Abstract
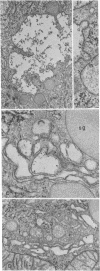
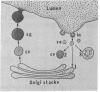
Dextran was used to trace membrane retrieved from the luminal surface after induced exocytosis in secretory cells of rat lacrimal and parotid glands. Two different approaches were used: (a) isolated acini were incubated in vitro with dextran followed by stimulation with carbamylcholine (lacrimal) or isoproterenol (parotid) and (b) rats were injected with isoproterenol followed by dextran infusion into the parotid duct in vivo. The main findings were the same regardless of the gland source or experimental approach. Dextran was taken up initially via coated pits into smooth-surfaced apical vesicles. Shortly thereafter it was found in multiple cell compartments: within the stacked Golgi cisternae, in condensing vacuoles, and in lysosomes. Uptake was more rapid and uniform in vivo; dextran was seen in multiple cisternae of numerous Golgi complexes within 5 min after infusion. In acini incubated in vitro uptake into Golgi cisternae was more delayed and occurred with increasing frequency up to 60 min; also, more dextran was taken up into lysosomes, which were more numerous in vitro than in vivo. The results demonstrate that, after exocytosis, membrane is removed from the cell surface via vesicles that fuse with multiple cell compartments. The two novel findings are: (a) the demonstration that the tracer can reach most of the Golgi cisternae in a given stack and (b) the demonstration of the rapidity with which the process takes place (i.e., within 5 min). The findings imply that at least some membrane retrieved from the cell surface after exocytosis fuses with the stacked Golgi cisternae.
Keywords: membrane recycling, dextran, parotid, lacrimal (gland)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams S. J., Holtzman E. Secretion and endocytosis in insulin-stimulated rat adrenal medulla cells. J Cell Biol. 1973 Feb;56(2):540–558. doi: 10.1083/jcb.56.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle J. D., Jamieson J. D., Palade G. E. Radioautographic analysis of the secretory process in the parotid acinar cell of the rabbit. J Cell Biol. 1972 May;53(2):290–311. doi: 10.1083/jcb.53.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol. 1974 Dec;63(3):883–903. doi: 10.1083/jcb.63.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Peluchetti D., Meldolesi J. Dynamic changes of the luminal plasmalemma in stimulated parotid acinar cells. A freeze-fracture study. J Cell Biol. 1976 Jul;70(1):59–74. doi: 10.1083/jcb.70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967 Nov;35(2):357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze J. J., Kramer M. F. Function of coated membranes and multivesicular bodies during membrane regulation in stimulated exocrine pancreas cells. Cell Tissue Res. 1974;156(1):1–20. doi: 10.1007/BF00220098. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Steiber A., Kim S. U., Graham D. I., Avrameas S. Internalization of neuronal plasma membrane ricin receptors into the Golgi apparatus. Exp Cell Res. 1975 Sep;94(2):426–431. doi: 10.1016/0014-4827(75)90508-x. [DOI] [PubMed] [Google Scholar]
- Hand A. R. Morphology and cytochemistry of the Golgi apparatus of rat salivary gland acnar cells. Am J Anat. 1971 Feb;130(2):141–157. doi: 10.1002/aja.1001300203. [DOI] [PubMed] [Google Scholar]
- Herzog V., Sies H., Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. J Cell Biol. 1976 Sep;70(3):692–706. doi: 10.1083/jcb.70.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E., Teichberg S., Abrahams S. J., Citkowitz E., Crain S. M., Kawai N., Peterson E. R. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J Histochem Cytochem. 1973 Apr;21(4):349–385. doi: 10.1177/21.4.349. [DOI] [PubMed] [Google Scholar]
- Kalina M., Robinovitch R. Exocytosis couples to endocytosis of ferritin in parotid acinar cells from isoprenalin stimulated rats. Cell Tissue Res. 1975 Nov 12;163(3):373–382. doi: 10.1007/BF00219471. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Holtzman E., Walter R. Hormone-stimulated exocytosis in the toad urinary bladder. Some possible implications for turnover of surface membranes. J Cell Biol. 1972 Jan;52(1):211–219. doi: 10.1083/jcb.52.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W., Schulz R. A. Micropinocytotic origin of coated and smooth microvesicles ("synaptic vesicles") in neurosecretory terminals of posterior pituitary glands demonstrated by incorporation of horseradish peroxidase. Nature. 1971 Jul 30;232(5309):341–342. doi: 10.1038/232341a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W. Thorium dioxide uptake into adrenal medullary cells and the problem of recapture of granule membrane following exocytosis. Brain Res. 1972 Feb 11;37(1):141–145. doi: 10.1016/0006-8993(72)90356-3. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B. The endoplasmic reticulum: a cytochemist's view (a review). Proc Natl Acad Sci U S A. 1976 Aug;73(8):2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Malaisse-Lagae F., Ravazzola M., Amherdt M., Renold A. E. Exocytosis-endocytosis coupling in the pancreatic beta cell. Science. 1973 Aug 10;181(4099):561–562. doi: 10.1126/science.181.4099.561. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pelletier G. Secretion and uptake of peroxidase by rat adenohypophyseal cells. J Ultrastruct Res. 1973 Jun;43(5):445–459. doi: 10.1016/s0022-5320(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Roth T. F., Cutting J. A., Atlas S. B. Protein transport: a selective membrane mechanism. J Supramol Struct. 1976;4(4):527–548. doi: 10.1002/jss.400040413. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972 May;53(2):365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis D. T., Dreifuss J., Harris M. C., Orci L. Secretion-related uptake of horseradish peroxidase in neurohypophysial axons. J Cell Biol. 1976 Aug;70(2 Pt 1):294–303. doi: 10.1083/jcb.70.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Kirshner N., Schramm M. Non-parallel transport of membrane proteins and content proteins during assembly of the secretory granule in rat parotid gland. Biochim Biophys Acta. 1975 Jan 14;375(1):87–105. doi: 10.1016/0005-2736(75)90074-7. [DOI] [PubMed] [Google Scholar]