Abstract
During tumor development, tumor cells constantly communicate with the surrounding microenvironment through both biochemical and biophysical cues. In particular, the tumor microenvironment can instruct carcinoma cells to undergo a morphogenesis program termed epithelial-to-mesenchymal transition (EMT) to facilitate local invasion and metastatic dissemination. Growing evidence uncovered a plethora of microenvironmental factors in promoting EMT, including pro-inflammatory cytokines secreted by locally activated stromal cells, hypoxia conditions, extracellular matrix components, and mechanical properties. Here, we review various biochemical and biophysical factors in the tumor microenvironment that directly impinge upon the EMT program. Specifically, cytokines such as TGFβ, TNFα and IL6 and hypoxia are capable of inducing EMT in various tumors. Several extracellular matrix (ECM) proteins, including Collagen-I, Fibronectin, and Hyaluronan, and ECM remodeling via extracellular Lysyl oxidase are also implicated in regulating EMT. In preclinical studies and ongoing clinical trials, targeting these tumor microenvironmental signals has shown promises in halting tumor progression in various human cancers.
BACKGROUND
During tumor metastasis, the EMT program has been indicated in giving rise to the dissemination of single tumor cells from primary epithelial tumors (1). EMT refers to a global cellular and molecular transition by which polarized epithelial cells gain mesenchymal properties to migrate. During EMT, epithelial cells reorganize cytoskeleton and resolve cell-cell junctions, which are accompanied with switching off the expression of epithelial markers and turning on mesenchymal genes. Although changes in epithelial and mesenchymal markers during EMT can vary significantly in different biological contexts, a network of transcription factors, including TWIST1/2, SNAIL1/2, ZEB1/2 and FOXC2 are consistently required to orchestrate the EMT program (2). Numerous studies have shown that expression of these transcription factors is associated with poor prognosis and distant metastasis in various human cancers (3). Besides its role in promoting tumor cell invasion, EMT is shown to confer tumor cells with resistance to apoptosis (4) and anoikis (5), thus allowing cell survival in the blood stream after intravasation. EMT could also facilitate tumor cells escape from the senescence program, especially through TWIST1 and ZEB1 (6,7). Furthermore, EMT has been shown to endow cancer cells with cancer stem cell (CSC)-like features, which further aid tumor dormancy and chemoresistance (8,9).
Studies with tumor samples or experimental tumor xenograft models have provided convincing evidence for the activation of EMT in various primary epithelial tumors. Interestingly, more recent studies reveal a dynamic requirement of EMT in tumor metastasis: activation of EMT promotes local tumor invasion, intravasation and extravasation of the systemic circulation, while reversion of EMT is essential to establish macrometastases in distant organs (1,10). The “reversible” EMT model implies that EMT is unlikely to be regulated by permanent genetic and epigenetic changes in tumor cells; instead, EMT is dynamically controlled by various pro-invasion signals from the tumor microenvironment (TME).
The TME is defined as the cellular and physical environment surrounding the primary tumor – including endothelial, inflammatory and immune cells, fibroblasts, ECM components and soluble factors. In this review, we discuss the most relevant and direct connections between TME signals and the EMT-inducing transcription factors in cancer. Based on the properties of the TME signals, we divide our discussion into four major categories: inflammatory signals, hypoxia, ECM components and ECM mechanical properties (Figure 1).
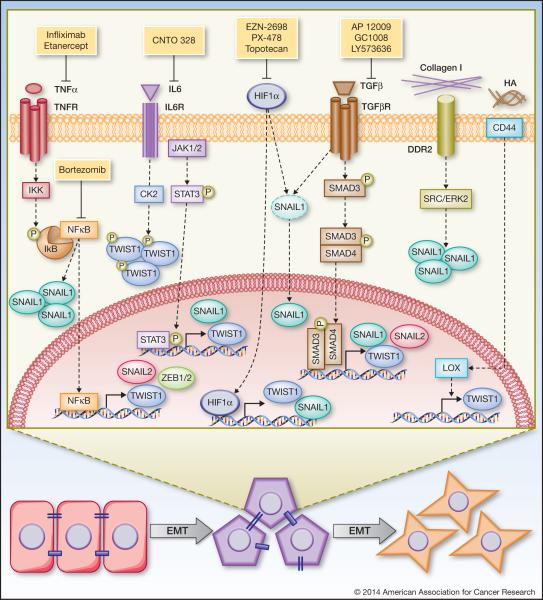
Figure 1. Regulation of EMT transcription factors by tumor microenvironmental signals.
Transforming growth factor-β (TGFβ) regulates up-regulation of TWIST1, SNAIL1, and SNAIL2 via the SMAD signaling pathway. Drugs that inhibit TGFβ are AP 12009, GC1008, LY573636, which are in clinical trial for advanced solid tumors. Tumor necrosis factors-α (TNFα) activates NFκB to induce TWIST1, SNAIL2, and ZEB1/2 expression and TNFα/NFκB activation also increases SNAIL1 protein stability. Therapeutic approaches to inhibit TGFβ signaling include TNFα antagonist (infliximab and etanercept) and NFkB inhibitor (bortezomib), all of which have been assessed in phase II clinical trial for several cancer types. Interleukin-6 (IL6) induces TWIST1 and SNAIL1 expression via JAK/STAT3 signaling and increases TWIST1 stability through CK2-dependent phosphorylation. An IL6 ligand-blocking antibody, CNTO 328, has been tested in phase I/II clinical trials with metastatic renal cell carcinoma. Hypoxia inducible factor-1α (HIF1α) induces TWIST1 and SNAIL1 expression and HIF1α either alone or in cooperation with TGFβ promotes SNAIL1 nuclear localization to stabilize SNAIL. Agents to inhibit HIF1α include EZN-2698, PX-478, and topotecan. Topotecan has been tested in phase I/II clinical trials in combination with conventional chemotherapy and EZN-2698 and PX-478 are currently being tested in phase I clinical trial. Collagen I can promote SNAIL1 stability through binding to its receptor DDR2 and activating SRC/ERK2 pathway. Hyaluronan (HA) binding to CD44 induces nuclear translocation of CD44 to directly induce Lysyl-Oxidase (LOX) expression, which in turn increases TWIST1 expression.
Inflammatory cytokines
An association between cancer development and inflammation has long being observed. During tumor progression, tumor cells recruit activated fibroblasts and immune cells that in turn secrete many cytokines to impact tumor development and metastasis (11). Interestingly, such cytokines have been shown to directly regulate the EMT program. Transforming growth factor-β (TGFβ), abundantly secreted by cancer-associated fibroblasts, platelets and tumor cells, is the best-characterized EMT inducer. TGFβ has been shown to induce TWIST1 and SNAIL2 expression in prostate and non-small cell lung cancer (12,13). TGFβ can also induce SNAIL1 and SNAIL2 via IKKα and SMAD signaling in pancreatic cancer cells (14). Furthermore, Vincent et al. showed that SNAIL-SMAD3/4 transcriptional repressor complex could promote TGFβ-mediated EMT in breast cancer (15). Tumor necrosis factor-α (TNFα) is a crucial activator of the NFκB signaling pathway and activated NFκB has been shown to induce multiple EMT transcription factors expression, including TWIST1, SNAIL2 and ZEB1/2 (16-18). Furthermore, Wu et al. found that NFκB activation could stabilize SNAIL1 to further promote cell migration and invasion (19). The release of interleukins by immune cells, endothelial cells and fibroblasts can also contribute to EMT. IL6 promotes EMT in head and neck cancer cells and correlates with increased TWIST1 and SNAIL1 expressions (20). Sullivan et al. showed that an IL6-TWIST1 positive feedback loop induces EMT in breast cancer cells (21). Taken together, various inflammatory cytokines from TME can regulate the expression and/or protein stability of EMT transcription factors to activate EMT and tumor invasion.
Hypoxia
Hypoxia condition has been shown to select tumor cells to become more invasive and metastatic. Specifically, hypoxia can promote EMT via hypoxia-inducible-factor-1α (HIF1α) (22). HIF1α is found to increase SNAIL1 protein stability, leading to suppression of E-cadherin in ovarian carcinoma (23). Yang et al. found that HIF1α could induce TWIST1 expression by binding directly to the TWIST1 promoter (24). In addition, HIF1α cooperates with inflammatory cytokines to promote EMT. For example, HIF1α, together with TGFβ, promotes SNAIL1 nuclear translocation to induce EMT through the suppression of estrogen receptor β in prostate carcinoma (25). Also HIF1α could enhance the expression of TWIST1 by up-regulating TNFα, IL6, and TGFβ in prostate cancer (26). Hypoxia, together with the Wnt/β-catenin signaling can also promote SNAIL1 stability by inhibiting GSK3β (27). Taken together, HIF1α, often in cooperation with additional TME factors, can induce EMT, suggesting a promising strategy to target hypoxic signaling for cancer therapeutics.
ECM components
ECM includes structural and non-structural components that can activate cellular signaling through membrane-bound receptors such as integrins. The critical role of ECM in promoting EMT was already evident in the original experiments conducted by Greenburg and Hay. They showed that epithelial cells from embryonic and adult anterior lens cultured in three-dimensional collagen gels can elongate and migrate as individual cells (28). Indeed, Dr. Hay concluded that “interactions with ECM may be a major factor in the ability of a cell to become mesenchymal”.
Recently, Zhang et al. unraveled a direct connection between ECM structural protein Collagen-I and SNAIL1 (29). They found that Collagen-I binds to its receptor DDR2 and activates downstream SRC/ERK2 to stabilize SNAIL1 in breast tumors cells. SNAIL1 further upregulates MT1-MMP and Collagen-I to promote tumor cell invasion. Another ECM structural component, Fibronectin, partly through binding to integrin receptors, induces SNAIL1 expression in tumor cells. This study demonstrated that cooperation of Fibronectin and TGFβ was required to activate the downstream SRC and ERK/MAPK kinases and induce EMT (30). Hyaluronan (HA) is a major component of ECM and signals through its membrane receptor CD44, which is overexpressed in many human cancers. HA binding to tumor cells was found to induce CD44 nuclear translocation and activate LOX expression, which in turn upregulates TWIST1 expression to promote breast cancer metastasis (31). Periostin, a non-structural ECM component highly expressed in human tumors, could signal through integrins to increase cell survival and promote metastatic progression of colon cancer in vivo (32). Kim et al. identified differential roles of Periostin in EMT: it induces SNAIL1 expression in prostate cancer cells whereas it inhibits TWIST1 expression in bladder cancer cells (33). These studies show that many ECM components are key regulators of EMT and tumor invasion.
ECM mechanical properties
During tumor progression, ECM is constantly remodeled by various cell types in the TME. Specifically, increasing matrix stiffness through LOX-mediated collagen crosslinking plays a critical role in tumor invasion and metastasis. Pioneer study by Paszek et al. showed that increasing ECM stiffness induced a malignant phenotype, associated with activated FAK and ERK signaling (34). LOX-mediated ECM stiffening promoted tumor progression in vivo partially via an activated FAK signaling (35). Conversely, treatment with a LOX inhibitor reduced focal adhesions and PI3K signaling, demonstrating that LOX modulates tumor progression through ECM stiffening to drive focal adhesions assembly. Furthermore, ECM stiffening was required to corporate with TGFβ to induce EMT in human breast tumor cells (36), further strengthening the notion that mechanical properties of the tumor microenvironment are key factors regulating EMT and promoting tumor progression.
CLINICAL-TRANSLATIONAL ADVANCES
Accumulating evidence supports a critical role of EMT in many aspects of tumor development, including resistance to apoptosis and senescence, CSCs, and invasion and metastasis, thus suggesting that targeting this process could be a promising therapeutic approach. However, the core EMT transcription factors remain technically challenging to target. Instead, a number of preclinical studies suggest that inhibiting EMT-inducing TME signals could serve as alternative approaches to impinge upon the EMT program. Here we summarize therapeutics in preclinical and clinical studies that target TME to prevent tumor progression (Table 1).
Table 1.
Clinical studies of drugs that target tumor microenvironment
| Target | Drug | Types of drug | Cancer types | Clinical Studies |
Response Median/Mo* |
PFS Median/Mo* |
OS Median/Mo* |
Ref. |
|---|---|---|---|---|---|---|---|---|
| TNFα | Infliximab | Monoclonal antibody | Renal cell carcinoma | Phase II |
Study 1: 32% PR+SD (7.7) Study 2: 61% SD (6.2) |
Study 1: 3.1 Study 2: 4.1 |
Study 1: 10 Study 2: 13.1 |
37 |
| Ovarian, renal, cervical cancer, endometrial stromal cell sarcoma, metastatic melanoma and metastatic colon cancer | Phase I | 6.2% SD (3.9) | 38 | |||||
| Etanercept (Enbrel) | Monoclonal antibody | Metastatic breast cancer | Phase II | 6.25% SD (4.1) | 39 | |||
| Recurrent ovarian cancer | Phase I | 33.3% SD (6.25) | 40 | |||||
| NFκB | Bortezomib | Proteasome inhibitor | Unresectable/metastatic gastric and gastroesophageal junction adenocarcinoma | Phase II | 6.25% SD (3.3) | 1.28 | 5.08 | 41 |
| Recurrent and/or metastatic head and neck squamous cell carcinoma | Phase II | 53% PR+SD (3.0) | 3.0 | 9.4 | 42 | |||
| IL6 | Siltuximab (CNTO-328) | Monoclonal antibody | Metastatic renal cell cancer | Phase I/II |
Part 2 54% PR+SD (7.6) |
3.4 | 5 | 43 |
| TGFβ | AP-12009 | Antisense oligonucleotide | Recurrent malignant glioma | Phase I/II | 29.1% SD (6) | 11 | 44 | |
| GC-1008 | Monoclonal Antibody | Metastatic melanoma and renal cell carcinoma | Phase I/II (Ongoing) | 45 | ||||
| LY-573636 (Tasisulam Sodium) | Small molecule inhibitor | Unresectable/metastatic non-small cell lung cancer | Phase II | 43.8% SD (4.21) | 2.69 | 8.48 | 46 | |
| Unresectable/metastatic soft tissue sarcoma | Phase II | 46.0% PR+SD (4.44) | 2.64 | 8.71 | 47 | |||
| Unresectable/metastatic melanoma | Phase II | 47.1% CR+PR+SD (6.6) | 2.6 | 9.6 | 48 | |||
| HIF1α | EZN-2698 | Antisense oligonucleotide | Advanced solid tumors and metastatic renal cell carcinoma | Phase I (Ongoing) | 49 | |||
| PX-478 | Small molecule inhibitor | Advanced metastatic cancer | Phase I (Ongoing) | 49 | ||||
| Topotecan | Small molecule inhibitor | Advanced, refractory non-small cell lung cancer | Phase I/II | 69.1% PR+SD (5.1) | 5.2 | 11.5 | 50 | |
| Tenascin C | 211Atch81C6 | Radioactive particles | Glioblastoma multiforme (GBM), anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO) | Pilot | GBM: SD (13.5) AA: SD (13) AO: SD (29) |
GBM: 37.5 Non-GBM: 60 |
55 | |
| FAK | PF-00562271 | Small molecule inhibitor | Advanced solid tumors | Phase I | 34% SD (1.5) 17% SD (9) |
61 |
Median/Mo: Median duration/months
PFS: Progress Free Survival; OS: Overall Survival, Ref: Reference
CR: Complete Response, PR: Partial Response, SD: Stable Disease
Inflammatory cytokines
Preclinical studies support the importance of inflammatory cytokines including TNFα and IL6 in promoting EMT and tumor invasion. Several TNFα inhibitors have been tested in clinical trial in different types of cancers. For example, infliximab, a TNFα monoclonal blocking antibody, has been tested in phase II clinical trials in renal cell carcinoma and advanced cancers (37,38). These studies suggested that TNFα inhibitor was effective to suppress the levels of IL6 and CCL2 in patients and improved progress-free survival. Two clinical studies examined the therapeutic effects of etanercept, a TNFα antagonist, in recurrent ovarian cancer and metastatic breast cancer. Etanercept is well tolerated in patients and significantly improved progress-free survival with consistent decrease in CCL and IL levels (39,40). Since NFκB is the essential downstream activator of the TNFα signaling, several clinical trials tested whether inhibition of NFκB signaling could suppress tumor progression and metastasis. Bortezomib, a proteasome inhibitor that suppresses NFκB activation, was tested in phase II clinical studies with metastatic gastric adenocarcinoma, and recurrent and metastatic head and neck squamous cell carcinoma (41,42). Although bortezomib alone showed poor response in patients, combination therapy with docetaxel or targeted inhibition of other oncogenic pathways are currently underway in solid tumors. Finally, various blocking antibodies against cytokines have been in various clinical studies. CNTO-328, an IL6 ligand-blocking antibody, was tested in phase I/II clinical trials for the treatment of metastatic renal cell carcinoma. This study showed that CNTO-328 could increase patient survival and more than 50% of progressive metastatic renal cell carcinoma patients presented stable diseases upon treatment (43). Together, these clinical trials in progress could bring a number of promising anti-inflammatory cytokine agents to the forefront of anti-metastasis therapeutics.
The TGFβ signaling is extensively targeted to block tumor progression and metastasis and various approaches have been taken to inhibit the TGFβ signaling. AP-12009, an antisense oligonucleotide against TGFβII, was tested in high-grade glioma patients and significantly improved survival compared to standard chemotherapy treatment (44). Furthermore, TGFβ neutralizing antibody GC-1008 showed promises in phase I trial for metastatic melanoma and renal cell carcinoma (45). Small molecule inhibitor, LY-573636, used in phase II clinical studies in patients with metastatic NSCLC, soft tissue sarcoma and melanoma, has also shown modest activity as a second/third line therapy (46-48). These studies showed that inhibiting TGFβ signaling pathway is safe, well tolerated in patients and could provide promising new therapeutics against tumor invasion.
Hypoxia
Several HIF1α inhibitors have also shown remarkable antitumor activities in a variety of preclinical and clinical trials. EZN-2698, an antisense oligonucleotide against of HIF1α, is being tested in phase I clinical trial with advanced solid tumors (49). Another HIF1α inhibitor, PX-478, which inhibit HIF1α expression, is currently tested in phase I clinical trials in patients with advanced metastatic cancer (49). Several novel compounds have also been identified in a high-throughput screen using a cell-based reporter of HIF1α transcriptional activity. One such compound Topotecan has been tested in phase I/II clinical trials with conventional chemotherapies such as cisplatin or bevacizumab in advanced lung cancer patients. Clinical results indicate that combination treatment is well tolerated and worthy of further clinical investigation (50), thus making them promising agents against tumor metastasis.
ECM components
Disruption of tumor ECM integrity has shown promising results in halting tumor metastasis in preclinical studies. Methylumbelliferone, a HA synthesis inhibitor, was effective in preventing bone metastasis of lung cancer in vivo (51). Neutralizing antibody directed against Periostin resulted in 40% inhibition of tumor growth (p<0.001), 80% inhibition of lung metastasis (p<0.001) and significant increase in survival (p<0.05) using mouse breast tumor xenografts (52).
Since cells that have undergone EMT secrete many unique ECM components, these ECM molecules have also been utilized for targeting drug delivery to tumors. For example, a promising approach has been used in clinical trials for Glioblastoma multiform patients, linking anti-Tenascin C antibody to radioactive particles to specifically target tumor cells. Result showed minimal toxicity associated with a promising antitumor benefit and encouraging overall outcomes (53). Recently, engineered HA-based conjugates have emerged as a promising strategy to efficiently target tumors with drugs exerting poor solubility and strong side effects, such as Paclitaxel (54). These strategies take advantage of unique EMT-associated TME components to achieve targeted delivery of traditional chemotherapeutics, thus presenting a new anti-cancer therapeutic strategy.
ECM mechanical properties
In patients, the presence of fibrotic foci in breast tumors is a prognostic marker of distant metastasis and correlates with poor survival (55). In addition, LOX is essential for hypoxia-induced breast cancer metastasis and its expression in patients is correlated to a poor outcome (56). Finally, a recent study shows that LOX is critical to establish a permissive microenvironment within fibrotic tissues, characterized by increased EMT, to favor the colonization of metastasizing tumor cells (57). Thus, anti-LOX strategies could suppress metastatic progression of the disease, not only by targeting the TME of the premetastatic niche, but also by targeting tumor cells themselves, as shown by the direct effect of LOX inhibition in attenuating FAK-dependent breast cancer cell invasion in a preclinical study (58). Therapeutic inhibition of FAK, recently validated in a phase I study, may also be a promising approach to prevent the effect of TME stiffness on metastatic progression of several types of cancer. Indeed the use of pharmacological inhibitor PF-00562271 in patients with advanced solid tumors unresponsive to existing therapies showed a significant stabilization of the disease, thus supporting FAK as a potential therapeutic target (59).
CONCLUSION and DISCUSSION
As discussed, a number of inhibitors targeting TME are being tested in preclinical and clinical trials and well-tolerated in patients and several showed promising results. Because these TME signals regulate various signaling pathways, the impacts of these inhibitors on tumor progression are likely beyond the EMT program. Given the critical role of EMT in multiple steps of tumor progression, targeting the EMT–inducing TME signals is indeed worth pursuing to combat metastatic cancers.
However, there are also a number of issues to be resolved to better decide how to effectively impact tumor progression by targeting the EMT program. Firstly, current clinical trials largely aim to shrink established metastases, in which the EMT program may not be involved. Instead, metastasis prevention trials in cancer patients with high metastasis risk would be the appropriate setting to test the effect of EMT inhibition on metastasis occurrence. Secondly, recent studies demonstrated the dynamic involvement of EMT in tumor metastasis: activation of EMT promotes tumor dissemination and reversion of EMT is essential for outgrowth of macrometastases. Therefore, EMT inhibitor alone could be counter-productive in preventing distant metastases if patients already have disseminated tumor cells in distant organs. In these cases, combining therapies targeting TME signals with traditional chemotherapy and targeted therapies to simultaneously inhibit EMT and cell proliferation could be a more powerful approach to eradicate both migrating as well as proliferating tumor cells, thus halting tumor progression.
ACKNOWLEDGMENTS
We apologize to the many researchers in this field whose work we were unable to cite due to space restrictions.
GRANT SUPPORT J. Yang is supported by the NCI of the NIH under award number 1RO1CA168689, American Cancer Society grant RSG-09-282-01-CSM, the Hartwell Foundation, and the U.S. Department of Defense Breast Cancer Program under award number W81XWH-13-1-0132. L. Fattet is supported by a postdoctoral fellowship from the Fondation pour la Recherche Medicale (SPE20130326547).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
REFERENCES
- 1.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–56. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Valdés F, Alvarez AM, Locascio A, Vega S, Herrera B, Fernández M, et al. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68–78. [PubMed] [Google Scholar]
- 5.Derksen PWB, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Ansieau S, Bastid J, Doreau A, Morel A-P, Bouchet BP, Thomas C, et al. Induction of EMT by TWIST proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Browne G, Sayan AE, Tulchinsky E. ZEB proteins link cell motility with cell cycle control and cell survival in cancer. Cell Cycle. 2010;9:886–91. doi: 10.4161/cc.9.5.10839. [DOI] [PubMed] [Google Scholar]
- 8.Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelialmesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–36. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakoff-Nahoum S. why cancer and inflammation? Yale J Biol Med. 2006;79:123–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Shiota M, Zardan A, Takeuchi A, Kumano M, Beraldi E, Naito S, et al. Clusterin mediates TGFβ-induced epithelial-mesenchymal transition and metastasis via TWIST1 in prostate cancer cells. Cancer Res. 2012;72:5261–72. doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 13.Slabáková E, Pernicová Z, Slavíčková E, Staršíchová A, Kozubík A, Souček K. TGFβ1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. Prostate. 2011;71:1332–43. doi: 10.1002/pros.21350. [DOI] [PubMed] [Google Scholar]
- 14.Brandl M, Seidler B, Haller F, Adamski J, Schmid RM, Saur D, et al. IKKα controls canonical TGFβ-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells. J Cell Sci. 2010;123:4231–9. doi: 10.1242/jcs.071100. [DOI] [PubMed] [Google Scholar]
- 15.Vincent T, Neve EPA, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGFβ mediated epithelialmesenchymal transition. Nat Cell Biol. 2009;11:943–50. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, et al. Epithelial-mesenchymal transition induced by TNFα requires NFkB-mediated transcriptional upregulation of TWIST1. Cancer Res. 2012;72:1290–300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storci G, Sansone P, Mari S, D'Uva G, Tavolari S, Guarnieri T, et al. TNFalpha up-regulates SLUG via the NFkappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol. 2010;225:682–91. doi: 10.1002/jcp.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NFκB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2006;26:711–24. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of SNAIL by NFκB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y-W, Xie T-X, Sano D, Myers JN. IL6 stabilizes TWIST and enhances tumor cell motility in head and neck cancer cells through activation of Casein kinase 2. PLoS One. 2011;6:e19412. doi: 10.1371/journal.pone.0019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, et al. Interleukin-6 induces an epithelial–mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–7. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vooijs M, Gort E, Groot A, Wall der E, van Diest P. Hypoxic regulation of metastasis via hypoxia-inducible factors. Curr Mol Med. 2008;8:60–7. doi: 10.2174/156652408783565568. [DOI] [PubMed] [Google Scholar]
- 23.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, et al. Hypoxia attenuates the expression of E-Cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–47. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M-H, Wu M-Z, Chiou S-H, Chen P-M, Chang S-Y, Liu C-J, et al. Direct regulation of TWIST by HIF1α promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 25.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERβ impedes prostate cancer EMT by destabilizing HIF1α and inhibiting VEGF-mediated SNAIL nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H-J, Park J-W, Cho Y-S, Cho C-H, Kim J-S, Shin H-W, et al. Pathogenic role of HIF1α in prostate hyperplasia in the presence of chronic inflammation. Biochim Biophys Acta. 2013;1832:183–94. doi: 10.1016/j.bbadis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Cannito S, Novo E, Compagnone A, Valfre di Bonzo L, Busletta C, Zamara E, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–78. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 28.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–9. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–87. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JH, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. 2013;33:1649–57. doi: 10.1038/onc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A. 2012;109:17460–5. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–39. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H. Opposite regulation of epithelial-to-mesenchymal transition and cell invasiveness by periostin between prostate and bladder cancer cells. Int J Oncol. 2011 doi: 10.3892/ijo.2011.997. [DOI] [PubMed] [Google Scholar]
- 34.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGFβ1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781–91. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, et al. Tumor Necrosis Factor α as a new target for renal cell carcinoma: two sequential phase II trials of Infliximab at standard and high dose. J Clin Oncol. 2007;25:4542–9. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- 38.Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNFα inhibitor, in patients with advanced cancer. Ann Oncol. 2008;19:1340–6. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- 39.Madhusudan S. A phase II study of Etanercept (Enbrel), a Tumor Necrosis Factor α inhibitor in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:6528–34. doi: 10.1158/1078-0432.CCR-04-0730. [DOI] [PubMed] [Google Scholar]
- 40.Madhusudan S. Study of Etanercept, a Tumor Necrosis Factor-Alpha inhibitor, in recurrent ovarian cancer. J Clin Oncol. 2005;23:5950–9. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 41.Shah MA, Power DG, Kindler HL, Holen KD, Kemeny MM, Ilson DH, et al. A multicenter, phase II study of Bortezomib (PS-341) in patients with unresectable or metastatic gastric and gastroesophageal junction adenocarcinoma. Invest New Drugs. 2010;29:1475–81. doi: 10.1007/s10637-010-9474-7. [DOI] [PubMed] [Google Scholar]
- 42.Chung CH, Aulino J, Muldowney NJ, Hatakeyama H, Baumann J, Burkey B, et al. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010;21:864–70. doi: 10.1093/annonc/mdp390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi J-F, Négrier S, James ND, Kocak I, Hawkins R, Davis H, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–62. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, et al. Inhibition of TGFβ2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–12. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 45.Nagaraj NS, Datta PK. Targeting the transforming growth factor-β signaling pathway in human cancer. Expert Opin Investig Drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scagliotti GV, Ilaria R, Jr, Novello S, Pawel von J, Fischer JR, Ermisch S, et al. Tasisulam Sodium (LY573636 Sodium) as third-line treatment in patients with unresectable, metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7:1053–7. doi: 10.1097/JTO.0b013e3182519d79. [DOI] [PubMed] [Google Scholar]
- 47.Ryan CW, Matias C, Agulnik M, Lopez-Pousa A, Williams C, Alwis DP, et al. A phase II study of tasisulam sodium (LY573636 sodium) as second-line or third-line treatment for patients with unresectable or metastatic soft tissue sarcoma. Invest New Drugs. 2012;31:145–51. doi: 10.1007/s10637-012-9819-5. [DOI] [PubMed] [Google Scholar]
- 48.Kirkwood JM, Gonzalez R, Reintgen D, Clingan PR, McWilliams RR, de Alwis DP, et al. A phase 2 study of tasisulam sodium (LY573636 sodium) as second-line treatment for patients with unresectable or metastatic melanoma. Cancer. 2011;117:4732–9. doi: 10.1002/cncr.26068. [DOI] [PubMed] [Google Scholar]
- 49.Onnis B, Rapisarda A, Melillo G. Development of HIF1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–6. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell SF, Beitinjaneh A, Tessema M, Bliss RL, Kratzke RA, Leach J, et al. Phase II study of topotecan and bevacizumab in advanced, refractory non-small-cell lung cancer. Clin Lung Cancer. 2013;14:495–501. doi: 10.1016/j.cllc.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Futamura N, Urakawa H, Arai E, Kozawa E, Ishiguro N, Nishida Y. Hyaluronan synthesis inhibitor supplements the inhibitory effects of zoledronic acid on bone metastasis of lung cancer. Clin Exp Metastasis. 2013;30:595–606. doi: 10.1007/s10585-012-9563-4. [DOI] [PubMed] [Google Scholar]
- 52.Morishita R. Role of periostin in cancer progression and metastasis: Inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28:181–6. doi: 10.3892/ijmm.2011.712. [DOI] [PubMed] [Google Scholar]
- 53.Zalutsky MR, Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, et al. Clinical experience with α-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2007;49:30–8. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arpicco S, Milla P, Stella B, Dosio F. Hyaluronic acid conjugates as vectors for the active targeting of drugs, genes and nanocomposites in cancer treatment. Molecules. 2014;19:3193–230. doi: 10.3390/molecules19033193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colpaert C, Vermeulen P, Van Marck E, Dirix L. The presence of a fibrotic focus is an independent predictor of early metastasis in lymph node-negative breast cancer patients. Am J Surg Pathol. 2001;25:1557. doi: 10.1097/00000478-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le Q-T, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 57.Cox TR, Bird D, Baker A-M, Barker HE, Ho MWY, Lang G, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–32. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L-C, Tu S-H, Huang C-S, Chen C-S, Ho C-T, Lin H-W, et al. Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway. Breast Cancer Res Treat. 2012;134:989–1004. doi: 10.1007/s10549-012-1986-8. [DOI] [PubMed] [Google Scholar]
- 59.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol. 2012;30:1527–33. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]