Abstract
Objective
In this study, we investigated the role of TFF1 in regulating cell proliferation and tumor development through β-catenin signaling using in vivo and in vitro models of gastric tumorigenesis.
Design
TFF1-Knockout mice, Immunohistochemistry, luciferase reporter, qRT-PCR, immunoblot and phosphatase assays were used to examine the role of TFF1 on β-catenin signaling pathway.
Results
Nuclear localization of β-catenin with transcriptional upregulation of its target genes, c-Myc and Ccnd1, was detected in hyperplastic tissue at early age of 4-6 weeks and maintained during all stages of gastric tumorigenesis in the Tff1-knockout mice. The reconstitution of TFF1 or TFF1 conditioned media significantly inhibited the β-catenin/TCF transcription activity in MKN28 gastric cancer cells. In agreement with these results, we detected a reduction in the levels of nuclear β-catenin with downregulation of c-MYC and CCND1 mRNA. Analysis of signaling molecules upstream of β-catenin revealed a decrease in p-GSK3β (Ser9) and p-AKT (Ser473) protein levels following the reconstitution of TFF1 expression; this was consistent with the increase of p-β-catenin (Ser33/37/Thr41) and decrease of p-β-catenin (Ser552). This TFF1-induced reduction in phosphorylation of GSK3β and AKT was dependent on PP2A activity. The treatment with okadaic acid or knockdown of PP2A abrogated these effects. Consistent with the mouse data, we observed loss of TFF1 and an increase in nuclear localization of β-catenin in stages of human gastric tumorigenesis.
Conclusion
Our data indicate that loss of TFF1 promotes β-catenin activation and gastric tumorigenesis through regulation of PP2A, a major regulator of AKT-GSK3β signaling.
Keywords: TFF1, β-catenin, gastric cancer, PP2A
Introduction
Gastric cancer is one of the most common cancers worldwide and second in mortality after lung cancer [1]. Cancer development and progression are a result of an accumulation of contributing events such as infectious, environmental, and genetic risk factors. For instance, Helicobacter pylori infection and dietary factors are important risk factors for gastric carcinogenesis, while several genetic polymorphisms are found to be associated with a predisposition to cancer development [2]. Although the diagnostic capabilities and therapeutic options have improved, the prognosis for gastric cancer patients remains poor, especially in advanced stages [3].
Trefoil factor 1 (TFF1) is a member of the trefoil factor family peptides that are cysteine-rich proteins and form a characteristic trefoil domain through disulfide bonds, which make them resistant to hazardous conditions such as low pH and proteolysis [4]. TFF1 is expressed predominantly in the gastric epithelia and secreted by the mucus-secreting pit cells of the corpus and antropyloric regions of the stomach [5, 6]. TFF1 has been reported as a gastric-specific tumor suppressor gene [7]. The TFF1 protein, which is secreted as a component of the protective mucus layer in the stomach, is highly expressed in response to mucosal injury [8]. Previous reports have shown loss of TFF1 protein expression in more than two-thirds of gastric adenocarcinomas because of mutation-independent mechanisms [9, 10]. The silencing of the TFF1 gene expression in gastric cancer is predominantly induced by loss of heterozygosity (LOH) and hypermethylation of the TFF1 promoter [9, 11, 12]. In addition, transcriptional regulation of TFF1 by COBRA1 have also been described in gastric cancers [10]. Using the Tff1-knockout mouse model, we and others have provided strong evidence supporting a tumor suppressor role for Tff1 in gastric tumorigenesis, and demonstrated that Tff1 is essential for normal differentiation of the antral and pyloric gastric mucosa [13, 14].
Activation of β-catenin is a frequent molecular event associated with the malignant transformation in the colon and upper gastrointestinal adenocarcinomas [15, 16]. Previous reports have indicated that β-catenin promotes cell proliferation by the induction of transcription of several genes such as CCND1 and c-MYC through the activation of T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factors [17, 18]. In normal epithelial cells, cytoplasmic β-catenin is tightly regulated by the adenomatous polyposis coli (APC) tumor-suppressor protein and glycogen synthase kinase-3β (GSK3β) leading to its phosphorylation at Ser33/34/Thr41 residues, ubiquitination, and proteasomal degradation [19, 20]. The accumulation of nuclear β-catenin is one of the hallmarks of activated β-catenin signaling in cancer; this activation was reported to be regulated by AKT-PP2A through phosphorylation of β-catenin at Ser552 and Ser675 [21]. In addition, AKT-dependent phosphorylation and inhibition of GSK3β activity enhances β-catenin protein stability through decreased phosphorylation of β-catenin at Ser33/34/Thr41 residues [22, 23]. Protein phosphatases play an important role in regulating the phosphorylation and activity of several kinases in cancer [24]. Protein phosphatase 2A (PP2A) is a highly complex trimeric holoenzyme, which consists of catalytic, structural and regulatory subunits. The catalytic domain of PP2A (PP2Ac) has been implicated in the regulation of the AKT-GSK3β-β-catenin signaling [25].
The role of TFF1 in regulating cell proliferation and the underlying mechanism in gastric tumorigenesis remain unclear. In this study, using the Tff1-knockout mouse model together with in vivo, ex vivo, and in vitro experiments; we demonstrated that TFF1 silencing enhances cell proliferation by activating β-catenin through regulation of the PP2A activity upstream of the AKT and GSK3β signaling in gastric cancer.
Materials & Methods
Animals: histologic evaluation and immunohistochemical assessment
The Tff1-knockout mouse model of gastric tumorigenesis [13], Tff1-heterozygote and normal wild-type mice were maintained under barrier conditions in a pathogen-free state in accordance with Institutional Animal Care and Use Committee approved protocol at Vanderbilt University. Frozen and formalin fixed paraffin-embedded stomach tissue samples were collected from Tff1-knockout (n=49), Tff1-heterozygote (n=10), and wild-type mice (n=28). Histopathological classification and grading of the gastric tissues were performed by our pathologists on H&E stained sections.
Primary gastric epithelial cell extraction and short-term culture
For preparation of short-term cultures of primary gastric epithelial cells from the Tff1-knockout and wild-type mice, stomachs were removed from 8-week-old mice and opened as described above. After washing with HBSS, the gastric antrum was cut and incubated in 10 ml of 1 mmol/L dithiothreitol for 15 minutes at 37°C with shaking, washed in HBSS 3 times at 37°C, and incubated in 0.5 mg/ml collagenase for 30 minutes at 37°C. After the first collagenase digestion, tissues were washed again with HBSS 3 times and incubated for an additional 30 minutes in collagenase (0.37 mg/ml) at 37°C. Tissues were triturated using a wide-mouthed pipette, and larger fragments of tissue were allowed to settle under gravity for 45 seconds. The supernatant containing isolated gastric cells was removed and transferred to a clean 50-ml conical tube and left on ice to sediment for 45 minutes. The supernatant was then carefully removed and discarded, and isolated cell colonies were plated in chamber slides. Colonies of gastric epithelial cells were cultured in F-12 (Ham's medium) supplemented with 10% FBS and 1% of antibiotic-antimycotic solution (Invitrogen Life Technologies). The cells were cultured for up to 72 hours in a humidified incubator at 37°C under an atmosphere of 5% CO2, and the medium was changed every 24 hours.
Reconstitution of TFF1 expression in cell lines
MKN28 cells were obtained from ATCC and were cultured in Ham's F-12 supplemented with 10% FBS (Invitrogen Life Technologies) at 37°C in an atmosphere containing 5% CO2. In order to reconstitute the expression of TFF1 in MKN28 cells using adenovirus system, The TFF1 coding sequence from pcDNA3.1/TFF1 plasmid was subcloned into the adenoviral shuttle vector (pACCMV). The recombinant adenovirus-expressing TFF1 was generated by co-transfecting HEK-293 cells with the shuttle and backbone adenoviral (pJM17) plasmids using the Calcium Phosphate Transfection kit (Applied Biological Materials). After infection, the MKN28 cells were analyzed for the expression of TFF1 by qRT-PCR.
Statistical analysis
Using GraphPad Prism software, a 1-way ANOVA Newman-Keuls Multiple Comparisons Test was used to compare the differences between 3 groups or more, and a 2-tailed Student's test was used to compare the statistical difference between 2 groups. The correlation between 2 parameters was determined by Spearman correlation. The differences were considered statistically significant when the P value was < 0.05.
Supplementary Methods
The methods that describe conditioned media, immunofluorescence, quantitative PCR, luciferase reporter, Western blotting, proliferation, phosphatase assay immunoprecipitation, shRNA knockdown, and immunohistochemistry on tissue microarrays assays are included in the Supplementary Materials and Methods.
Results
Loss of Tff1 activates β-catenin in gastric hyperplasia and subsequent tumorigenesis cascade in mouse
Immunohistochemistry analysis of β-catenin staining of the pyloric antrum gastric tissues of the Tff1-knockout (Tff1-KO) mice revealed a moderate-strong cytosolic and nuclear immunostaining of β-catenin as early as 4-6 weeks of age. In contrast, the expression of β-catenin was limited to the cell membrane in Tff1-wild-type (Tff1-WT) and Tff1-heterozygote (Tff1-HET) mice of similar age (Figure 1A). To confirm the activation of β-catenin at an early age, the mRNA expression of β-catenin target genes, c-Myc and Ccnd1, was evaluated by qRT-PCR. The results showed a significant increase of mRNA expression of c-Myc (P < 0.01) and Ccnd1 (P < 0.05) in Tff1-KO as compared to Tff1-WT and Tff1-HET (Figure 1B). Notably, the mRNA expression of c-Myc and Ccnd1 genes was not significantly different between Tff1-WT and Tff1-HET. The mRNA expression of the Tff1 gene was confirmed in all three groups of mice using qRT-PCR (Supplemental Figure S1). Indeed, the results demonstrated no significant correlation between the mRNA expression of c-Myc or Ccnd1 and different age groups in Tff1-KO mice (Supplemental Figure S2). Next, the immunohistochemistry analysis of gastric tissues revealed an increase of nuclear β-catenin expression in hyperplasia, low-grade dysplasia (LGD), high-grade dysplasia (HGD), and adenocarcinoma (AC) in the pyloric antrum of Tff1-KO mice as compared to normal Tff1-WT mice (Figure 1C). To confirm the activation of β-catenin in the tissue samples, the mRNA expression of β-catenin target genes, c-Myc and Ccnd1, was evaluated by qRT-PCR. The results showed a significant increase of mRNA expression of c-Myc (P < 0.001) and Ccnd1 (P < 0.05) in hyperplasia and remained elevated in advanced lesions (LGD, HGD, and AC) in Tff1-KO mice as compared to normal wild-type (Figure 1D). These results are concordant with previous reports indicating that β-catenin nuclear translocation is an early event in gastric adenocarcinoma and several other cancers [26]. Taken together, the data suggest that loss of Tff1 promotes β-catenin activation at an early age (4-6 weeks) and stage of gastric tumorigenesis independent of age increase in the Tff1-KO gastric cancer mouse model.
Figure 1. Loss of TFF1 promotes (β-catenin activation in hyperplastic tissue prior to onset of tumorigenesis in a gastric cancer mouse model.
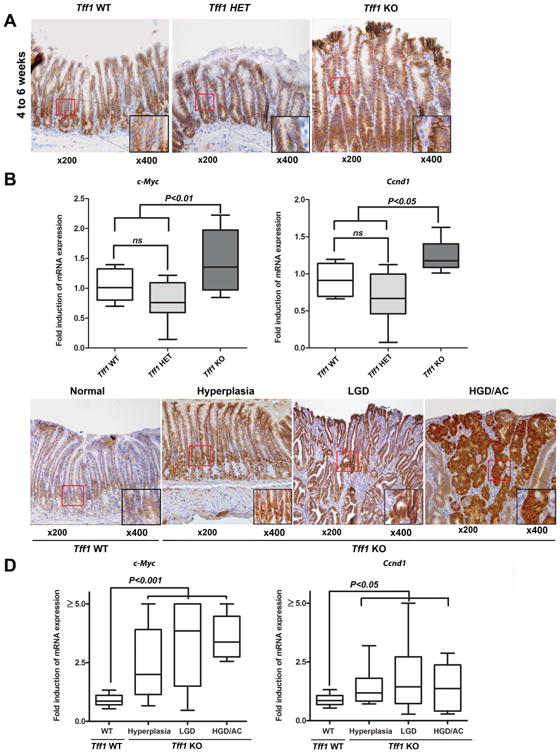
A) Immunohistochemistry analysis of β-catenin expression in the antropyloric gastric mucosa in Tff1-wild-type (Tff1-WT), Tff1-heterozygote (Tff1-HET) and Tff1-knockout (Tff1-KO) recently weaned mice, 4-6 weeks of age. B) Quantitative real-time PCR analysis demonstrated c-Myc and Ccnd1 mRNA expression levels in Tff1-WT, Tff1-HET and Tff1-KO 4-6 weeks of age. C) Immunohistochemistry analysis of β-catenin protein expression in the antropyloric gastric mucosa of representative histological features from wild-type demonstrated membranous β-catenin expression. The Tff1-KO mice with hyperplasia, low-grade dysplasia (LGD), and high-grade dysplasia or adenocarcinoma (HGD/AC) showed strong cytosolic and nuclear immunostaining of β-catenin. D) Quantitative real-time PCR analysis demonstrated c-Myc and Ccnd1 mRNA expression levels in histological stages (WT, Hyperplasia, LGD, and HGD/AC). Original magnification (×200) and insets (×400) are shown (A and C). Box-and-whisker plots used in (B and D) depict the smallest value, lower quartile, median, upper quartile, and largest value.
TFF1 regulates β-catenin localization, transcriptional activity, and downstream targets
To explore the role of TFF1 in regulating β-catenin, we evaluated β-catenin cellular localization following modulation of TFF1 expression in primary gastric epithelial cells isolated from Tff1-WT and Tff1-KO mice, and human gastric cancer epithelial cells (MKN28). The immunofluorescence data showed that β-catenin was predominantly localized in the nucleus in Tff1-KO mouse gastric epithelial cells more than in wild-type cells (P < 0.001) (Figure 2A). In agreement with the ex vivo mouse data, the MKN28 cell model results revealed strong nuclear β-catenin staining that was significantly abrogated following reconstitution of TFF1 (P < 0.001) (Figure 2B). The results also indicated a substantial increase in β-catenin cell membrane staining in TFF1-expressing cells as compared to control cells (Figure 2B). Western blot analysis data of nuclear and cytosolic protein fractions from MKN28 cells infected with control or TFF1 adenoviruses indicated less nuclear β-catenin expression in TFF1-expressing cells than control cells, consistent with the immunofluorescence data (Supplemental Figure S3). We investigated whether the secreted TFF1 protein could regulate β-catenin expression in MKN28 cells. The immunofluorescence data showed that nuclear β-catenin expression was significantly reduced in cells treated with TFF1 conditioned media as compared to control cells (P < 0.001, Figure 3C). To characterize the suppressive effect of TFF1 on β-catenin transcriptional activity, the TCF transcription activity was measured using pTopFlash and its mutant pFopFlash luciferase reporters. The infection of MKN28 cells with the TFF1 adenovirus led to a 50% decrease (P < 0.01) in the pTopFlash reporter activity as compared to the control adenovirus (Figure 3A). We confirmed the suppressive effect of TFF1 on β-catenin activity by luciferase reporter assays following treatment of MKN28 cells with TFF1 conditioned media (Figure 3B) or transfection with PTT5 or PTT5-TFF1 plasmids (Figure 3C). Collectively, these data from ex vivo and in vitro studies indicated that TFF1 decreases the β-catenin nuclear localization and its transcriptional activity in gastric epithelial cells.
Figure 2. TFF1 expression regulates nuclear localization of β-catenin.
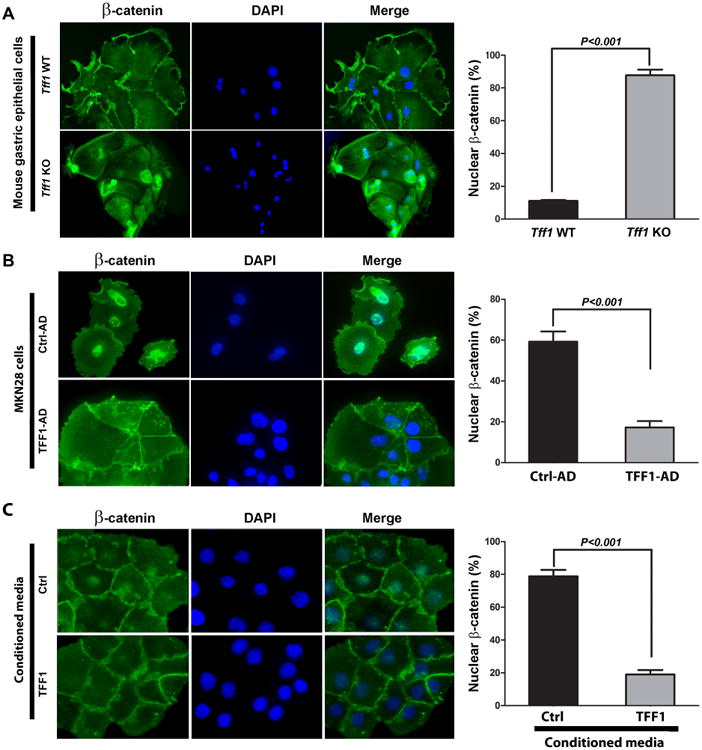
A) Ex vivo immunofluorescence staining of β-catenin in normal gastric epithelial cells isolated from the antropyloric region in wild-type or Tff1-KO mice. B) In vitro immunofluorescence assay of β-catenin in MKN28 cells infected with control (Ctrl-AD) or TFF1 (TFF1-AD) adenoviruses. (C) In vitro immunofluorescence assay of β-catenin in MKN28 cells treated with control or TFF1 conditioned media. Nuclear localization of β-catenin is shown in green. DAPI (blue) was used as a nuclear counterstain. Original magnification, ×400. The quantification of nuclear β-catenin positive staining in at least 200 counted cells presented as percentage ± SEM are shown in the right panels of (A, B and C). P < 0.001, Student's t test.
Figure 3. TFF1 attenuates transcriptional activation of β-catenin.
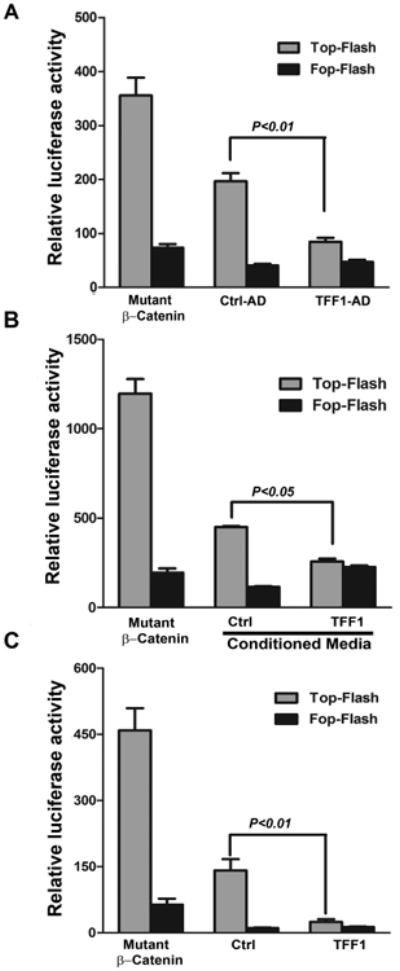
A-C) p-TOP-flash and its mutant p-FOP-flash luciferase reporter assays showing β-catenin and TCF-LEF transcriptional activity. A) MKN28 cells infected with control or TFF1 adenoviruses. B) MKN28 cells treated with control or TFF1 conditioned media. C) MKN28 cells transiently transfected with PTT5 control or PTT5-TFF1 expression plasmids. Mutant β-catenin was used as positive control. Data are presented as mean ± SEM of at least three experiments, with each condition performed in triplicate.
To investigate whether the TFF1-induced suppression of β-catenin transcriptional activity leads to downregulation of downstream targets such as c-Myc and cyclin D1, we evaluated mRNA and protein expression of these targets in in vivo and in vitro models. The results revealed a significant increase in mRNA levels of Ccnd1 and c-Myc (P < 0.01, Figure 4A), and Axin-2 (P < 0.05, Supplemental Figure S4A) in Tff1-KO mice as compared to wild-type. In accordance with mRNA results, Western blot analysis demonstrated substantially increased c-Myc and cyclin D1 protein expression in the Tff1-KO mice as compared to wild-type (Figure 4B). The β-catenin protein level was higher in the Tff1-KO mice than wild-type (Figure 4B).
Figure 4. TFF1 negatively regulates mRNA and protein expression of β-catenin target genes in vivo and in vitro.
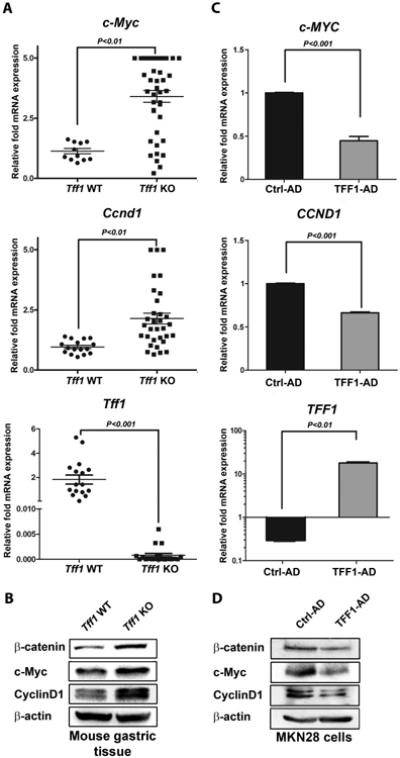
A) qRT-PCR data demonstrate mRNA upregulation of β-catenin target genes (c-Myc and Ccnd1) in Tff1-KO mice as compared to wild-type. The Tff1 gene expression was verified in wild-type and Tff1-KO mice. Horizontal bars indicate the mean values. P < 0.05 was considered statistically significant. B) Western blot analysis indicated an increase in protein levels of β-catenin, c-Myc, and cyclin D1 in Tff1-KO mice relative to wild-type. C) qRT-PCR data indicate mRNA down-regulation of β-catenin target genes (c-MYC and CCND1) in MKN28 cells infected with TFF1 adenovirus as compared to control. Reconstitution of TFF1 expression in MKN28 cells was confirmed. The bar graphs represent the mean ± SEM of 3 independent experiments. D), Western blot analysis showed that transient expression of TFF1 in MKN28 cells decreased protein levels of β-catenin, c-MYC, and Cyclin D1 as compared to control. Protein loading was normalized for equal levels of β-actin. The Western blot results represent 1 of 3 independent experiments.
In line with the in vivo data, the MKN28 cell model qRT-PCR data showed a significant decrease in mRNA expression of c-MYC and CCND1 (P < 0.001, Figure 4C), and AXIN-2 (P < 0.001, Supplemental Figure S4B) in TFF1-expressing cells as compared to control cells. Western blot analysis results confirmed the decrease in protein expression of β-catenin, c-MYC, and cyclin D1 in TFF1-expressing cells as compared to control cells (Figure 4D). To determine if secreted TFF1 is functional, we treated MKN28 cells with conditioned media and evaluated protein expression by Western blot analysis. The results showed that secreted TFF1 induced a decrease in β-catenin, cyclin D1, and c-MYC protein levels (Supplemental Figure S5). To ascertain that c-Myc is a β-catenin target gene, we performed c-Myc luciferase reporter assay in MKN28 cell infected with control or TFF1 adenoviruses. The data indicated that TFF1 expression significantly decreased c-Myc transcriptional activity (P < 0.01). The c-Myc luciferase reporter with a mutation in the TCF binding site was used as a negative control (Supplemental Figure S6). Taken together, the data demonstrated that TFF1 is a negative regulator of β-catenin activity and downstream targets in vivo and in vitro.
TFF1 regulates gastric epithelial cell proliferation in vivo and in vitro
The promotion of cellular proliferation is one of the important functions of β-catenin signaling in cancer [27]. To investigate the effect of TFF1-dependent regulation of β-catenin on cell growth and proliferation, we subjected gastric tissues from mice, 4-6 weeks of age and up, to immunohistochemical staining of Ki-67, and MKN28 cells to EdU (5-ethynyl-2′-deoxyuridine) staining. In the wild-type gastric tissues, Ki-67 staining was observed in the basal proliferative region of the glands or crypts (Figure 5A-B). On the other hand, the Tff1-KO gastric tissues exhibited Ki-67 staining that extended to the surface of the mucosa (Figure 5A-B). The quantification data demonstrated that in Tff1-KO mice 70-80% of gastric epithelial cells exhibited Ki-67 staining per gland; however, in wild type mice 32% or less of cells were positive for Ki-67 per gland (Figure 5A-B). In line with these data, the EdU results showed that incorporation of EdU was significantly lower in MKN28 cells infected with TFF1 adenovirus or treated with TFF1 conditioned media than control cells (P < 0.01, Figure 5C-D). These results demonstrated the role of TFF1 in regulating gastric epithelial cell proliferation in vivo and in vitro.
Figure 5. TFF1 regulates gastric cell proliferation in vivo and in vitro.
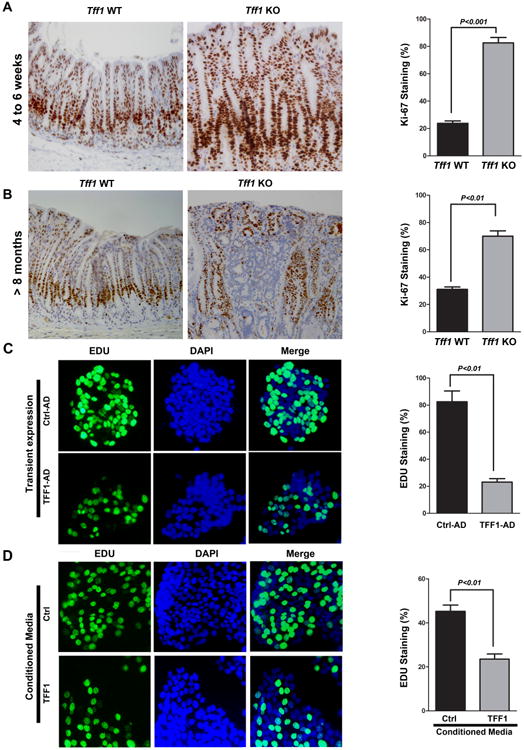
A-B) Representative images of Ki-67 immunostaining (brown nuclei) in gastric glands of wild-type and Tff1-KO mice, 4-6 weeks of age (A) and 8 months and up (B). Original magnification (×200) is shown. The quantitative data of Ki-67 immunostaining in at least 200 counted cells were presented as percentage ± SEM in the right panels. C-D) Representative images of EdU immunostaining in MKN28 cells infected with control or TFF1 adenoviruses (C), or incubated with TFF1 or control conditioned media (D). Cells were subjected to ClickiT® EdU Assay to evaluate cell proliferation. EdU staining is depicted by green fluorescence. DAPI (blue) was used as nuclear counterstain. The quantitative data of at least 200 counted cells indicating a significant decrease of cell proliferation in TFF1-expressing cells or cells treated with TFF1 conditioned media as compared to control cells were presented in the right panels of (C and D).
TFF1 inhibits β-catenin signaling through activation of GSK3β
Our next step was to investigate if TFF1 inhibits β-catenin through regulation of GSK3β. Therefore, we transiently expressed TFF1 in MKN28 cells in the presence or absence of LiCl, a specific GSK3β inhibitor that leads to the phosphorylation of GSK3β at Ser9 with inhibition of its kinase and subsequent activation of β-catenin. As expected, the luciferase reporter assay data showed that the β-catenin activity in control cells treated with LiCl was significantly higher than non-treated cells (P < 0.05, Figure 6A). On the other hand, TFF1-expressing cells exhibited significantly less β-catenin activity than control cells (P < 0.05, Figure 6A). However, treatment with LiCL significantly abrogated TFF1 effect, rescuing β-catenin activity to control level (Figure 6A). The Western blot results indicated that TFF1 expression significantly decreased p-p-GSK3β (Ser9), p-β-catenin (Ser552), β-catenin, and increased p-β-catenin (Ser33/37/Thr41) protein levels as compared to control. However, in TFF1-expressing cells, LiCl counteracted the effects of TFF1 (Figure 6B). Taken together, these results indicated that TFF1 can inhibit β-catenin signaling through activation of GSK3β.
Figure 6. TFF1 suppresses AKT-β-catenin signaling through activation of GSK3β and PP2A.
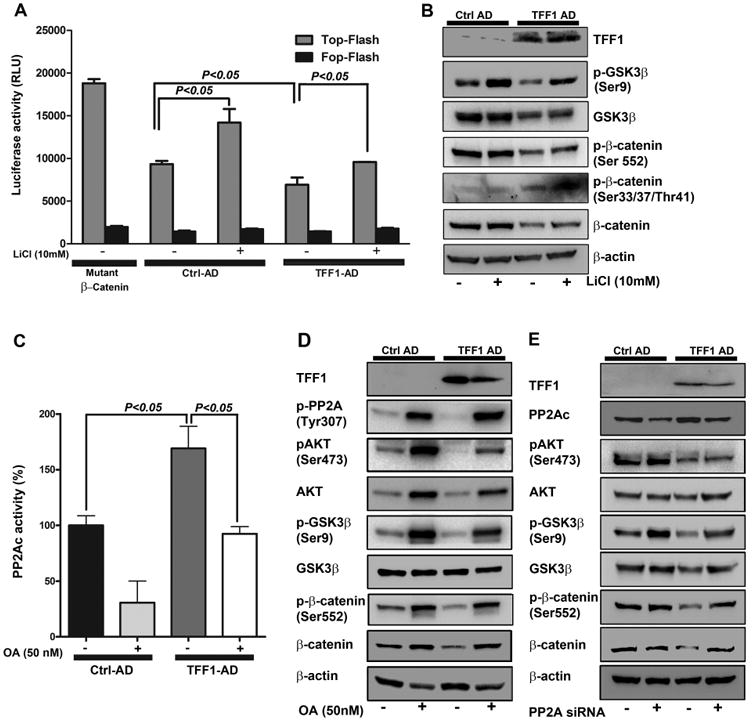
A-B) Reconstitution of TFF1 attenuates β-catenin transcriptional activity and signaling through activation of GSK3β. A) p-TOP-flash and its mutant p-FOP-flash luciferase reporter assays showing β-catenin/TCF transcriptional activity in MKN28 cells infected with Control or TFF1 adenoviruses and treated overnight with vehicle or LiCl (10 mM). Data are presented as mean ± SEM of at least three experiments, with each condition performed in triplicate. P<0.05 was considered significant. B) Western Blot analysis of p-β-catenin (Ser552) and (Ser33, 37, Thr41), β-catenin, p-GSK3β (Ser9), GSK3β, TFF1 and β-actin proteins. C, D&E) Reconstitution of TFF1 suppresses β-catenin signaling through activation of PP2A. C) MKN28 cells were infected with control or TFF1 adenoviruses, treated with vehicle or okadaic acid (OA, 50 nM) for 16 h, and subjected to PP2A phosphatase activity assay. Data are presented as mean ± SEM of at least three experiments. D&E) MKN28 cells were infected with control or TFF1 adenoviruses treated with vehicle or OA (D) or transfected with control or PP2Ac siRNA (E); all samples were subjected to Western blot analysis. The Western blot results represent 1 of 3 independent experiments.
TFF1 suppresses the AKT-β-catenin pathway through PP2A activation
In addition to kinases, protein phosphatases such as PP2A are implicated in β-catenin regulation [28]. The results showed that the PP2A phosphatase activity was significantly higher in TFF1-expressing MKN28 cells than control cells (P < 0.05). Of note, the TFF1-induced increase of PP2A activity was completely abrogated upon treatment with okadaic acid (OA), a selective inhibitor of PP2A (P < 0.05, Figure 6C). In a control experiment, the treatment of cells with OA decreased PP2A activity by 69% relative to control cells (P < 0.05, Figure 6C). To investigate the involvement of PP2A in TFF1-β-catenin signaling, we treated control or TFF1-expressing MKN28 cells with or without OA followed by Western blot analysis. The data showed that, in control cells, there was an increase of p-AKT (Ser473), p-GSK3β (Ser9), p-β-catenin (Ser552), and β-catenin protein levels after treatment with OA (Figure 6D). As expected, in TFF1-expressing cells, there was a decrease in the expression levels of all these proteins. However, inhibition of PP2A activity with OA counteracted the TFF1-induced decrease of p-GSK3β (Ser9), p-AKT (ser473), p-β-catenin (Ser552), and β-catenin protein levels in MKN28 cells (Figure 6D). It has been reported that the phosphorylation of PP2A catalytic subunit at Tyr307 residue indicates inhibition of PP2A [29]. Our Western blot data revealed that transient expression of TFF1 in MKN28 cells decreased p-PP2AC (Tyr307) protein level as compared to control (Figure 6D). Treatment with OA reversed the effect of TFF1 on PP2AC phosphorylation in these cells (Figure 6D). To confirm the role of PP2A in TFF1-β-catenin signaling, we transfected control or TFF1-expressing MKN28 cells with control siRNA or PP2Ac siRNA. Western blot analysis data showed an increase in the protein levels of p-GSK3β (Ser9), p-β-catenin (Ser552), and β-catenin in MKN28/TFF1 cells transfected with PP2Ac siRNA as compared to MKN28/TFF1 cells transfected with control siRNA (Figure 6E). This confirms the results of PP2A inhibition with OA (Figure 6D). Taken together, the results indicate that activation of PP2A by TFF1 is an important step that mediates TFF1-induced suppression of β-catenin signaling in gastric cancer cells.
TFF1 Loss and β-catenin activation during the multistep human gastric carcinogenesis cascade
Using immunohistochemistry on tissue microarrays, we evaluated the protein expression of TFF1 and β-catenin in human gastric lesions and normal mucosa. The data showed strong immunostaining of TFF1 in normal gastric glands, while β-catenin staining was mostly restricted to the cell membrane (Figure 7A). Conversely, in dysplasia and gastric adenocarcinomas, TFF1 expression was substantially attenuated whereas β-catenin was mostly expressed in the nucleus (Figure 7B-C). Our quantitative results showed a significant decrease in TFF1 expression in intestinal metaplasia (IM), dysplasia (Dys), and adenocarcinoma as compared to normal glands (NG) (P < 0.001, Figure 7D). In contrast, we demonstrated a significant increase in nuclear β-catenin expression along the multistep gastric carcinogenesis cascade from IM, Dys, to adenocarcinoma as compared to NG (P < 0.001) (Figure 7E). Of note, we detected a significant inverse correlation between nuclear β-catenin and TFF1 expression (r=-0.5; P < 0.001) (Figure 7F).
Figure 7. Immunohistochemistry for β-catenin and TFF1 in human tissue samples.
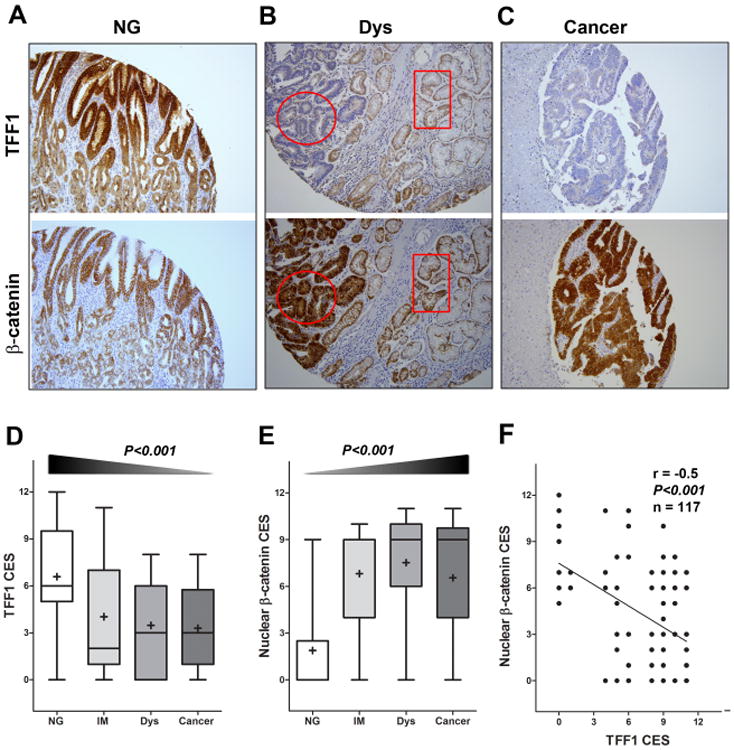
A-C) Immunohistochemical staining for TFF1 (upper panels) and β-catenin (lower panels) was performed on serial tissue sections from human gastric mucosa with (A) normal gland (NG), (B) dysplasia (Dys), and (C) cancer. A progressive decrease of TFF1 expression was observed from normal mucosa to adenocarcinoma, along with a progressive increase of nuclear localization of β-catenin expression. The circular and rectangular areas demonstrate an inverse relationship in the immunostaining of TFF1 and β-catenin in serial sections from the same tissue. Original magnification (×200) is shown. D-E) The graphs summarize the immunohistochemical staining of TFF1 and nuclear β-catenin results on gastric tissue microarrays, respectively. F) The results from Pearson's correlation test between TFF1 and β-catenin nuclear staining are shown.
Discussion
Gastric cancer ranks as the fourth most common cancer and the second most frequent cause of cancer deaths worldwide [30]. Understanding of the molecular basis of gastric tumorigenesis is a crucial step for the development of novel anticancer therapeutic approaches. In this study, we investigated the role of the TFF1 protein in regulating β-catenin signaling in gastric tumorigenesis.
Deficient TFF1 gene expression has been shown to promote gastric tumorigenesis in human and mouse [14, 31]. The development of gastric cancer involves complex and interacting signaling pathways that promote gastric tumorigenesis [2]. In this report, we provide, for the first time, experimental evidence of TFF1 regulation of β-catenin-mediated gastric tumorigenesis in Tff1-KO mouse model, and characterize the underlying molecular mechanism in human in vitro cell models. The activation of the β-catenin pathway has been reported as an early initiating event in gastric tumorigenesis [32]. In this regard, our findings confirm that β-catenin activation occurs during early stages of tumorigenesis in the Tff1-KO mice. The accumulation of nuclear β-catenin started in hyperplastic gastric tissues in 4-6 week old mice. This was maintained during the progression from low-grade dysplasia to high-grade dysplasia and adenocarcinoma, suggesting the importance of β-catenin in all stages of gastric cancer. Immunohistochemical analysis of human gastric tissues demonstrated similar findings supporting the notion that TFF1 loss and nuclear β-catenin expression are early molecular events associated with the onset of the multistep gastric tumorigenesis cascade in human.
The activity of β-catenin signaling depends on the accumulation and translocation of β-catenin to the nucleus, one of the hallmarks for the initiation of tumorigenesis in a variety of human cancers [18, 33, 34]. Our ex vivo data demonstrated significant nuclear localization of β-catenin, indicative of its activity, in pyloro-antral epithelial cells from Tff1-KO mice. In contrast, the reconstitution of TFF1 expression or TFF1 conditioned media reduced nuclear accumulation of β-catenin and enhanced its cell membrane localization in the in vitro cell model. The pTOP-flash luciferase reporter data further validated the role of TFF1 as a negative regulator of β-catenin/TCF transcription activity.
In the nucleus, the β-catenin/TCF transcription complex regulates the expression of several genes that are involved in human carcinogenesis such as CCND1 and c-MYC [18, 35]. Our results demonstrated that loss of Tff1 gene expression induces an increase of mRNA and protein expression of β-catenin target genes, c-Myc and Ccnd1, as early as 4-6 weeks of age in the Tff1-KO mice. This confirms that activation of β-catenin signaling occurs at early stages of gastric tumorigenesis. Conversely, the reconstitution of TFF1 expression in vitro significantly decreased mRNA and protein levels of c-Myc and cyclin D1. The β-catenin target genes, c-Myc and Ccnd1, are known oncogenes that drive cell growth and proliferation [36]. Our findings demonstrated that loss of Tff1 gene expression in vivo increased cell proliferation in mouse gastric tissues, whereas the reconstitution of TFF1 expression in vitro abrogated the cancer cell proliferative activity. The results provide strong evidences supporting that, loss of TFF1 expression enhances nuclear translocation and transcriptional activity of β-catenin, the outcome of which is reflected on the increased cell proliferation capacity. Taken together, our findings suggest that TFF1 exhibits a tumor suppressor function through negative regulation of the β-catenin pathway, a common mechanism for tumor suppressor genes [37].
The phosphorylation of β-catenin (Ser33/37/Thr41) by GSK3β leads to its degradation via the ubiquitin/proteasome pathway [20]. Our results demonstrated that the decrease of the β-catenin protein level and activity after the reconstitution of TFF1 expression was associated with a concomitant decrease of p-GSK3β (Ser9). Treatment with LiCl, a GSK3β specific inhibitor, abrogated TFF1-induced decrease of pTOP luciferase activity, and p-GSK3β (Ser9) and β-catenin protein levels. These data suggested that activation of GSK3β by TFF1 is a possible mechanism that mediates the inhibition of β-catenin. In fact, we confirmed that TFF1-induced activation of GSK3β leads to phosphorylation of β-catenin at Ser33/37/Thr41 residues, known to mediate ubiquitination and degradation of β-catenin [19, 20]. Since GSK3β is a substrate of AKT, we established that TFF1-induced dephosphorylation and activation of GSK3β involves the negative regulation of AKT by TFF1. Our data showed that the decrease in the p-AKT (Ser473) protein level by TFF1 abrogated the AKT-induced phosphorylation of β-catenin Ser552 residue [23, 38]. Collectively, these results suggest that TFF1 attenuates β-catenin activity through negative regulation of the AKT-GSK3β axis.
The regulation of β-catenin signaling has been shown to involve serine/threonine kinases and phosphatases [39]. The catalytic domain of PP2A (PP2Ac) has been implicated in the regulation of the AKT-GSK3β-β-catenin axis [22, 25, 40]. Our data demonstrated for the first time that TFF1 increased PP2Ac phosphatase activity, whereby treatment with okadaic acid (a PP2A inhibitor) or genetic knockdown of PP2Ac counteracted the signaling effects of TFF1 leading to enhanced AKT-GSK3β-β-catenin signaling. We also showed that TFF1 decreased p-PP2A (Tyr307), confirming PP2A activation by TFF1. The fact that TFF1 is a secreted protein [41] suggests that TFF1 could regulate PP2A through a cell surface receptor, the regulation of which leads to activation of PP2A. However, the precise underlying mechanism that may involve direct or indirect regulation of PP2A requires further investigation. Collectively, our data clearly indicate that TFF1 mediates β-catenin inhibition through regulation of the PP2A-AKT pathway (Figure 8).
Figure 8. Schematic representation of the role of TFF1 in suppressing AKT-β-catenin pathway through activation of PP2A in gastric epithelial cells.
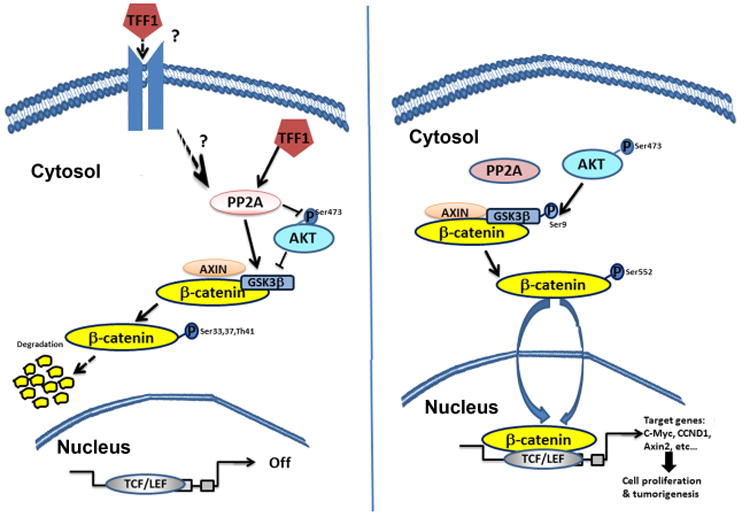
In normal gastric epithelial cells, TFF1 activates PP2A leading to dephosphorylation of AKT, activation of GSK3β, and degradation of the β-catenin protein. With the silencing of the TFF1 gene in gastric cancer, β-catenin becomes active and translocates into the nucleus where it binds to TCF/LEF transcription factors and transactivate β-catenin target genes involved in proliferation and tumorigenesis.
In summary, these novel findings underscore the role of TFF1 loss in activating β-catenin signaling and promoting gastric tumorigenesis through regulation of the PP2A-AKT axis. The close similarities in the Tff1-KO mouse model and human gastric cancer suggest that this experimental system may be highly valuable for understanding the biology and developing new therapeutic strategies against gastric cancer.
Supplementary Material
Figure S1. Tff1 mRNA expression in gastric tissues in 4-6 weeks old wild-type, Tff1-heterozygotes, and Tff1-Knockout mice
Quantitative RT-PCR data demonstrated mRNA levels in the antropyloric gastric mucosa of Tff1 in 4-6 weeks old wild-type (n=10), Tff1-heterozygote (n=10), and Tff1-knockout (n=10) mice.
Figure S2. No overall correlation between c-Myc and Ccnd1 mRNA expression and age in Tff1-knockout mice
(A-B) Pearson's correlation test of mRNA expression of β-catenin target genes, c-Myc (A) and Ccnd1 (B) between different age groups. 4-8 weeks old mice exhibit hyperplastic gastric tissue, and 4 months and older mice develop dysplastic or malignant gastric lesions.
Figure S3. TFF1 expression regulates nuclear localization of β-catenin
Western blot analysis of total β-catenin and TFF1 in the nuclear and cytosolic protein fractions of infected MKN28 cells with control or TFF1 adenoviruses is shown. Gel loading was normalized to Histone 3 and β-actin.
Figure S4. Secreted TFF1 attenuates β-catenin and its targets protein expression in vitro
MKN28 cells were treated with control or TFF1 conditioned media, and subjected to immunoblot analysis. The data showed a marked decrease of β-catenin, c-Myc, and CyclinD1 protein levels in cells treated with TFF1 conditioned media relative to control cells. The levels of TFF1 expression in the conditioned media are shown.
Figure S5. TFF1 negatively regulates AXIN-2 mRNA expression in vivo and in vitro
(A-B) QRT-PCR data demonstrated mRNA up-regulation of β-catenin target gene Axin-2 in Tff1-KO mice as compared to wild-type (A), and down-regulation of AXIN-2 in MKN28 cells infected with TFF1 adenovirus as compared to control cells (B).
Figure S6. TFF1 expression negatively regulates transcriptional activation of c-Myc
PBV-Luc-Myc-Wild type and its mutant luciferase reporter assays showing c-Myc transcriptional activity in MKN28 cells infected with control or TFF1 adenoviruses.
Supplemental Table 1. Mouse primer sequences used in quantitative real time PCR
Human primer sequences used in quantitative real time PCR
What is already known about this subject?
TFF1 is a gastric tumor suppressor gene.
TFF1 is expressed in the gastric epithelia and secreted by the mucus-secreting pit cells of the corpus and antropyloric regions of the stomach
Loss of TFF1 leads to the development of gastric cancer.
What are the new findings?
This study demonstrates that loss of Tff1 induces β-catenin activation in hyperplastic gastric mucosa and along stages of gastric tumorigenesis in mice
TFF1 regulates β-catenin localization, transcriptional activity, and downstream targets c-Myc and CyclinD1
TFF1 regulates gastric epithelial cell proliferation
TFF1 negatively regulates the AKT-β-catenin pathway through activation of PP2A
Loss of TFF1 increases nuclear β-catenin expression during human gastric carcinogenesis cascade
How might it impact on clinical practice in the foreseeable future?
This study provides the role of TFF1 in regulating cell proliferation and tumor development through β-catenin signaling using in vivo and in vitro models of gastric tumorigenesis.
TFF1 plays a pivotal role in maintaining the integrity of the gastric mucosa, and the fact that TFF1 could suppress proliferation of cancer cells, may offer a rationale for new therapeutic targets and treatment modalities of gastric cancer.
Acknowledgments
Grant Support: This study was supported by grants from the Department of Veterans Affairs (WER), Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485), and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, Department of Veterans Affairs, or Vanderbilt University.
Abbreviations used in this paper
- TFF1
trefoil factor 1
- GSK3
glycogen synthase kinase 3
- PP2A
protein phosphatase 2A
- TCF/LEF
T cell factor/lymphoid enhancement factor
- OA
okadaic acid
- LiCl
lithium chloride
Footnotes
Authors' contribution: MS: design of in vitro and in vivo experiments and acquisition of data; analysis and interpretation of data; drafting of the manuscript; technical and material support
DP: analysis of immunohistochemical data
AK: assisted in in vitro experiments and interpretation of data
ZC: assisted in in vivo experiments and interpretation of data
MBP: histopathology analysis of mouse and human tissues
MKW: histopathology analysis of mouse and human tissues
AB: analysis and interpretation of data; experimental troubleshooting; drafting of the manuscript; critical revision of the manuscript
PC: histopathology analysis of mouse and human tissues
WER: study concept and design; obtained funding; study supervision; experimental troubleshooting; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittoni A, Maccaroni E, Scartozzi M, Berardi R, Cascinu S. Chemotherapy for locally advanced and metastatic gastric cancer: state of the art and future perspectives. European review for medical and pharmacological sciences. 2010;14:309–14. [PubMed] [Google Scholar]
- 4.Thim L. Trefoil peptides: from structure to function. Cellular and molecular life sciences : CMLS. 1997;53:888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thim L, May FE. Structure of mammalian trefoil factors and functional insights. Cell Mol Life Sci. 2005;62:2956–73. doi: 10.1007/s00018-005-5484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, Chenard MP, et al. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705–8. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- 7.Escargueil AE, Larsen AK. Mitosis-specific MPM-2 phosphorylation of DNA topoisomerase IIalpha is regulated directly by protein phosphatase 2A. The Biochemical journal. 2007;403:235–42. doi: 10.1042/BJ20061460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taupin D, Pedersen J, Familari M, Cook G, Yeomans N, Giraud AS. Augmented intestinal trefoil factor (TFF3) and loss of pS2 (TFF1) expression precedes metaplastic differentiation of gastric epithelium. Lab Invest. 2001;81:397–408. doi: 10.1038/labinvest.3780247. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho R, Kayademir T, Soares P, Canedo P, Sousa S, Oliveira C, et al. Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab Invest. 2002;82:1319–26. doi: 10.1097/01.lab.0000029205.76632.a8. [DOI] [PubMed] [Google Scholar]
- 10.McChesney PA, Aiyar SE, Lee OJ, Zaika A, Moskaluk C, Li R, et al. Cofactor of BRCA1: a novel transcription factor regulator in upper gastrointestinal adenocarcinomas. Cancer Res. 2006;66:1346–53. doi: 10.1158/0008-5472.CAN-05-3593. [DOI] [PubMed] [Google Scholar]
- 11.Park WS, Oh RR, Park JY, Lee JH, Shin MS, Kim HS, et al. Somatic mutations of the trefoil factor family 1 gene in gastric cancer. Gastroenterology. 2000;119:691–8. doi: 10.1053/gast.2000.16483. [DOI] [PubMed] [Google Scholar]
- 12.Tomita H, Takaishi S, Menheniott TR, Yang X, Shibata W, Jin G, et al. Inhibition of gastric carcinogenesis by the hormone, gastrin, is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 14.Soutto M, Belkhiri A, Piazuelo MB, Schneider BG, Peng D, Jiang A, et al. Loss of TFF1 is associated with activation of NF-kappaB-mediated inflammation and gastric neoplasia in mice and humans. J Clin Invest. 2011;121:1753–67. doi: 10.1172/JCI43922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogasawara N, Tsukamoto T, Mizoshita T, Inada K, Cao X, Takenaka Y, et al. Mutations and nuclear accumulation of beta-catenin correlate with intestinal phenotypic expression in human gastric cancer. Histopathology. 2006;49:612–21. doi: 10.1111/j.1365-2559.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 16.Takayama T, Shiozaki H, Shibamoto S, Oka H, Kimura Y, Tamura S, et al. Beta-catenin expression in human cancers. Am J Pathol. 1996;148:39–46. [PMC free article] [PubMed] [Google Scholar]
- 17.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. The Journal of biological chemistry. 2006;281:9971–6. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 18.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 19.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 20.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. The EMBO journal. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang LY, Jiang LN, Li FF, Li H, Liu F, Gu Y, et al. Reduced beta-catenin expression is associated with good prognosis in Astrocytoma. Pathology oncology research : POR. 2010;16:253–7. doi: 10.1007/s12253-009-9219-0. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Pandurangan AK, Lu F, Fyrst H, Zhang M, Byun HS, et al. Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3beta pathway in colon cancer. Carcinogenesis. 2012;33:1726–35. doi: 10.1093/carcin/bgs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. The Journal of biological chemistry. 2007;282:11221–9. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Wang X, Yue P, Tao H, Ramalingam SS, Owonikoko TK, et al. Protein phosphatase 2A and DNA-dependent protein kinase are involved in mediating rapamycin-induced Akt phosphorylation. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M113.463679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer letters. 2013 doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:1401–8. doi: 10.1158/1078-0432.ccr-0157-03. [DOI] [PubMed] [Google Scholar]
- 27.Vangamudi B, Zhu S, Soutto M, Belkhiri A, El-Rifai W. Regulation of beta-catenin by t-DARPP in upper gastrointestinal cancer cells. Mol Cancer. 2011;10:32. doi: 10.1186/1476-4598-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Yang J, Liu Y, Chen X, Yu T, Jia J, et al. PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation. The Journal of biological chemistry. 2009;284:22649–56. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–4. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 30.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 31.Buache E, Etique N, Alpy F, Stoll I, Muckensturm M, Reina-San-Martin B, et al. Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice. Oncogene. 2011;30:3261–73. doi: 10.1038/onc.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita H, Yamada Y, Oyama T, Hata K, Hirose Y, Hara A, et al. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer research. 2007;67:4079–87. doi: 10.1158/0008-5472.CAN-06-4025. [DOI] [PubMed] [Google Scholar]
- 33.Ying Y, Tao Q. Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers. Epigenetics : official journal of the DNA Methylation Society. 2009;4:307–12. [Google Scholar]
- 34.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cellular signalling. 2008;20:581–9. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Liao DJ, Thakur A, Wu J, Biliran H, Sarkar FH. Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Critical reviews in oncogenesis. 2007;13:93–158. doi: 10.1615/critrevoncog.v13.i2.10. [DOI] [PubMed] [Google Scholar]
- 37.Akiyama T. Wnt/beta-catenin signaling. Cytokine & growth factor reviews. 2000;11:273–82. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 38.Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, et al. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–32. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 39.Verheyen EM, Gottardi CJ. Regulation of Wnt/beta-catenin signaling by protein kinases. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polakis P. Wnt signaling and cancer. Genes & development. 2000;14:1837–51. [PubMed] [Google Scholar]
- 41.Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J. 2002;16:592–4. doi: 10.1096/fj.01-0498fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Tff1 mRNA expression in gastric tissues in 4-6 weeks old wild-type, Tff1-heterozygotes, and Tff1-Knockout mice
Quantitative RT-PCR data demonstrated mRNA levels in the antropyloric gastric mucosa of Tff1 in 4-6 weeks old wild-type (n=10), Tff1-heterozygote (n=10), and Tff1-knockout (n=10) mice.
Figure S2. No overall correlation between c-Myc and Ccnd1 mRNA expression and age in Tff1-knockout mice
(A-B) Pearson's correlation test of mRNA expression of β-catenin target genes, c-Myc (A) and Ccnd1 (B) between different age groups. 4-8 weeks old mice exhibit hyperplastic gastric tissue, and 4 months and older mice develop dysplastic or malignant gastric lesions.
Figure S3. TFF1 expression regulates nuclear localization of β-catenin
Western blot analysis of total β-catenin and TFF1 in the nuclear and cytosolic protein fractions of infected MKN28 cells with control or TFF1 adenoviruses is shown. Gel loading was normalized to Histone 3 and β-actin.
Figure S4. Secreted TFF1 attenuates β-catenin and its targets protein expression in vitro
MKN28 cells were treated with control or TFF1 conditioned media, and subjected to immunoblot analysis. The data showed a marked decrease of β-catenin, c-Myc, and CyclinD1 protein levels in cells treated with TFF1 conditioned media relative to control cells. The levels of TFF1 expression in the conditioned media are shown.
Figure S5. TFF1 negatively regulates AXIN-2 mRNA expression in vivo and in vitro
(A-B) QRT-PCR data demonstrated mRNA up-regulation of β-catenin target gene Axin-2 in Tff1-KO mice as compared to wild-type (A), and down-regulation of AXIN-2 in MKN28 cells infected with TFF1 adenovirus as compared to control cells (B).
Figure S6. TFF1 expression negatively regulates transcriptional activation of c-Myc
PBV-Luc-Myc-Wild type and its mutant luciferase reporter assays showing c-Myc transcriptional activity in MKN28 cells infected with control or TFF1 adenoviruses.
Supplemental Table 1. Mouse primer sequences used in quantitative real time PCR
Human primer sequences used in quantitative real time PCR


