Abstract
Declining physical function is a major health problem for older adults as it is associated with multiple comorbidities and mortality. Exercise has been shown to improve physical function, though response to exercise is variable. Conversely, drugs targeting the renin–angiotensin system (RAS) pathway, including angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs), are also reported to improve physical function. In the past decade, significant strides have been made to understand the complexity and specificity of the RAS system as it pertains to physical function in older adults. Prior findings have also determined that interactions between antihypertensive medications and exercise may influence physical function above and beyond either factor alone. We review the latest research on RAS, exercise, and physical function for older adults. We also outline future research aims in this area, including genetic influences and clinical phenotyping, for the purpose of maintaining or improving physical function through tailored treatments.
Keywords: Angiotensin, Receptor, Function, Muscle, Genetics
Introduction
Physical function is an important predictor of health outcomes in older adults. The ability to perform basic physical function tasks is an important component of health-related quality of life (Muszalik et al. 2011) and a predictor of hospitalization, surgical outcomes, and mortality (Penninx et al. 2000; Dumurgier et al. 2009; Afilalo et al. 2010; Studenski et al. 2011). Accordingly, maintaining physical function is critical to the preservation of health and the well-being of older adults. In the USA, nearly half of the 37.3 million persons aged ≥65 years report having one or more physical limitations in performing essential daily tasks (Seeman et al. 2010). The adverse outcomes associated with these limitations have created a significant burden on health-care systems, which is likely to become more substantial given that older adults represent the fastest-growing segment of the population (US Census 2009). As a result, the development of methods to maintain the health and independence of older persons is an important public health goal.
A number of studies have shown physical exercise to have beneficial effects on the physical function of older adults (Cress et al. 1999; Brown et al. 2000; Miszko et al. 2003; Nelson et al. 2004; LIFE Study Investigators et al. 2006). However, to date, no pharmacologic therapies have proven to be effective in attenuating age-related functional decline. An efficacious pharmacologic intervention could have significant clinical impact since such regimens typically require minimal effort on the part of patients—an important point given that the initial effort to begin an intervention program is a primary barrier to lifestyle-based treatments (Lees et al. 2005). Several years ago, Carter et al. (2005) reported on the potential for angiotensin-converting enzyme inhibitors (ACEi) to improve body composition and physical performance among older adults. In the years following, scientists have made significant strides in the basic understanding of renin–angiotensin system (RAS) physiology, as well as the effects of RAS modulation on physical performance. Collectively, these studies have increased understanding and raised new questions regarding the potential of RAS-modulating drugs in preventing age-related disability. The objective of the present review is to concisely highlight these recent developments and highlight key remaining gaps in the literature related to determining the potential utility of ACEi, as well as angiotensin receptor blockers (ARBs), in the preservation of physical function and prevention of disability in older persons.
RAS pathway and physiology: recent developments
Conventional RAS pathway and introduction of Ang (1–7) axis
Classically, RAS has been described as the progressive cleavage of liver-secreted angiotensinogen into the peptide angiotensin I (Ang I). Kidney-secreted renin is necessary for the cleavage, which itself is inhibited by atrial and brain natriuretic peptides (ANP and BNP, respectively). When present, Ang I is converted by angiotensin-converting enzyme (ACE) into the peptide angiotensin II (Ang II), which acts on angiotensin II receptor type 1 (AT1) (Fig. 1(A)). ACE also breaks down the peptide bradykinin (Fig. 1(B)), a key vasodilatory peptide. Ang I can also be converted to Ang II by the chymase family of enzymes, although the mechanisms of this pathway are considered separate from the conventional RAS pathway (Balcells et al. 1997; Miyazaki and Takai 2006; Lorenz 2010).
Fig. 1.
Renin angiotensin pathway. Simplified overview of the RAS. A Conventional RAS pathway: angiotensinogen is cleaved by renin to form Ang1, is converted to angiotensin II peptide (Ang II) by angiotensin-converting enzyme (ACE), and acts on angiotensin II receptor type 1 (AT1) to cause vascular dysfunction, inflammation, and oxidative damage. B Bradykinin breakdown: vasodilatory peptide broken down by ACE. C Adjunctive RAS axis: angiotensin-converting enzyme 2 (ACE2) converts Ang II to peptide angiotensin (1–7) [Ang (1–7)], acts on the Mas (AT7) receptor, and induces cardioprotective mechanisms. D Ang (1–7) breakdown: ACE hydrolyzes Ang (1–7) to Ang (1–5), which inhibits cardioprotective mechanisms (Schindler et al. 2007)
Angiotensin II is both formed in, and acts in, a large variety of tissue types and is involved in many cellular processes other than blood pressure regulation—most notably cell growth and extracellular matrix formation (de Gasparo et al. 2000). Also important is that Ang II and nitric oxide signaling pathways reportedly overlap and that, with ACE inhibitors, there is a protective attenuation of ROS production, inflammation signaling pathways, and cell death pathways (Marzetti et al. 2011). Over a decade ago, a second ACE form was discovered (ACE2), which acts upon Ang II to make Ang (1–7) (Donoghue et al. 2000). Since that time, research has elucidated details of an adjunctive RAS axis, where ACE2 and other endopeptidases (neprilysin) catalyze Ang I or II conversion into the peptide angiotensin (1–7) (Velez et al. 2009), which acts on the Mas (AT7) receptor to induce cardioprotective mechanisms (Santos 2014; Fig. 1(C)). In contrast to the Ang II–AT1 axis, Ang (1–7) signaling is connected to downstream vasodilation action, which counteracts the hypertensive effects of AT1 signaling. The Ang (1–7) axis is significant due to the plieotropic effects of antihypertensive medications like ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs) (Passos-Silva et al. 2013). For example, both AT1 signaling inhibition and Mas signaling activation induce antihyperglycemic effects in humans, highlighting commonalities between downstream signal transduction of Ang (1–7) and insulin. The Ang (1–7) axis is also involved in antiangiogenic, antifibrotic, and anti-inflammatory properties (Machado et al. 2001; Katovich et al. 2008; Passos-Silva et al. 2013). Research has also eluded to detrimental axis triggered when ACE breaks down Ang (1–7) into Ang (1–5), which inhibits the cardioprotective mechanisms of the Ang (1–5) axis (Schindler et al. 2007). However, research on mechanisms and clinical effect of this axis is limited. While the classical RAS pathway has been vastly studied, in this review, we will focus on how it has been investigated in muscle and physical function and using different antihypertensive drugs to determine novel strategies to improve patient care.
Local tissue-specific RAS
Systemic RAS involves reaction of circulating enzymes and substrates derived from characteristic organs (Cushman et al. 1989). However, the independent RAS exists localized within specific tissues (Abadir et al. 2012). Notably, local RAS have been identified in a number of tissues including, but not limited to, cardiac, nervous, renal, and gastrointestinal (Paul et al. 2006; Bader and Ganten 2008). While possessing the same biochemical activity as circulating systems, local RAS effectors vary in response to physiological perturbation. Therefore, local effects of RAS-modifying interventions may occur despite similar systemic exposure to the drug. Furthermore, the activity and structure of local RAS change with age. For example, compared to younger mice, Ang II receptor density is decreased within skeletal muscle mitochondria of older mice (Abadir 2011). Chronic Ang II elevation without hypertension has been shown to produce age-related cardiomyopathy in transgenic mice, suggesting specificity to cardiac muscle (Domenighetti et al. 2005). Related to renal RAS, urinary Ang II and Ang (1–7)—as well as protein excretion—have been found to increase in older versus younger Fischer-344 rats (Gilliam-Davis et al. 2007). Renal RAS also appears to undergo age-related influence by other local systems, most notable brain RAS. Glial angiotensinogen-deficient transgenic rats have demonstrated lower systolic blood pressure, lower serum leptin, and lower glucose–insulin index with aging compared to normal aging animal models (Kasper et al. 2005, 2006). Baroreflex sensitivity is also preserved in older glial angiotensinogen-deficient transgenic rats, demonstrating contributions of brain RAS to age-related cardiac function decline (Arnold et al. 2008, 2013). Finally, age-related comorbid conditions can have significant influence on the structure of local RAS, as diabetes has been associated with increased intracellular RAS activity and subsequent fibrosis within cardiac muscle (Singh et al. 2008).
Skeletal muscle accounts for ~40 % of total body mass and is the primary tissue responsible for movement (Smith and Muscat 2005). As such, skeletal muscle is a critical tissue for the regulation and maintenance of physical function. In addition to the systemic RAS, both animal and human studies have demonstrated the existence of a local RAS within skeletal muscle tissue (Reneland and Lithell 1994; Jones and Woods 2003). These studies have identified several important physiologic effects for the local skeletal muscle RAS. For example, muscle RAS may be integral to the muscle formation, as Ang II inhibition has been associated with decreased capillary and myoblast formation (Bellamy et al. 2010; Johnston et al. 2011). Emerging evidence also suggests muscle RAS differentially influences skeletal muscle based on fiber type (Johnston et al. 2010). A study by Westerkamp and Gordon (2005) found Ang II attenuation inhibited satellite cell response to overload of the soleus, but not plantaris, muscle of Sprague Dawley rats, suggesting a specificity to slow- versus fast-twitch muscle fibers. Still, despite these findings, the full extent of physiologic impact of the local RAS on muscle function remains undetermined. Likewise, a paucity of evidence exists to determine age-related effects to local muscle RAS. Future investigations are warranted to determine how advanced age influences activity of skeletal muscle and other local RAS. Studies are also needed to determine how these local systems interact with the systemic RAS and may contribute to changes in physical function in advanced age.
Angiotensin-converting enzyme inhibitors and physical function
Angiotensin-converting enzyme inhibitors (ACEi) prevent Ang I to Ang II conversion by inhibiting ACE activity, effectively preventing the constriction of blood vessels, and lowering blood pressure. Thus, ACEi are widely utilized for managing hypertension. Moreover, ACEi—either independently or in combination with other drugs—have shown effects for a variety of other conditions including acute myocardial infarction, cardiac failure, diabetic nephropathy, and cognitive decline (McFarlane et al. 2003; Brugts et al. 2009; Sink et al. 2009). These benefits manifest at least partially as a result of improved endothelial function and reduced oxidative stress and inflammation (Krysiak and Okopień 2008; Ismail et al. 2010). While such beneficial effects on cardiovascular outcomes are well documented, the ability of ACEi to improve physical function remains somewhat in question.
A number of preclinical studies have suggested that ACEi may have a protective effect in preserving muscle strength, endurance, and body composition in late life (Coirault et al. 1999; Carter et al. 2005). Several studies have also evaluated the potential utility of ACEi for persevering physical function in humans; however, the findings have been mixed. In a randomized controlled trial of ACEi effects, functionally impaired seniors on ACEi demonstrated 6-min walk test times equivalent to the improvements seen after 6 months of exercise training (Sumukadas et al. 2007). Continuous ACEi use has also been associated with attenuated 3-year rates in decline of muscle strength and walking speed compared to intermittent or alternative antihypertensive medication (Onder et al. 2002). Conversely, a recent clinical trial of older individuals at high cardiovascular risk showed that physical function was similar after 6 months of ACEi administration compared to placebo (Cesari et al. 2010). A large observational study of older women also found that ACEi administration was not associated with frailty incidence (Gray et al. 2009). In other cases, ACEi have been shown to be associated with overall weight maintenance but not muscle strength maintenance (Schellenbaum et al. 2005).
Multiple factors may influence the capacity for ACEi to influence physical function, including the health of the cardiovascular system (Cesari et al. 2010). The complexity of discerning ACEi effects is also increased by the possibility of multiple mechanisms. For example, endothelial function improves with ACEi, and the resultant improvements in blood flow and/or glucose delivery may, therefore, be associated with improvements in muscle function (Henriksen and Jacob 2003). Further, associations between ACE and body composition may underlie improved physical function mechanisms. For example, older adults taking ACEi have been found to have greater lean body mass in the lower extremities (Di Bari et al. 2004). By contrast, common ACE-related genetic polymorphisms in older men (e.g., ACE I/D gene, angiotensinogen M235T gene, AT1 A1166C gene) are associated with a greater likelihood of being overweight or having higher abdominal adiposity (Strazzullo et al. 2003). Finally, insulin-like growth factor-1 (IGF-1) may mediate the relationship between body composition and physical function. Muscle wasting related to Ang II has been attributed to decreased IGF-1 (Song et al. 2005), and in older adults using ACEi, IGF-1 is higher than those not taking ACEi (Maggio et al. 2006; Giovannini et al. 2010).
ARBs and physical function
Like ACEi, ARBs are traditionally used to treat hypertension and have similar downstream effects, albeit through different mechanisms. While ACEi and Ang (1–7) inhibit Ang I to Ang II conversion and subsequent bradykinin breakdown, ARBs work directly by inhibiting ligand binding to the AT1 receptor. ARBs vary in pharmacokinetic properties and receptor affinity; however, all drugs work by competing with Ang II for receptor binding (Burnier 2001). Like ACEi, ARBs have shown effects for conditions other than hypertension that are pertinent to older adults, including heart failure, stroke, retinal protection, and diabetes (Cernes et al. 2011). A recent study by Li et al. (2010) found ARBs to be associated with a reduced incidence and progression of cognitive decline, which was not observed with ACEi. And while ARBs have comparable efficacy to ACE inhibitors, they generally do not face the same potential issue of intolerance (Grothusen et al. 2009).
As with ACEi, ARB administration may directly or indirectly improve physical function in the elderly (Carter 2011). In the case of metabolic syndrome, ARBs decrease the pressor response to exercise (Nashar et al. 2004). In patients with severe congestive heart failure, ARBs reportedly improve exercise capacity (Hamroff et al. 1999). More recent clinical trials have extended these results. For example, 4 weeks of ARB treatment improved diastolic heart failure patients’ tolerability to exercise (Kato et al. 2008), while 12 months of ARB treatment improved exercise capacity in patients with peripheral artery disease (Zankl et al. 2010). Moreover, ARB use may act synergistically with exercise to improve blood pressure, thereby improving overall health. For example, in a recent clinical trial, exercise alongside ARB administration was shown to improve blood pressure of patients with chronic obstructive pulmonary disease, whereas exercise alone could not (Marquis et al. 2008).
The mechanisms by which ARBs improve physical function are less understood. In animals, long-term ARB administration improves aerobic exercise ability through a mechanism dependent on the PPAR-δ/AMPK pathway (Feng et al. 2011). However, other studies highlight negative tissue-specific effects of ARBs on exercise performance. For example, ARB injection into the right lateral cerebral ventricle, but not injection peripherally, increased thermogenesis in rats subjected to exhaustive training (Leite et al. 2006) as well as oxygen consumption demands (Leite et al. 2007), thereby reducing performance. These effects may be due in part to disruption of AT1 signaling and downstream influence on serotonin and dopamine secretion in select regions of the brain (Leite et al. 2010). Further, ARB effects are not limited to the AT1 receptor. Rodrigues et al. (2007) found that antagonizing angiotensin II effects on the medullary AT2 receptor of hypertensive rats diminished the hypotensive effects of exercise, which implicate the AT2 arm of the brain RAS in managing blood pressure. Moreover, differential ARB effects occur, depending on location of the AT2 receptor blockade in the medulla (Tedesco and Ally 2009), which highlights both the complexity of this system and current limitations in exploiting its dynamics with ARB-based clinical treatments.
ACEi–exercise interaction
Prior suggestions that ACEi improves physical function have led to more recent studies investigating potential moderating effects of ACEi on exercise response. In previous animal work, ACEi administration was found to have no effect on endurance time, oxidative capacity, or mitochondrial function in sedentary rats (Bahi et al. 2004). Habouzit et al. (2009) replicated this lack of effect in sedentary rats. However, the same study also reported enhanced running capacity in rats that were endurance trained and administered ACEi compared to those only endurance trained (Habouzit et al. 2009). Specific to older animals, preliminary indication is that ACEi administration enhances exercise tolerance above and beyond exercise use alone (Carter et al. 2012). Moreover, the combination of exercise and ACEi has been linked to physiologic changes related to physical function such as improved capillary density and increased type 1 fiber percentage (Kanazawa et al. 2006; Guo et al. 2010).
Emerging research suggests that such an ACEi–exercise interaction in late life may translate to humans. Buford et al. (2012) retrospectively examined the effects of chronic exercise on physical function in seniors. Increased exercise response (i.e. changes in walking speed, standing balance, chair-rise ability) was observed in older adults administered ACEi, compared to individuals either not using antihypertensive medication or on alternative antihypertensive medications (Buford et al. 2012). However, a more recent study using a prospective design did not report enhanced exercise response after 20 weeks of ACEi administration (Sumukadas et al. 2014). However, several key questions remain to be answered. For instance, in the latter study, an indicator of aerobic capacity was chosen as the primary indicator of function but the training regimen did not include a component specifically designed to improve aerobic capacity. Additionally, the inclusion of both antihypertensive and non-hypertensive individuals, as well as participant use of a variety of concomitant medications, may have also limited the ability to detect differences between groups. Future studies are necessary to determine the relative influence of these potentially confounding factors.
ARB–exercise interaction
ARBs may also moderate the influence of exercise on physical function, much like ACEi, and these interactions have been observed in animal models. Combining ARBs with exercise has conferred 1) the best renoprotective effects in a rat model of diabetic nephropathy (Tufescu et al. 2008) and 2) protection against insulin resistance induced by high fat diet (Wang et al. 2012). However, continual eccentric contraction training in conjunction with ARBs was found to prevent gains in muscle mass and contractile force, suggesting that ARBs may have some negative tissue effects (McBride 2006). Similarly, oral ARB administration was associated with suppression of exercise-induced hippocampal neurogenesis in rats (Mukuda and Sugiyama 2007).
To date, data regarding an interaction between ARBs and exercise in humans is sparse. One trial comparing ARBs to calcium channel blockers in hypertensive patients found ARBs to attenuate indicators of exercise-induced inflammatory response and thrombogenic factor activation (Liakos et al. 2012). However, similar interactions are not universal. For individuals with left ventricle hypertrophy (Gerdts et al. 2006) or right ventricle dysfunction (Dore et al. 2005), exercise capacity was not improved with ARB treatment. Moreover, disease states like insulin resistance may be more responsive to ACEi. Unlike ARBs, application of ACEi in animals facilitates a return to the normal state of glucose transport. This differential effect is mechanistically related to the ability of ACE inhibitors and/or Ang (1–7) to modulate bradykinin-mediated signaling by preventing its degradation by ACE (Fig. 1(B)) (Carter et al. 2005).
Genetics
Genetic research of RAS, as it pertains to physical function, has focused primarily on a variant characterized by insertion (I) or the deletion (D) of a 287-bp region of DNA (I/D allele) in the ACE gene locus (Heled et al. 2004; Puthucheary et al. 2011; de Souza et al. 2013). In Caucasians, nearly 50 % variation of ACE in circulating serum is attributed to the I/D variant (Rigat et al. 1990). Moreover, II genotype of the ACE gene has been associated with lower ACE levels, and the ID and DD genotypes with intermediate and higher ACE levels, respectively (Rigat et al. 1990). The ACE I/D genetic variant is also purported to play a role in cardiovascular disease processes. Specifically, the DD genotype has been associated with increased Ang II due to increases in ACE, resulting in predisposition to cardiovascular dysfunction (Yanai et al. 1996; Wang et al. 2007). However, an ACE II genotype with lower ACE levels has shown to be less of an indicator of cardiovascular problems (Bauters and Amouyel 1998). In addition, the ACE I/D variant has been linked to variability in insulin sensitivity. Dengel et al. (2002) found that homozygous genotypes (II and DD) were less sensitive to insulin as compared to the ID genotype and that exercise improved insulin sensitivity in the II genotype as compared to the ID or DD genotypes.
Multiple studies have ascertained a link between ACE I/D genetic variant and either training and/or exercise response. Early work by Montgomery et al. (1998) found that high-altitude mountain climbers possessing the I allele had enhanced ascent endurance compared to those without the allele. In the same study, soldiers with I allele demonstrated higher weightlifting endurance compared to those without. Similarly, Myerson et al. (1999) observed increased frequency of the I allele in Olympic endurance runners compared to athletes from non-endurance sports. Since that time, research has elucidated a differential influence of the I and D alleles on exercise performance. Specifically, the I allele has been associated with more long-term endurance sports like long-distance running or triathlon, while the D allele has been associated with more short-term and/or strength-related sports like competitive swimming and weightlifting (Puthucheary et al. 2011). Along the same lines, the D allele has been associated with training-related growth of cardiac muscle compared to the I allele (Puthucheary et al. 2011).
Compared to athletes, age-related examination of the ACE I/D genetic variant and exercise response is under-investigated. However, a recent publication by Buford et al. (2014) suggests that ACE I/D genotype regulates changes in physical function after long-term exercise training in older adults. Specifically, those with the II genotype demonstrated impaired functional responses to regular exercise compared to those with the ID or DD genotype. These findings are similar to those of Kritchevsky et al. (2005) who reported that the II genotype was associated with an increased risk of incident mobility limitation among physically active, but not sedentary, older adults. Notably, subsequent training studies appeared to indicate, as in younger individuals, a genotype effect that was dependent on the mode of exercise utilized. For instance, Giaccaglia et al. (2008) reported that, while responses were robust among DD carriers, older adults with the II genotype did not experience significant increases in knee extensor strength following 18 months of resistance training. In contrast, however, Defoor et al. (2006) reported that improvements in aerobic power were greatest in II carriers following 3 months of aerobic training. Though more research is needed to provide clarity in this area, the mode of exercise appears to have an important influence on functional responses among individuals with particular genotypes. Moreover, future research may ascertain the extent to which genetics mediate or moderate associations between antihypertensive medication (i.e., ACEi or ARBs) and exercise responsiveness for older adults.
Conclusions and future directions
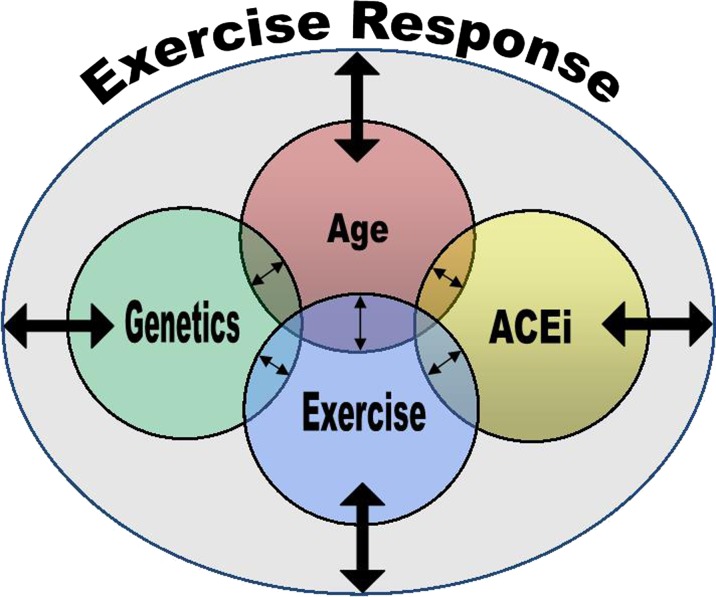
In the present review, we highlight the intricate network between aging, physical function, genetics, and antihypertensive pharmacotherapies. Collectively, these factors appear to be influential in the exercise response capacity of older adults and, in turn, clinical care (Fig. 2). Accounting for factors underlying exercise response capacity may eventually provide a clinical phenotype, which can be translated to treatment tailored to the individual, for the purpose of improving physical function. However, research is yet to advance this significantly toward the realm of personalized medicine. Traditionally, publications have examined the effect of ACEi or ARBs on physical function without accounting for the influence of genetics. To our knowledge, there has not been a systematic study to evaluate all variables in a single study. Future research should aim to taper the misappropriation of different drugs and identify the correct treatment (exercise regimen and/or HTD) based on individual factors. This type of personalized treatment has the potential to both improve quality of life and prolong life through enhancement of physical function.
Fig. 2.
Conceptual model of individual influences on exercise response. Age, genetic, exercise, and antihypertensive pharmacotherapy (ACEi), as well as mediation/moderation among these factors, influence exercise response capacity. Improved understanding of factors underlying exercise response could assist in the development of tailored treatments for older adults to optimize improvements in physical function
Acknowledgments
This work was supported by grants from Claude D. Pepper Older Americans Independence Centers (University of Florida, P30AG028740), University of Florida Clinical and Translational Science Institute (National Center for Research Resources, UL1RR029890, KL2RR029888), and NIH/NCATS Clinical and Translational Science Awards to the University of Florida (TL1 TR000066 and UL1 TR000064).
References
- Abadir PM. The frail renin-angiotensin system. Clin Geriatr Med. 2011;27:53–65. doi: 10.1016/j.cger.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012;38:437–445. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afilalo J, Eisenberg MJ, Morin J-F, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Arnold AC, Sakima A, Ganten D, et al. Modulation of reflex function by endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension. 2008;51:1326–1331. doi: 10.1161/HYPERTENSIONAHA.107.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Gallagher PE, Diz DI. Brain renin-angiotensin system in the nexus of hypertension and aging. Hypertens Res Off J Jpn Soc Hypertens. 2013;36:5–13. doi: 10.1038/hr.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Ganten D. Update on tissue renin–angiotensin systems. J Mol Med. 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- Bahi L, Koulmann N, Sanchez H, et al. Does ACE inhibition enhance endurance performance and muscle energy metabolism in rats? J Appl Physiol Bethesda MD 1985. 2004;96:59–64. doi: 10.1152/japplphysiol.00323.2003. [DOI] [PubMed] [Google Scholar]
- Balcells E, Meng QC, Johnson WH, et al. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769–1774. doi: 10.1152/ajpheart.1997.273.4.H1769. [DOI] [PubMed] [Google Scholar]
- Bauters C, Amouyel P (1998) Association between the ACE genotype and coronary artery disease. Insights from studies on restenosis, vasomotion and thrombosis. Eur Heart J 19 Suppl J:J24–29 [PubMed]
- Bellamy LM, Johnston APW, De Lisio M, Parise G. Skeletal muscle-endothelial cell cross talk through angiotensin II. Am J Physiol Cell Physiol. 2010;299:C1402–1408. doi: 10.1152/ajpcell.00306.2010. [DOI] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Ehsani AA, et al. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–965. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- Brugts JJ, Ferrari R, Simoons ML. Angiotensin-converting enzyme inhibition by perindopril in the treatment of cardiovascular disease. Expert Rev Cardiovasc Ther. 2009;7:345–360. doi: 10.1586/erc.09.2. [DOI] [PubMed] [Google Scholar]
- Buford TW, Hsu FC, Brinkley TE, Carter CS, Church TS, Dodson JA, Goodpaster BH, McDermott MM, Nicklas BJ, Yank V, Johnson JA, Pahor M, LIFE Research Group (2014) Genetic influence on exercise-induced changes in physical function among mobility-limited older adults. Physiol Genomics 46(5):149–158 [DOI] [PMC free article] [PubMed]
- Buford TW, Manini TM, Hsu F-C, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60:1244–1252. doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103:904–912. doi: 10.1161/01.cir.103.6.904. [DOI] [PubMed] [Google Scholar]
- Carter CS, Giovannini S, Seo DO, DuPree J, Morgan D, Chung HY, Lees H, Daniels M, Hubbard GB, Lee S, Ikeno Y, Foster TC, Buford TW, Marzetti E (2011) Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 x Brown Norway rats. AGE 33(2):167–183 [DOI] [PMC free article] [PubMed]
- Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60:1437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- Carter CS, Marzetti E, Leeuwenburgh C, et al. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67A:17–27. doi: 10.1093/gerona/glr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernes R, Mashavi M, Zimlichman R. Differential clinical profile of candesartan compared to other angiotensin receptor blockers. Vasc Health Risk Manag. 2011;7:749–759. doi: 10.2147/VHRM.S22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11:26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coirault C, Langeron O, Lambert F, et al. Impaired skeletal muscle performance in the early stage of cardiac pressure overload in rabbits: beneficial effects of angiotensin-converting enzyme inhibition. J Pharmacol Exp Ther. 1999;291:70–75. [PubMed] [Google Scholar]
- Cress ME, Buchner DM, Questad KA, et al. Exercise: effects on physical functional performance in independent older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M242–248. doi: 10.1093/gerona/54.5.m242. [DOI] [PubMed] [Google Scholar]
- Cushman DW, Wang FL, Fung WC, et al. Differentiation of angiotensin-converting enzyme (ACE) inhibitors by their selective inhibition of ACE in physiologically important target organs. Am J Hypertens. 1989;2:294–306. doi: 10.1093/ajh/2.4.294. [DOI] [PubMed] [Google Scholar]
- Defoor J, Vanhees L, Martens K, et al. The CAREGENE study: ACE gene I/D polymorphism and effect of physical training on aerobic power in coronary artery disease. Heart Br Card Soc. 2006;92:527–528. doi: 10.1136/hrt.2004.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gasparo M, Catt KJ, Inagami T, et al. International union of pharmacology: XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Dengel DR, Brown MD, Ferrell RE, et al. Exercise-induced changes in insulin action are associated with ACE gene polymorphisms in older adults. Physiol Genomics. 2002;11:73–80. doi: 10.1152/physiolgenomics.00048.2002. [DOI] [PubMed] [Google Scholar]
- Di Bari M, van de Poll-Franse LV, Onder G, et al. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, et al. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Dore A, Houde C, Chan K-L, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- Dumurgier J, Elbaz A, Ducimetière P, et al. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Luo Z, Ma L, et al. Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR-δ/AMPK pathway. J Cell Mol Med. 2011;15:1572–1581. doi: 10.1111/j.1582-4934.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts E, Björnstad H, Devereux RB, et al. Exercise performance during losartan- or atenolol-based treatment in hypertensive patients with electrocardiographic left ventricular hypertrophy (a LIFE substudy) Blood Press. 2006;15:220–226. doi: 10.1080/08037050600911957. [DOI] [PubMed] [Google Scholar]
- Giaccaglia V, Nicklas B, Kritchevsky S, et al. Interaction between angiotensin converting enzyme insertion/deletion genotype and exercise training on knee extensor strength in older individuals. Int J Sports Med. 2008;29:40–44. doi: 10.1055/s-2007-964842. [DOI] [PubMed] [Google Scholar]
- Gilliam-Davis S, Payne VS, Kasper SO, et al. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol Heart Circ Physiol. 2007;293:H1327–1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Cesari M, Marzetti E, et al. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J Nutr Health Aging. 2010;14:457–460. doi: 10.1007/s12603-010-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, LaCroix AZ, Aragaki AK, et al. Angiotensin-converting enzyme inhibitor use and incident frailty in women aged 65 and older: prospective findings from the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2009;57:297–303. doi: 10.1111/j.1532-5415.2008.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothusen A, Divchev D, Luchtefeld M, Schieffer B. Angiotensin II type 1 receptor blockade: high hopes sent back to reality? Minerva Cardioangiol. 2009;57:773–785. [PubMed] [Google Scholar]
- Guo Q, Minami N, Mori N, et al. Effects of estradiol, angiotensin-converting enzyme inhibitor and exercise training on exercise capacity and skeletal muscle in old female rats. Clin Exp Hypertens N Y N 1993. 2010;32:76–83. doi: 10.3109/10641960902993046. [DOI] [PubMed] [Google Scholar]
- Habouzit E, Richard H, Sanchez H, et al. Decreased muscle ACE activity enhances functional response to endurance training in rats, without change in muscle oxidative capacity or contractile phenotype. J Appl Physiol Bethesda MD 1985. 2009;107:346–353. doi: 10.1152/japplphysiol.91443.2008. [DOI] [PubMed] [Google Scholar]
- Hamroff G, Katz SD, Mancini D, et al. Addition of angiotensin II receptor blockade to maximal angiotensin-converting enzyme inhibition improves exercise capacity in patients with severe congestive heart failure. Circulation. 1999;99:990–992. doi: 10.1161/01.cir.99.8.990. [DOI] [PubMed] [Google Scholar]
- Heled Y, Moran DS, Mendel L, et al. Human ACE I/D polymorphism is associated with individual differences in exercise heat tolerance. J Appl Physiol. 2004;97:72–76. doi: 10.1152/japplphysiol.01087.2003. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol. 2003;196:171–179. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- Ismail H, Mitchell R, McFarlane SI, Makaryus AN. Pleiotropic effects of inhibitors of the RAAS in the diabetic population: above and beyond blood pressure lowering. Curr Diab Rep. 2010;10:32–36. doi: 10.1007/s11892-009-0081-y. [DOI] [PubMed] [Google Scholar]
- Johnston APW, Baker J, Bellamy LM, et al. Regulation of muscle satellite cell activation and chemotaxis by angiotensin II. PLoS One. 2010;5:e15212. doi: 10.1371/journal.pone.0015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston APW, Bellamy LM, Lisio MD, Parise G. Captopril treatment induces hyperplasia but inhibits myonuclear accretion following severe myotrauma in murine skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;301:R363–369. doi: 10.1152/ajpregu.00766.2010. [DOI] [PubMed] [Google Scholar]
- Jones A, Woods DR. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol. 2003;35:855–866. doi: 10.1016/s1357-2725(02)00342-4. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Kawamura T, Li L, et al. Combination of exercise and enalapril enhances renoprotective and peripheral effects in rats with renal ablation. Am J Hypertens. 2006;19:80–86. doi: 10.1016/j.amjhyper.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Kasper SO, Carter CS, Ferrario CM, et al. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin-angiotensin systems. Physiol Genomics. 2005;23:311–317. doi: 10.1152/physiolgenomics.00163.2005. [DOI] [PubMed] [Google Scholar]
- Kasper SO, Ferrario CM, Ganten D, Diz DI. Rats with low brain angiotensinogen do not exhibit insulin resistance during early aging. Endocrine. 2006;30:167–174. doi: 10.1385/ENDO:30:2:167. [DOI] [PubMed] [Google Scholar]
- Kato S, Onishi K, Yamanaka T, et al. Exaggerated hypertensive response to exercise in patients with diastolic heart failure. Hypertens Res Off J Jpn Soc Hypertens. 2008;31:679–684. doi: 10.1291/hypres.31.679. [DOI] [PubMed] [Google Scholar]
- Katovich MJ, Grobe JL, Raizada MK. Angiotensin-(1-7) as an antihypertensive, antifibrotic target. Curr Hypertens Rep. 2008;10:227–232. doi: 10.1007/s11906-008-0043-9. [DOI] [PubMed] [Google Scholar]
- Kritchevsky SB, Nicklas BJ, Visser M, et al. Angiotensin-converting enzyme insertion/deletion genotype, exercise, and physical decline. JAMA J Am Med Assoc. 2005;294:691–698. doi: 10.1001/jama.294.6.691. [DOI] [PubMed] [Google Scholar]
- Krysiak R, Okopień B. Pleiotropic effects of angiotensin-converting enzyme inhibitors in normotensive patients with coronary artery disease. Pharmacol Rep PR. 2008;60:514–523. [PubMed] [Google Scholar]
- Lees FD, Clarkr PG, Nigg CR, Newman P. Barriers to exercise behavior among older adults: a focus-group study. J Aging Phys Act. 2005;13:23–33. doi: 10.1123/japa.13.1.23. [DOI] [PubMed] [Google Scholar]
- Leite LHR, Lacerda ACR, Marubayashi U, Coimbra CC. Central angiotensin AT1-receptor blockade affects thermoregulation and running performance in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R603–607. doi: 10.1152/ajpregu.00038.2006. [DOI] [PubMed] [Google Scholar]
- Leite LHR, Lacerda ACR, Balthazar CH, et al. Central AT(1) receptor blockade increases metabolic cost during exercise reducing mechanical efficiency and running performance in rats. Neuropeptides. 2007;41:189–194. doi: 10.1016/j.npep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Leite LHR, Rodrigues AG, Soares DD, et al. Central fatigue induced by losartan involves brain serotonin and dopamine content. Med Sci Sports Exerc. 2010;42:1469–1476. doi: 10.1249/MSS.0b013e3181d03d36. [DOI] [PubMed] [Google Scholar]
- Liakos CI, Vyssoulis GP, Michaelides AP, et al. The effects of angiotensin receptor blockers vs. calcium channel blockers on the acute exercise-induced inflammatory and thrombotic response. Hypertens Res Off J Jpn Soc Hypertens. 2012;35:1193–1200. doi: 10.1038/hr.2012.134. [DOI] [PubMed] [Google Scholar]
- LIFE Study Investigators. Pahor M, Blair SN, et al. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- Li N-C, Lee A, Whitmer RA, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz JN. Chymase: the other ACE? Am J Physiol - Ren Physiol. 2010;298:F35–F36. doi: 10.1152/ajprenal.00641.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RD, Santos RA, Andrade SP. Mechanisms of angiotensin-(1-7)-induced inhibition of angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2001;280:R994–R1000. doi: 10.1152/ajpregu.2001.280.4.R994. [DOI] [PubMed] [Google Scholar]
- Maggio M, Ceda GP, Lauretani F, et al. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study) Am J Cardiol. 2006;97:1525–1529. doi: 10.1016/j.amjcard.2005.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis K, Maltais F, Lacasse Y, et al. Effects of aerobic exercise training and irbesartan on blood pressure and heart rate variability in patients with chronic obstructive pulmonary disease. Can Respir J J Can Thorac Soc. 2008;15:355–360. doi: 10.1155/2008/894712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, DuPree J, Lees HA, Giovannini S, Seo D, Buford TW, Sweet K, Morgan D, Strehler KYE, Diz D, Borst SE, Moningka M, Krotova K, Carter CS (2013) Late-life enalapril administration induces nitric oxidedependent and independent metabolic adaptations in the rat skeletal muscle. AGE 35(4):1061–1075 [DOI] [PMC free article] [PubMed]
- McBride TA. AT1 receptors are necessary for eccentric training-induced hypertrophy and strength gains in rat skeletal muscle. Exp Physiol. 2006;91:413–421. doi: 10.1113/expphysiol.2005.032490. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol. 2003;91:30H–37H. doi: 10.1016/s0002-9149(03)00432-6. [DOI] [PubMed] [Google Scholar]
- Miszko TA, Cress ME, Slade JM, et al. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58:171–175. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci. 2006;100:391–397. doi: 10.1254/jphs.cpj06008x. [DOI] [PubMed] [Google Scholar]
- Montgomery HE, Marshall R, Hemingway H, et al. Human gene for physical performance. Nature. 1998;393:221–222. doi: 10.1038/30374. [DOI] [PubMed] [Google Scholar]
- Mukuda T, Sugiyama H. An angiotensin II receptor antagonist suppresses running-enhanced hippocampal neurogenesis in rat. Neurosci Res. 2007;58:140–144. doi: 10.1016/j.neures.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Muszalik M, Dijkstra A, Kędziora-Kornatowska K, et al. Independence of elderly patients with arterial hypertension in fulfilling their needs, in the aspect of functional assessment and quality of life (QoL) Arch Gerontol Geriatr. 2011;52:e204–209. doi: 10.1016/j.archger.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Myerson S, Hemingway H, Budget R, et al. Human angiotensin I-converting enzyme gene and endurance performance. J Appl Physiol Bethesda Md 1985. 1999;87:1313–1316. doi: 10.1152/jappl.1999.87.4.1313. [DOI] [PubMed] [Google Scholar]
- Nashar K, Nguyen JP, Jesri A, et al. Angiotensin receptor blockade improves arterial distensibility and reduces exercise-induced pressor responses in obese hypertensive patients with the metabolic syndrome. Am J Hypertens. 2004;17:477–482. doi: 10.1016/j.amjhyper.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Layne JE, Bernstein MJ, et al. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol A Biol Sci Med Sci. 2004;59:154–160. doi: 10.1093/gerona/59.2.m154. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BWJH, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- Passos-Silva DG, Verano-Braga T, Santos RAS. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci Lond Engl 1979. 2013;124:443–456. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Ferrucci L, Leveille SG, et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- Puthucheary DZ, Skipworth JRA, Rawal J, et al. The ACE gene and human performance. Sports Med. 2011;41:433–448. doi: 10.2165/11588720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Reneland R, Lithell H. Angiotensin-converting enzyme in human skeletal muscle. A simple in vitro assay of activity in needle biopsy specimens. Scand J Clin Lab Invest. 1994;54:105–111. doi: 10.3109/00365519409086516. [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MC, Campagnole-Santos MJ, Machado RP, et al. Evidence for a role of AT(2) receptors at the CVLM in the cardiovascular changes induced by low-intensity physical activity in renovascular hypertensive rats. Peptides. 2007;28:1375–1382. doi: 10.1016/j.peptides.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Santos RA. Angiotensin-(1–7) Hypertension. 2014;63:1138–1147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- Schellenbaum GD, Smith NL, Heckbert SR, et al. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertension. J Am Geriatr Soc. 2005;53:1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x. [DOI] [PubMed] [Google Scholar]
- Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3:125–137. [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health and Nutrition Examination Surveys, 1988-1994 and 1999-2004. Am J Public Health. 2010;100:100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Le B, Khode R, et al. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KM, Leng X, Williamson J, et al. Angiotensin converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Muscat GEO. Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease. Int J Biochem Cell Biol. 2005;37:2047–2063. doi: 10.1016/j.biocel.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Song Y-H, Li Y, Du J, et al. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza JC, Tibana RA, de Sousa NMF, et al. Association of cardiovascular response to an acute resistance training session with the ACE gene polymorphism in sedentary women: a randomized trial. BMC Cardiovasc Disord. 2013;13:3. doi: 10.1186/1471-2261-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzullo P, Iacone R, Iacoviello L, et al. Genetic variation in the renin–angiotensin system and abdominal adiposity in men: the Olivetti Prospective Heart Study. Ann Intern Med. 2003;138:17–23. doi: 10.7326/0003-4819-138-1-200301070-00007. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA J Am Med Assoc. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumukadas D, Witham MD, Struthers AD, McMurdo MET. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ Can Med Assoc J J Assoc Medicale Can. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumukadas D, Band M, Miller S, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69:736–743. doi: 10.1093/gerona/glt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco A, Ally A. Angiotensin II type-2 (AT2) receptor antagonism alters cardiovascular responses to static exercise and simultaneously changes glutamate/GABA levels within the ventrolateral medulla. Neurosci Res. 2009;64:372–379. doi: 10.1016/j.neures.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Tufescu A, Kanazawa M, Ishida A, et al. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J Hypertens. 2008;26:312–321. doi: 10.1097/HJH.0b013e3282f2450b. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau (2009) Population: elderly, racial and Hispanic origin population profiles. The 2012 statistical abstract. http://www.census.gov/compendia/statab/cats/population/elderly_racial_and_hispanic_origin_population_profiles.html. Accessed 3 Jul 2014
- Velez JCQ, Ryan KJ, Harbeson CE, et al. Angiotensin I is largely converted to angiotensin (1-7) and angiotensin (2–10) by isolated rat glomeruli. Hypertension. 2009;53:790–797. doi: 10.1161/HYPERTENSIONAHA.109.128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Chen Z, Jin S, Su Q. Correlation between angiotensinogen gene and primary hypertension with cerebral infarction in the Li nationality of China. Neurosci Bull. 2007;23:287–292. doi: 10.1007/s12264-007-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Koike T, Li P, et al. Effects of angiotensin II AT1 receptor inhibition and exercise training on insulin action in rats on high-fat diet. Life Sci. 2012;90:322–327. doi: 10.1016/j.lfs.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Westerkamp CM, Gordon SE. Angiotensin-converting enzyme inhibition attenuates myonuclear addition in overloaded slow-twitch skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1223–R1231. doi: 10.1152/ajpregu.00730.2004. [DOI] [PubMed] [Google Scholar]
- Yanai K, Nibu Y, Murakami K, Fukamizu A. A cis-acting DNA element located between TATA box and transcription initiation site is critical in response to regulatory sequences in human angiotensinogen gene. J Biol Chem. 1996;271:15981–15986. doi: 10.1074/jbc.271.27.15981. [DOI] [PubMed] [Google Scholar]
- Zankl AR, Ivandic B, Andrassy M, et al. Telmisartan improves absolute walking distance and endothelial function in patients with peripheral artery disease. Clin Res Cardiol Off J Ger Card Soc. 2010;99:787–794. doi: 10.1007/s00392-010-0184-0. [DOI] [PubMed] [Google Scholar]