in the homolytic scission of the C1 and C2 bond of pyruvate by accepting (or donating) an electron to form formate (or CO2). Subsequent attack via the amino group at C2 of m1G on C2 of pyruvate followed by transimination and tautomerization drive formation of imG-14 [18]. An alternative mechanism proposed in the literature is shown in Figure 4. In this scheme homolytic cleavage of the C1-C2 bond of pyruvate results in formation of CO and a pyruvate m1G adduct. Subsequent attack by m1G C2 amine on C2 of pyruvate creates the tricyclic ring. The second FeS cluster is proposed to facilitate the elimination of water to generate the product [19]. The two proposals have in common activation of the substrate by H-atom abstraction by 5□-dAdo• formed by reductive cleavage of SAM. They differ, however, in whether they invoke covalent catalysis and the proposed role of the auxiliary cluster.
Figure 3.
Mechanistic proposal for catalysis by TYW1. This mechanistic proposal is adapted from Young et al. [18] and involves covalent catalysis and proposes a redox role for the auxiliary cluster.
FIgure 4.
Alternative mechanistic proposal for TYW1. This mechanistic proposal was adapted from Perche-Letuvee et al. [19] and proposes a role for the auxiliary cluster in promoting aromatization.
Both of the mechanistic proposals for the reaction catalyzed by TYW1 utilize the auxiliary cluster in facilitating the cleavage of the C1-C2 bond of pyruvate. The precise role of the cluster will require additional studies, which in particular include determining the fate of the C1 of pyruvate. Loss of C1 as CO2 would require that the cluster be reduced in the course of catalysis and constrains the starting cluster to the +3 or the +2 oxidation states. The formation of formate, however, requires that the cluster be oxidized from the +1 or the +2 states. The spectroscopic studies suggest that under reducing conditions, the resting state of the protein contains two clusters in the +1 oxidation state, which is most consistent with a model whereby the cluster is oxidized in catalysis.
The reaction catalyzed by TYW1 is clearly complex and many aspects of the reaction remain to be established. In addition to the mechanistic complexities, TYW1 from higher organisms has a unique domain architecture consisting of an N-terminal flavodoxin and a C-terminal catalytic domain. The archaeal homologs only have a catalytic domain [25]. The role of the flavodoxin like domain has not been examined; however, all radical SAM enzymes require reductive activation of the radical SAM [4Fe-4S]+2 cluster to the +1 state (see FIgure 2) by an external reductant which, in vivo, is thought to be flavodoxin or a related protein while in vitro a chemical reductant such as dithionite is used [35]. It is possible that the protein in yeast and higher organisms carries its own reductive activation machinery in cis. This is an intriguing possibility as this protein may be a convenient model system for exploring the mechanism of reductive activation of radical SAM enzymes. Moreover, the ability to reduce the cluster as needed by the flavodoxin domain may reduce the potential for abortive cleavage of SAM, which is observed to be generally more prevalent when non-natural reducing systems are used [36].
The identification of the source of the 2-carbon fragment that is required for the formation of imG-14 paves the way for future mechanistic studies of this fascinating radical-mediated transformation.
Future Directions
While nearly all the enzymes in the pathway catalyze reactions requiring SAM, for the most part, these are generally methylation reactions that are precedented. The role of SAM in the radical-mediated conversion of m1G to imG-14 is arguably the most mechanistically novel reaction in the pathway. The availability of structural information, the ability to produce protein replete with iron-sulfur cofactors, and identification of the second substrate in the reaction should accelerate future molecular level insights into this fascinating transformation.
Highlights.
Wybutosine is a hypermodified tRNA base
Biosynthesis of wybutosine requires pyruvate
TYW1 is a member of the radical SAM superfamily
TYW1 catalyzes a complex radical-mediated ring formation of the unique tricyclic ring of wybutosine
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Science of the National Institutes of Health under award number R01 GM72623. Additional support from a Career Award in Biomedical Sciences from the Burroughs Wellcome Fund (to V.B.) is also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FAP, Fabris D, Agris PF. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 201039:D195–D201. doi: 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustilo EM, Vendeix FA, Agris PF. tRNA's modifications bring order to gene expression. Curr Opin in Microbiol. 2008;11:134–140. doi: 10.1016/j.mib.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarty RM, Bandarian V. Biosynthesis of pyrrolopyrimidines. Bioorg Chem. 2012;43:15–25. doi: 10.1016/j.bioorg.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noma A, Kirino Y, Ikeuchi Y, Suzuki T. Biosynthesis of wybutosine, a hypermodified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006;25:2142–2154. doi: 10.1038/sj.emboj.7601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi K, Furutachi N, Funamizu M, Grunberger D, Weinstein IB. Structure of the Fluorescent Y Base From Yeast Phenylalanine Transfer Ribonucleic Acid. J Am Chem Soc. 1970;92:7617–7619. doi: 10.1021/ja00729a035. [DOI] [PubMed] [Google Scholar]
- 6.RajBhandary UL, Chang SH. Studies on polynucleotides. LXXXII. Yeast phenylalanine transfer ribonucleic acid: partial digestion with ribonuclease T1 and derivation of the total primary structure. J Biol Chem. 1968;243:598–608. [PubMed] [Google Scholar]
- 7.Fink LM, Lanks KW, Goto T, Weinstein IB. Comparative studies on mammalian and yeast phenylalanine transfer ribonucleic acids. Biochemistry. 1971;10:1873–1878. doi: 10.1021/bi00786a022. [DOI] [PubMed] [Google Scholar]
- 8.Dudock BS, Katz G, Taylor EK, Holley RW. Primary structure of wheat germ phenylalanine transfer RNA. Proc Natl Acad Sci USA. 1969;62:941–945. doi: 10.1073/pnas.62.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Crecy-Lagard V, Brochier-Armanet C, Urbonavicius J, Fernandez B, Phillips G, Lyons B, Noma A, Alvarez S, Droogmans L, Armengaud J, et al. Biosynthesis of Wyosine Derivatives in tRNA: An Ancient and Highly Diverse Pathway in Archaea. Mol Biol Evol. 2010;27:2062–2077. doi: 10.1093/molbev/msq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCloskey JA, Graham DE, Zhou S, Crain PF, Ibba M, Konisky J, Söll D, Olsen GJ. Post-transcriptional modification in archaeal tRNAs: identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic Methanococcales. Nucleic Acids Res. 2001;29:4699–4706. doi: 10.1093/nar/29.22.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCloskey JA, Liu XH, Crain PF, Bruenger E, Guymon R, Hashizume T, Stetter KO. Posttranscriptional modification of transfer RNA in the submarine hyperthermophile Pyrolobus fumarii. Nucleic Acids Symp Ser. 2000 doi: 10.1093/nass/44.1.267. [no volume] [DOI] [PubMed] [Google Scholar]
- 12.McCloskey JA, Crain PF, Edmonds CG, Gupta R, Hashizume T, Phillipson DW, Stetter KO. Structure determination of a new fluorescent tricyclic nucleoside from archaebacterial tRNA. Nucleic Acids Res. 1987;15:683–693. doi: 10.1093/nar/15.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmonds CG, Crain PF, Gupta R, Hashizume T, Hocart CH, Kowalak JA, Pomerantz SC, Stetter KO, McCloskey JA. Posttranscriptional Modification of tRNA in Thermophilic Archaea (Archaebacteria). J Bacteriol. 1991;173:3138–3148. doi: 10.1128/jb.173.10.3138-3148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiebe R, Poralla K. Origin of the nucleoside Y in yeast tRNAPhe. FEBS Lett. 1973;38:27–28. doi: 10.1016/0014-5793(73)80504-6. [DOI] [PubMed] [Google Scholar]
- 15.Waas WF, de Crécy-Lagard VV, Schimmel PP. Discovery of a Gene Family Critical to Wyosine Base Formation in a Subset of Phenylalanine-Specific Transfer RNAs. J Biol Chem. 2005;280:37616–37622. doi: 10.1074/jbc.M506939200. [DOI] [PubMed] [Google Scholar]
- 16.Umitsu M, Nishimasu H, Noma A, Suzuki T, Ishitani R, Nureki O. Structural basis of AdoMet-dependent aminocarboxypropyl transfer reaction catalyzed by tRNA-wybutosine synthesizing enzyme, TYW2. Proc Natl Acad Sci USA. 2009;106:15616–15621. doi: 10.1073/pnas.0905270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki Y, Noma A, Suzuki T, Ishitani R, Nureki O. Structural basis of tRNA modification with CO2 fixation and methylation by wybutosine synthesizing enzyme TYW4. Nucleic Acids Res. 2009;37:2910–2925. doi: 10.1093/nar/gkp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Young AP, Bandarian V. Pyruvate Is the Source of the Two Carbons That Are Required for Formation of the Imidazoline Ring of 4-Demethylwyosine. Biochemistry. 2011;50:10573–10575. doi: 10.1021/bi2015053. This paper identified the second substrate of TYW1 as pyruvate allowing mechanistic studies of the protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Perche-Letuvee P, Kathirvelu V, Berggren G, Clemancey M, Latour J-M, Maurel V, Douki T, Armengaud J, Mulliez E, Fontecave M, et al. 4-Demethylwyosine Synthase from Pyrococcus abyssi is a Radical-S-adenosyl-L-methionine Enzyme with an Additional [4Fe-4S]+2 Cluster That Interacts with the Pyruvate Co-substrate. J Biol Chem. 2012;287:41174–41185. doi: 10.1074/jbc.M112.405019. This study showed the presence of two iron sulfur clusters in TYW1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey PA, Hegeman AD, Ruzicka FJ. The radical SAM superfamily. Crit Rev Biochem Mol Biol. 2008;43:63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 21*.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. This paper recognized for the first time the CxxxCxxC sequence motif that is common to all enzymes in the radical SAM superfamily. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowling DP, Vey JL, Croft AK, Drennan CL. Structural diversity in the AdoMet radical enzyme superfamily. Biochim Biophys Acta. 2012;1824:1178–1195. doi: 10.1016/j.bbapap.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booker SJ. Radical SAM enzymes and radical enzymology. Biochim Biophys Acta. 2012;1824:1151–1153. doi: 10.1016/j.bbapap.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Roach PL. Radicals from S-adenosylmethionine and their application to biosynthesis. Curr Opin Chem Biol. 2011;15:267–275. doi: 10.1016/j.cbpa.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Noma A, Suzuki T, Senda M, Senda T, Ishitani R, Nureki O. Crystal Structure of the Radical SAM Enzyme Catalyzing Tricyclic Modified Base Formation in tRNA. J Mol Biol. 2007;372:1204–1214. doi: 10.1016/j.jmb.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Goto-Ito S, Ishii R, Ito T, Shibata R, Fusatomi E, Sekine S, Bessho Y, Yokoyama S. Structure of an archaeal TYW1, the enzyme catalyzing the second step of wye-base biosynthesis. Acta Cryst. 2007:D63. doi: 10.1107/S0907444907040668. 2007, doi:10.1107/S0907444907040668. [DOI] [PubMed] [Google Scholar]
- 27*.Lanz ND, Booker SJ. Identification and function of auxiliary iron-sulfur clusters in radical SAM enzymes. Biochim Biophys Acta. 2012;1824:1196–1212. doi: 10.1016/j.bbapap.2012.07.009. This is an excellent review on the role(s) of auxiliary clusters in radical SAM enzymes. [DOI] [PubMed] [Google Scholar]
- 28.Bui BT, Florentin D, Fournier F, Ploux O, Méjean A, Marquet A. Biotin synthase mechanism: on the origin of sulphur. FEBS Lett. 1998;440:226–230. doi: 10.1016/s0014-5793(98)01464-1. [DOI] [PubMed] [Google Scholar]
- 29.Cicchillo RM, Iwig DF, Jones AD, Nesbitt NM, Baleanu-Gogonea C, Souder MG, Tu L, Booker SJ. Lipoyl synthase requires two equivalents of S-adenosyl-L-methionine to synthesize one equivalent of lipoic acid. Biochemistry. 2004;43:6378–6386. doi: 10.1021/bi049528x. [DOI] [PubMed] [Google Scholar]
- 30.Hänzelmann P, Schindelin H. Binding of 5′-GTP to the C-terminal FeS cluster of the radical S-adenosylmethionine enzyme MoaA provides insights into its mechanism. Proc Natl Acad Sci USA. 2006;103:6829–6834. doi: 10.1073/pnas.0510711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lees NS, Hänzelmann P, Hernandez HL, Subramanian S, Schindelin H, Johnson MK, Hoffman BM. ENDOR Spectroscopy Shows That Guanine N1 Binds to [4Fe− 4S] Cluster II of the S-Adenosylmethionine-Dependent Enzyme MoaA: Mechanistic Implications. J Am Chem Soc. 2009;131:9184–9185. doi: 10.1021/ja903978u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grove TL, Ahlum JH, Sharma P, Krebs C, Booker SJ. A Consensus Mechanism for Radical SAM-Dependent Dehydrogenation? BtrN Contains Two [4Fe-4S] Clusters. Biochemistry. 2010;49:3783–3785. doi: 10.1021/bi9022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grove TL, Lee K-H, St Clair J, Krebs C, Booker SJ. In Vitro Characterization of AtsB, a Radical SAM Formylglycine-Generating Enzyme That Contains Three [4Fe-4S] Clusters. Biochemistry. 2008;47:7523–7538. doi: 10.1021/bi8004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieder KW, Booker S, Ruzicka FJ, Beinert H, Reed GH, Frey PA. S-Adenosylmethionine-Dependent Reduction of Lysine 2,3-Aminomutase and Observation of the Catalytically Functional Iron-Sulfur Centers by Electron Paramagnetic Resonance. Biochemistry. 1998;37:2578–2585. doi: 10.1021/bi972417w. [DOI] [PubMed] [Google Scholar]
- 35.Duschene KS, Veneziano SE, Silver SC, Broderick JB. Control of radical chemistry in the AdoMet radical enzymes. Curr Opin Chem Biol. 2009;13:74–83. doi: 10.1016/j.cbpa.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grove TL, Benner JS, Radle MI, Ahlum JH, Landgraf BJ, Krebs C, Booker SJ. A Radically Different Mechanism for S-Adenosylmethionine-Dependent Methyltransferases. Science. 2011;332:604–607. doi: 10.1126/science.1200877. [DOI] [PubMed] [Google Scholar]