Abstract
Seed dormancy is imposed by one or more of the embryo, endosperm, and maternal tissues that belong to two generations and represent two ploidy levels. Many quantitative trait loci (QTL) have been identified for seed dormancy as measured by gross effects on reduced germination rate or delayed germination in crop or model plants. This research developed an endosperm genotype−based genetic approach to determine specific tissues through which a mapped QTL regulates germination using rice as a model. This approach involves testing germination velocity for partially after-ripened seeds harvested from single plants heterozygous for a tested QTL and genotyping endosperms from individual germinated and nongerminated seeds with a codominant DNA marker located on the QTL peak region. Information collected about the QTL includes genotypic frequencies in germinated and/or nongerminated subpopulations; allelic frequency distributions during a germination period; endosperm or embryo genotypic differences in germination velocity; and genotypic frequencies for gametes involved in the double fertilization to form the sampled seeds. Using this approach, the seed dormancy loci SD12, SD1-2, and SD7-1 were determined to regulate germination through the embryo, endosperm, and maternal tissues, respectively; SD12 and SD1-2 acted additively on germination velocity in the offspring tissues; and SD12 also was associated with the preferential fertilization of male gametes in rice. This new genetic approach can be used to characterize mapped genes/QTL for tissue-specific functions in endospermic seeds and for marker-assisted selection of QTL alleles before or immediately after germination in crop breeding.
Keywords: seed dormancy, quantitative trait locus, endosperm, segregation distortion, rice
Seed dormancy developed on the mother plant (i.e., primary dormancy) is imposed by one or more of the embryo, endosperm, and maternal tissues that belong to two (maternal and offspring) generations and represent two (diploid and triploid) ploidy levels. Genotypic variation of seed dormancy exits in natural populations and crop germplasm as an adaptive mechanism for seed-bearing plants to regulate the timing of germination. The natural variation in seed dormancy or germination-related traits has been associated with multiple quantitative trait loci (QTL) in barley (e.g., Ullrich et al. 1993), wheat (e.g., Anderson et al. 1993), rice (e.g., Lin et al. 1998), oats (Fennimore et al. 1999), sorghum (e.g., Lijavetzky et al. 2000), Arabidopsis (e.g., Alonso-Blanco et al. 2003), lettuce (e.g., Argyris et al. 2005), sunflower (e.g., Gandhi et al. 2005), rye (Masojć et al. 2007), oilseed rape (Schatzki et al. 2013), and peach (Blaker et al. 2013). Of the QTL reported in the listed and other research, only those in peach were detected based on marker-trait associations in the same generation (i.e., F2 seeds, Blaker et al. 2013); the remaining loci in all other species were claimed based on associations between plant genotypes and germination capabilities of seeds from the plants in mapping populations, which ignored the difference in generation or genotype between seed component tissues. Technically, it is difficult to genotype both plants and individual seeds from the plants in a mapping population. Statistical models, which combine maternal with offspring (embryo or endosperm) genotypes to map QTL associated with seed traits (e.g., Zhang et al. 2004), have not been used for research on seed dormancy. Thus, additional research is needed to determine specific tissues in which a QTL underlying gene is expressed to impose seed dormancy. This information is critical to further understand cellular and molecular mechanisms of seed dormancy and is also useful to develop selection strategies to manipulate germinability (e.g., the resistance to preharvest sprouting in cereal crops) in breeding programs using the QTL alleles.
Experimental approaches that have been used to infer the involvement of a seed component tissue in dormancy imposition can be grouped into somatic, molecular and genetic categories. Somatic approaches have to resort to exercising embryos or physically removing the maternal tissues testa and pericarp (also known as seed and fruit coats) covering caryopses in grass species. The isolated embryos or naked caryopses were germinated on a selected medium to infer the presence of embryo or coat-imposed dormancy, as did in Arabidopsis, barley, oat, rice, and wheat (Takahashi 1963; Morris et al. 1989; Foley 1992; Wang et al. 1995; Lee et al. 2010). Molecular approaches include various methods for gene tissue-specific expression analyses, such as RNA in situ hybridization and GUS reporter assay, which require DNA/mRNA sequences of a known gene to prepare probes (vectors) for hybridization (transformation). Via the use of molecular approaches, genes cloned from the rice qLTG3-1 and Sdr4 and the wheat QPhs.ocs-3A1 (TaMFT) QTL were implicated to regulate dormancy or low-temperature germination in embryos (Fujino et al. 2008; Sugimoto et al. 2010; Nakamura et al. 2011). Genetic approaches start with crosses between genotypes different in seed dormancy or QTL allele to obtain hybrid F1 or F2 seeds. F1 seed samples from reciprocal crosses were tested for the difference in germinability to infer a maternal tissue effect on seed dormancy, as reported for rice and wheat (Nair et al. 1965; Noll et al. 1982; Flintham 2000). The F2 seed samples were partially after-ripened to test embryo genotypic frequencies for a QTL marker in the germinated and/or nongerminated subpopulations; the QTL was inferred to be involved in the maternal tissue-imposed dormancy if the genotypic/allelic frequencies kept constant between the subpopulations, or involved in the offspring tissue-imposed dormancy if the dormancy-reducing allele had a greater frequency in the germinated than in the nongerminated subpopulation (Gu et al. 2008). By use of the embryo genotype-based genetic approach, the qSD7-1 and qSD12 QTL were associated with maternal and offspring tissue-imposed dormancies, respectively, in rice.
Advantages of the genetic over the somatic and molecular approaches are nondestructive testing and applicability to a large sample of seeds. Concerns about the genetic approaches are some uncertainties in inferences for tissue specificity or about interference by a linked segregation distortion locus (SDL; Gu et al. 2008). First, a maternal tissue effect on germination inferred by the difference between reciprocal F1s may be confounded with a cytoplasmic or endospermic effect, because hybrid seeds have female cytoplasm in all their cells and two of the three chromosomal sets in an endosperm cell from the female parent. The uncertainty about a cytoplasmic effect can be resolved by the embryo genotype-based genetic approach, as F2 seeds self-pollinated from an F1 plant are identical in cytoplasm. Second, the embryo genotype-based approach cannot distinguish an embryonic from an endospermic effect. An embryo is developed from a fertilized egg (2n) fused between the egg cell (n) in an embryo sac and one of the two sperms (n) in a pollen tube, whereas the endosperm is developed from the primary endosperm nucleus (3n) fused between two genetically identical polar nuclei (n) in the sac and the other sperm. Thus, from an F1 plant heterozygous for dormancy locus (D/d), the F2 seeds with the Dd genotype embryo have either DDd or Ddd genotype endosperm. Theoretically, if the dormancy gene expresses in the triploid tissue, the endospermic effect, which could be confounded with the maternal tissue effect as inferred by the reciprocal F1 approach or cannot be detected by the embryo genotype-based approach, could be estimated by the association between germination velocity of individual F2 seeds and their endosperm genotypes. In case that the gene expresses in the diploid offspring tissue, the endosperm genotypes can be transformed into embryo genotypes to estimate the embryonic effect. Third, genotypic frequencies for a dormancy gene in a germinated or nongerminated subpopulation of partially after-ripened F2 seeds also can be affected by a linked SDL. An SDL was often associated with preferential dysfunction/fertilization of the male or female gamete (Lyttle 1991). Fortunately, gamete genotypic frequencies of an SDL for both male and female sides can be estimated based on frequencies of the four endosperm (but not the three embryo) genotypes in a random sample of F2 seeds. The estimates can help determine if a distorted segregation observed in a germinated or nongerminated subpopulation arises from the selection for less or more dormancy seeds, a linked SDL, or both.
This research was devoted to develop an endosperm genotype-based genetic approach to characterize functions of previously mapped seed dormancy QTL in regulating germination through the embryo, endosperm, or maternal tissues. Genetic and seed biology principles for the new approach are same as those described for the embryo genotype-based approach (Gu et al. 2008), except that genotyping information is collected from endosperms, rather than embryos. It is relatively easy to genotype embryos, because DNA samples for the genotyping can be prepared from embryonic leaves or seedlings after a germination test and there are only three embryo genotypes for a locus in an F2 seed population of a diploid species. Thus, challenges to this research would be techniques used to extract endospermic DNAs from individual seeds without a negative impact on standard germination testing and to display all four endosperm genotypes for a locus in an F2 seed population. In this research, rice (Oryza sativa L.) was used as a model to develop a marker-genotyping system to meet the technical challenges, and the seed dormancy QTL SD1-2, SD7-1, and SD12, which were isolated as single Mendelian factors into the same genetic background in the previous research, were selected to demonstrate the efficacy of the new genetic approach. This article summarized techniques and analytic strategies for the endosperm genotype-based genetic approach, presented new information about the three selected loci, and discussed implications of some discoveries from this research.
Materials and Methods
Parental lines and F2 seed populations
Isogenic lines (ILs):
Four ILs, including the recipient parent EM93-1, were used to develop hybrid F1s that are heterozygous for each of the SD1-2, SD7-1, and SD12 loci or both SD7-1 and SD12 (Table 1). EM93-1 is a line of cultivated rice (Oryza sativa subsp. indica) with a semi-dwarf plant height controlled by the semidwarf1 (sd1) gene and is homozygous for the dormancy-enhancing allele at SD1-2 and the dormancy-reducing alleles at both SD7-1 and SD12. The ILs were developed by introducing single chromosome (chr) segments from SS18-2, a line of wild-like weedy rice (O. sativa), into the recipient genetic background. Specifically, ILsd1-2 has an introgression segment of ~3000 kb in physical length containing both Sd1 and sd1-2 on chr 1 (Ye et al. 2013); ILSD7-1 has an introgression segment of ~2 kb intragenic to the pleiotropic gene SD7-1/Rc on chr 7 and the introgression converts the mutant allele in EM93-1 (sd7-1/rc) into a functional allele for both seed dormancy and red pericarp color (Gu et al. 2011); and ILSD12 has an introgression segment of ~200 kb containing the SD12 allele on chr 12 (Gu et al. 2010).
Table 1. Genotypic and phenotypic information about isogenic lines (IL) and hybrid F1s used to develop F2 seed populations.
| Parental Line or F1a | Genotypea | Segment/Markerb | Pericarp Colorc | Plant Heightd |
|---|---|---|---|---|
| A. ILSD1-2 (EM93-1) | SD1-2SD1-2sd7-1sd7-1sd12sd12 | Recipient | White (rcrc) | Semidwarf (sd1sd1, ~80 cm) |
| B. ILsd1-2 | sd1-2sd1-2sd7-1sd7-1sd12sd12 | ~3000 kb | White (rcrc) | Tall (Sd1Sd1, ~100 cm) |
| C. ILSD7-1 | SD1-2SD1-2SD7-1SD7-1sd12sd12 | ~2 kb | Red (RcRc) | Semidwarf (sd1sd1) |
| D. ILSD12 | SD1-2SD1-2sd7-1sd7-1SD12SD12 | ~200 kb | White (rcrc) | Semidwarf (sd1sd1) |
| F1_SD1-2 (A×B) | SD1-2sd1-2sd7-1sd7-1sd12sd12 | RM315/3602 | White (rcrc) | Tall (Sd1sd1, ~90 cm) |
| F1_SD7-1 (A×C) | SD1-2SD1-2SD7-1sd7-1sd12sd12 | RID12 | Red (Rcrc) | Semidwarf (sd1sd1) |
| F1_SD12 (A×D) | SD1-2SD1-2sd7-1sd7-1SD12sd12 | SD12m13 | White (rcrc) | Semidwarf (sd1sd1) |
| F1_SD7-1SD12 (C×D) | SD1-2SD1-2SD7-1sd7-1SD12sd12 | RID12 and SD12m13 | Red (Rcrc) | Semidwarf (sd1sd1) |
EM93-1 was the recipient of single introgression segments from a line of “red” weedy rice in ILs for the SD1-2, SD7-1 or SD12 locus. Upper or lower case letters indicate dormancy-enhancing (SD) or -reducing (sd) alleles at the three loci.
Physical lengths of the introgression segments in kilobases or DNA markers selected to tag the loci.
Rc, red pericarp color gene, which belongs to the same locus as SD7-1, with the functional allele responsible for both red pigment and enhanced seed dormancy (Gu et al. 2011).
sd1, semidwarf1 gene located on the SD1-2-containing region, with the EM93-1−derived allele responsible for both reduced plant height and enhanced seed dormancy (Ye et al. 2013).
F2 seed populations:
About 20 F1 hybrid plants from each of the four crosses were grown in a greenhouse, verified for genotypes at SD1-2, SD7-1, and SD12 using markers on the introgression segments (Table 1), and self-pollinated to produce F2 seed populations. Seeds were harvested from individual F1 plants at 40 d after flowering, air-dried at the greenhouse for 3 d, and stored at a freezer (−20°) to maintain the primary dormancy.
Seed after-ripening, germination, and subpopulatons
Seed samples from the freezer were after-ripened at the room temperature (24−25°) for 0−35 d to release part of the primary dormancy prior to germination testing. The time period of a partially after-ripening treatment varied with seed populations and was determined in preliminary experiments to manipulate relative sizes of germinated and nongerminates subpopulations. Two or more independent germination experiments were conducted for each of the four F2 populations and an experiment consisted of 600−2000 fully developed seeds. About 60 seeds derived from an F1 plant were distributed in a 9-cm Petri dish lined with a filter paper, soaked with 8 mL of deionized water, and incubated at 30° and 100% relative humidity in dark. Germination (radicle emerged >3 mm) counting started at 48 hr after imbibition and continued every 12 or 24 hr for 7 or ~10 d. Germinated seeds were transferred to new Petri dishes to collect the endosperm tissue. All germinated seeds from an experiment were formed a germinated subpopulation, whereas seeds that did not germinate and were not contaminated within 7 or 10 d were grouped as a nongerminated subpopulation.
DNA microextraction and marker genotyping
For a germinated subpopulation, a newly germinated intact seed (spikelet) was cut in a cross section at 1/3 to the endosperm end. The embryo-less portion was cleaned by removing the maternal tissues and the endosperm tissue transferred into a 1.5-mL centrifuge tube and stored in a −20° freezer for DNA extraction. This method was also used to sample the endosperm tissue from nongerminated or dry seeds. In preliminary experiments to genotype both endosperm and embryo tissues, the sectioned seeds with an emerged radicle were transferred to 24-well cell culture plates (Corning 15.6-mm diameter) lined with wetted filter papers and placed in an illuminated growth chamber for several days to collect embryonic leaves for DNA extraction.
Endospermic DNA was extracted using methods modified from Chunwongse et al. (1993) and Kang et al. (1998). Primarily, the endosperm tissue was incubated in 200 µL of of lysis buffer (0.5% sodium dodecyl sulfate and 20 µg of Proteinase K) at 37° for 1 hr and ground with a spatula. The crude sample was mixed with 400 µL of 2% CTAB solution [2% (w/v) cetyltrimethylammonium bromide, 100 mM Tris-HCl (pH 8.0), 20 mM ethylenediaminetetraacetic acid (pH 8.0), 1.4 M NaCl, and 1% polyvinylpyrrolidone] and phase-separated with the 24:1 ratio of chloroform:isoamyl alcohol mixture. DNA in the aqueous phase was precipitated with cold isopropanol and washed with 70% ethanol. Air-dried DNA was dissolved in 50 µL of TE buffer (10 mM Tris-HCl and 1 mM EDTA at pH 8.0) and treated with 1 µL of RNaseA (10 mg/µL) to remove RNA. Alternatively, endosperm samples were placed in a 96-well plate and heated in 80 µL of lysis buffer (0.1 M NaOH) on a Thermocycler set at 95° for 15 min. The lysing solutions were mixed with 80 µL of neutralization buffer (10 mM Tris-HCl and 0.1 M HCl at pH 2.0) and the supernatant was used as DNA templates for polymerase chain reaction (PCR). DNA extraction from the embryonic leaves was conducted using the previously described method (Gu et al. 2008).
The simple sequence repeat or insertion/deletion markers on partial high-resolution maps for the SD1-2 (Ye et al. 2013), SD7-1 (Gu et al. 2011), or SD12 (Gu et al. 2010) regions were selected to optimize a genotyping system. The selected markers are codominant, different in size by 10 or more bp between the two alleles, and capable of distinguishing four endosperm genotypes of F2 seeds by regular PCR and gel electrophoresis. The PCR amplification was performed using a 20-μL volume containing 40 ng of DNA template, 4 µL of 5× Green GoTaq reaction buffer (Promaga), 200 µM dNTP, 50 nM each primer, and 2 units of Taq polymerase, in a BIO-RAD Thermocycler. The PCR program was initiated at 94° for 5 min, followed by 35 cycles of denature at 94° for 30 sec, annealing at 55° for 30 sec and elongation at 72° for 45 sec, and ended with a final elongation at 72° for 7 min. PCR products were separated in a 6% nondenaturing polyacrylamide gel at ~300 V for ~3 hr and displayed and recorded using the AlphaEaseFC (Alpha Innotech) gel imaging system.
Data analysis and genetic inferences
Genotypic and allelic frequencies:
Seeds (F2) self-pollinated from a hybrid F1 plant are identical for the maternal tissue genotype (Dd), but vary in embryo (DD, Dd, and dd) and endosperm (DDD, DDd, Ddd, and ddd) genotypes for a locus with two alleles that enhance (D) or reduce (d) seed dormancy (Table 2). The endosperm genotypic frequencies FDDD, FDDd, FDdd, and Fddd in a subpopulation or a random sample were calculated as described in Table 2 and used to estimate the overall allelic frequencies FD and Fd in the sample and the genotypic frequencies for male (FD’ and Fd’) and female (FD’’ and Fd’’) gametes involved in the double fertilization to develop the F2 seeds. The observed genotypic and allelic frequencies may or may not follow Hardy-Weinberg Equilibrium, depending on responses of the dormancy gene to the selection for germinated or nongerminated subpopulations and/or the presence or absence of a SDL in the gene-containing region (Gu et al. 2008). Thus, χ2 testing was used to determine the fitness of endosperm genotypic frequencies to the 0.25:0.25:0.25:0.25 expectation and binomial testing used to determine the fitness of allelic and gamete genotypic frequencies to the 0.5 expectation. Genetic inferences from the statistical tests were: 1) the gene was involved in offspring tissue-imposed dormancy and responded to the selection when genotypic frequencies deviate from the expectations with Fd greater in the germinated than in the nongerminated subpopulation; 2) the gene was involved in maternal tissue-imposed dormancy and did not responded to the selection when genotypic frequencies fit the expectations with Fd constant across subpopulations; and 3) there is a SDL in the dormancy gene-containing region when genotypic frequencies deviate from the expectations in the joined population of germinated and nongerminated seeds or in a random sample.
Table 2. List of genotypes for seed component tissues and endosperm genotypic frequencies in an F2 seed population segregating for a dormancy locus (D/d).
| Female Gamete | Male Gamete | Genotypic Frequencya | |
|---|---|---|---|
| d (0.5) | D (0.5) | ||
| d (0.5)b | ddd (dd) [Dd]c | Ddd (Dd) [Dd] | Fd’’ = Fddd + FDdd |
| Fddd = Nddd/N (0.25)b | FDdd = NDdd/N (0.25) | ||
| D (0.5) | DDd (Dd) [Dd] | DDD (DD) [Dd] | FD’’ = FDDd + FDDD |
| FDDd = NDDd/N (0.25) | FDDD = NDDD/N (0.25) | ||
| Genotypic frequencya | Fd’ = Fddd + FDDd | FD’ = FDdd + FDDD | |
| Overall allelic frequency | Fd = Fddd + 0.5FDdd + 0.5FDDd | FD = FDDD + 0.5FDDd + 0.5FDdd | |
Genotypic frequencies for male (Fd’ and FD’) and female (Fd’’ and FD’’) gametes involved in the double fertilization to form the seed population.
The value in the parentheses is the Mendelian expectation for the endosperm (0.25) or gamete (0.5) genotypic frequency, or the overall allelic frequency (0.5).
Genotypes for the endosperm (triploid), embryo (parentheses), and maternal (brackets) tissues of a seed at a dormancy locus with the two functionally differentiated alleles D and d. Fddd, FDdd, FDDd, and FDDD are genotypic frequencies for the ddd, Ddd, DDd, and DDD endosperms, respectively, estimated based on the number of seeds for individual genotypes (Nddd, NDdd, NDDd, and NDDD) and the population size (N).
Binomial testing also was used to determine the equity or difference in genotypic frequency between male and female gametes (e.g., Fd’ = Fd’’). SE used for a test was calculated as (2Fd × FD/N)1/2, where N is the number of genotyped seeds in a subpopulation or random sample. Significant differences in the tests were used to infer underlying mechanisms, such as a dosage effect of the dormancy gene on germination velocity in endosperms (Fd’’ > Fd’), or a differentiation of the SDL in preferential fertilization between male and female gametes (Fd’ > Fd’’).
Germination distributions:
A sample of partially after-ripened seeds is characterized by germination heterogeneity, i.e., some germinate earlier than others and some dormant seeds never germinate in an experiment. The heterogeneity occurs in a sample of seeds from a pure line (due to nongenetic factors) and a segregating (e.g., F2) population (due to both genetic and nongenetic factors). To quantify the heterogeneity in an experiment, the daily counted germination data from all samples were used to develop a germination distribution:
| (1) |
where, yj is the cumulative germination rate at day j (j = 2 to 7 or 10), ΣjNgj is the summation of seeds germinated from day 2 to day j, and N is the total number of seeds tested in the experiment. The germination distribution was compared with allelic frequency distributions in the germinated subpopulation to infer whether the dormancy gene is involved in the regulation of germination through the offspring or maternal tissues.
For experiments genotyped for both germinated and nongerminated seeds, the germination distribution was calculated for individual endosperm genotypes:
| (2) |
where, yij is the cumulative germination percentage for the ith endosperm genotype (i = 0, 1, 2, and 3, the copy number of D allele in the genotype) on day j; ΣjNgij is the summation of the ith genotypic seeds germinated from days 2 to j; Nng is the total number of nongerminated seeds (including those with missing genotyping data) in the experiment; and Fngi is the frequency of genotype i in the nongerminated subpopulation. The difference in germination distribution pattern among the genotypes was used to infer whether the gene is involved in the genetic control of seed dormancy imposed by the embryo, endosperm or maternal tissues.
Genetic effect estimation:
For genes involved in the regulation of germination through the embryo or endosperm tissue, their component effects in germinated subpopulations were estimated using linear regression models for embryo genotypes:
| (3) |
or for endosperm genotypes (Mo 1988):
| (4) |
where: yik is the incubation time required to complete germination for seed k (k = 1 to Ng, the number of genotyped germinated seeds) of the ith endosperm genotype; µ is the model mean; xi is the dummy variable for the additive component and is coded as −1, 0, and 1 for the embryo genotypes dd, Dd, and DD, respectively, or coded as −1.5, −0.5, 0.5, and 1.5 for the endosperm genotypes ddd, Ddd, DDd, and DDD, respectively; zi is the dummy variable for the dominance component and is coded as −0.5 for both dd and DD or 0.5 for Dd; z1i is the dummy variable for the first dominance component of the two D alleles over the d allele in the DDd genotype and is coded as 1 for DDd or 0 for the remaining three endosperm genotypes; z2i is the dummy variable for the second dominance component of the D allele over the two d alleles in the Ddd genotype and is coded as 1 for Ddd or 0 for the remaining three endosperm genotypes; a, d, d1, and d2 are partial regression coefficients for corresponding variables and the estimates of gene additive or dominance effects; and εik is the residual effect of the model. Regression analysis was implemented using the REG procedure of SAS 9.3 (SAS Institute 2011) with a stepwise selection set at the significance level of probability <5%.
For the germinated subpopulation segregating for both SD7-1 and SD12, two-way analysis of variance was used to detect their main and interactional effects on germination velocity. The variance analysis was conducted based on embryo genotypes using the two-factor factorial model:
| (5) |
where, yijk is the incubation time required to complete germination for the kth seed having the ith genotype at SD7-1 and the jth genotype at SD12, µ is the model mean; αi and βj are main effects of SD7-1 and SD12, respectively; (αβ)ij is the interactional effect between the two loci; and εijk is the error term of the model.
Results
Reliability of the marker genotyping system to distinguish endospermic genotypes
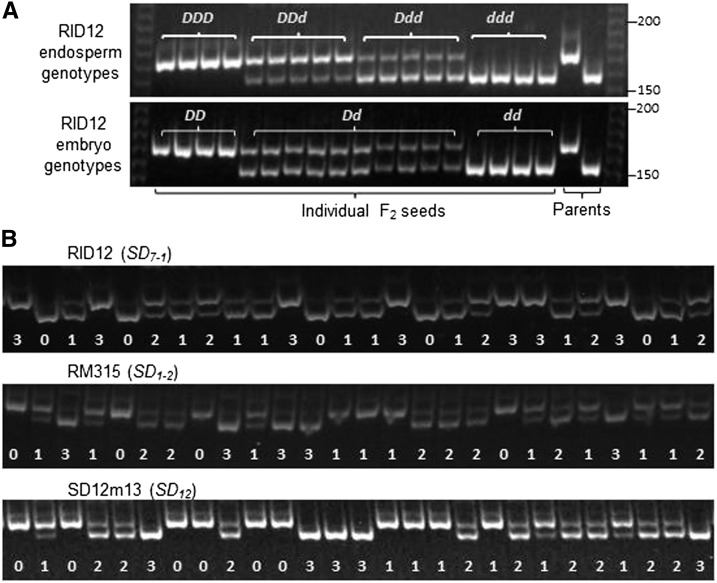
The markers RID12, RM315/3602, and SD12m13, which were selected to tag SD7-1, SD1-2, and SD12, respectively, could separate both embryo and endosperm genotypes in F2 seed populations. As shown on gel images for RID12 (Figure 1A), the two homozygous (DDD and ddd) endosperms displayed one of the two alleles (bands), which are same as the homozygous (DD and dd) embryos; whereas the two heterozygotes (DDd and Ddd) showed both bands (codominance) with one brighter than the other (dosage effect), which are different from the heterozygous (Dd) embryos that have the two bands equal in signal intensity. All the selected markers were consistent in gel image patterns (i.e., codominance and dosage effect) in F2 seed populations (Figure 1B). Of the 11 germination experiments, 9 had >90% germinated or nongerminated seeds genotyped with this marker-genotyping system, and the success rate was similar to that (93%) for a random sample of seeds segregating for SD12 (Table 3). These results demonstrated that the quality of endospermic DNAs from germinated seeds is good for genotyping with the PCR-based markers and the genotypes are readily converted into embryo genotypes.
Figure 1.
Electrophoresis patterns of endosperm genotypes for codominant markers. (A) Comparison between endosperm and embryo genotypes of same seeds. (B) Segregation patterns for four endosperm genotypes of F2 seeds. RID12, RM315, and SD12m13 were selected to mark the seed dormancy loci SD7-1, SD1-2, and SD12, respectively. Gel images show electrophoresis patterns for individual F2 seeds from germinated subpopulations. The genotypes are indicated by combinations of the dormancy-enhancing (D) and/or -reducing (d) alleles, or by the copy number of the D allele (0−3) at a locus.
Table 3. Summary of genotypic and allelic frequencies for the SD7-1, SD1-2, or SD12 locus in F2 seed subpopulations or joined populations.
| Experimenta | Subpopulation (Genotyped Seeds)b | Endosperm Genotypic Frequencyc | χ2 Value (Probability)c | Allelic/Gametic Frequencyd | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fddd | FDdd | FDDd | FDDD | Fd | Fd’ | Fd’’ | |||
| SD7-1 | |||||||||
| Ex. #1 (720, 0 DAR, 6%) | G (43, 100%) | 0.256 | 0.256 | 0.256 | 0.232 | 0.07 (0.995) | 0.512ns | 0.512ns | 0.511ns |
| Ex. #2 (810, 3 DAR, 22%) | G (176, 98%) | 0.256 | 0.216 | 0.307 | 0.222 | 3.68 (0.298) | 0.517ns | 0.563ns | 0.472ns |
| Ex. #3 (631, 10 DAR, 77%) | G (481, 99%) | 0.262 | 0.247 | 0.249 | 0.241 | 0.44 (0.932) | 0.510ns | 0.511ns | 0.509ns |
| NG (138, 93%) | 0.283 | 0.232 | 0.246 | 0.239 | 0.84 (0.839) | 0.522ns | 0.529ns | 0.514ns | |
| G+NG (619) | 0.267 | 0.244 | 0.249 | 0.241 | 0.99 (0.804) | 0.513ns | 0.515ns | 0.511ns | |
| SD1-2 | |||||||||
| Ex. #1 (1091, 1 DAR, 25%) | G (250, 92%) | 0.376 | 0.296 | 0.200 | 0.128 | 35.4 (<0.0001) | 0.624*** | 0.576* | 0.672*** |
| Ex. #2 (1309, 10 DAR, 55%) | G (674, 93%) | 0.261 | 0.236 | 0.242 | 0.261 | 0.09 (0.710) | 0.500ns | 0.497ns | 0.503ns |
| Ex. #3 (1096, 1 DAR, 24%) | G (280, 100%) | 0.396 | 0.300 | 0.207 | 0.096 | 55.3 (<0.0001) | 0.650*** | 0.604*** | 0.696*** |
| NG (801, 99%) | 0.205 | 0.236 | 0.257 | 0.302 | 16.1 (0.0011) | 0.451** | 0.462* | 0.441*** | |
| G+NG (1081) | 0.254 | 0.253 | 0.244 | 0.249 | 0.26 (0.967) | 0.503ns | 0.499ns | 0.507ns | |
| SD12 | |||||||||
| Ex. #1 (1980, 7 DAR, 20%) | G (381, 98%) | 0.367 | 0.234 | 0.257 | 0.142 | 39.4 (<0.0001) | 0.613*** | 0.625*** | 0.601*** |
| Ex. #2 (639, 10 DAR, 34%) | G (215, 99%) | 0.381 | 0.195 | 0.228 | 0.195 | 20.4 (0.0001) | 0.593* | 0.609** | 0.577* |
| Ex. #3 (633, 14 DAR, 26%) | G (161, 99%) | 0.372 | 0.186 | 0.298 | 0.143 | 21.2 (<0.0001) | 0.615** | 0.671*** | 0.559ns |
| NG (234, 50%) | 0.269 | 0.209 | 0.291 | 0.231 | 3.77 (0.286) | 0.519ns | 0.560ns | 0.479ns | |
| G+NG (395) | 0.544ns | 0.588*** | 0.499ns | ||||||
| Ex. #4 (a random sample of 484 seeds) | 0.287 | 0.231 | 0.285 | 0.196 | 11.3 (0.0101) | 0.545* | 0.572** | 0.519ns | |
| SD7-1 & SD12 | |||||||||
| Ex. #1 (1529, 14 DAR, 29%) | G (436, SD12) | 0.477 | 0.181 | 0.216 | 0.126 | 127 (<0.0001) | 0.675*** | 0.693*** | 0.661*** |
| G (436, SD7-1) | 0.225 | 0.239 | 0.280 | 0.257 | 2.97 (0.396) | 0.484ns | 0.504ns | 0.463ns | |
| Ex. #2 (1019, 35 DAR, 81%) | NG (121, SD12) | 0.149 | 0.231 | 0.165 | 0.454 | 29 (<0.0001) | 0.347*** | 0.314*** | 0.380** |
| NG (121, SD7-1) | 0.314 | 0.256 | 0.198 | 0.231 | 3.46 (0.326) | 0.541ns | 0.512ns | 0.570ns | |
Listed in the parentheses are the total number of seeds received the number of days of after-ripening (DAR) treatment before the germination test and the mean germination rate at the seventh day after imbibition.
Listed in the parentheses are the number and proportion of genotyped seeds from the germinated (G) or nongerminated (NG) subpopulation.
The letters D or d in subscripts represent dormancy-enhancing or -reducing alleles in endosperm genotypes, which were tested against the 0.25:0.25:0.25:0.25 expectation.
The overall allelic frequency (Fd) and the male (Fd’) and female (Fd’’) gamete frequencies for the d allele are defined in Table 2. The estimates for SD12 in the joined population (G+NG in SD12 Ex. #3) are means weighted by the germination rate 26%. The superscripts indicate that the difference of the estimate from 0.5 was not significant at P = 0.05 (ns) or significant at P < 0.05 (*), <0.01(**), or <0.001 (***). The underlined estimates indicate Fd’’>Fd’ for SD1-2 or Fd’>Fd’’ for SD12 at the significance level of P < 0.05.
SD7-1 had neither endospermic nor embryonic effect on germination
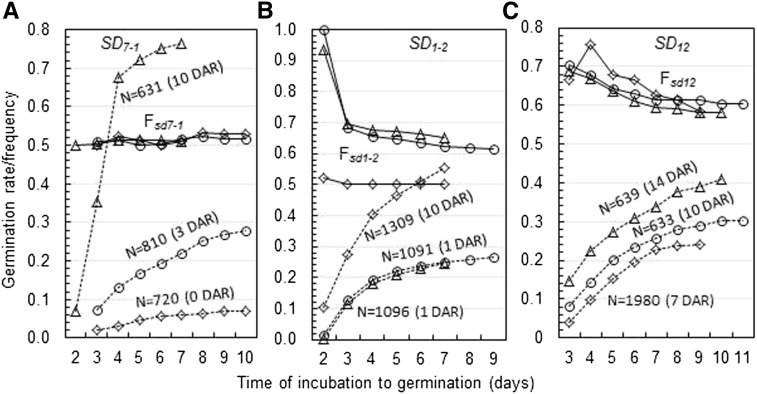
Three germination experiments were conducted for 0-(Ex. #1), 3-(Ex. #2), or 10-(Ex. #3) day after-ripened (DAR) seeds derived from the hybrid F1_SD7-1 (Table 1). Germination rate was 6% for Ex. #1 and 22% for Ex. #2, and germinated seeds were genotyped with the marker RID12. In the germinated subpopulations, four endosperm genotypes fit the expectation (Table 3), and frequencies for the dormancy-enhancing (FSD7-1) and -reducing (Fsd7-1) alleles distributed around 0.5 during the germination period (Figure 2A). Thus, the selection for early germinated seeds did not alter the genetic equilibrium at SD7-1, and endosperm or embryo genotypic variation did not contribute to the phenotypic variation in germination velocity.
Figure 2.
Germination and allelic frequency distributions in germinated subpopulations segregating for the seed dormancy loci SD7-1 (A), SD1-2 (B), and SD12 (C). Dotted lines indicate germination distributions for three independent experiments (open diamonds, circles, and triangles), which were conducted for each locus using the indicated number (N) of F2 seeds received given days of after-ripening (DAR) treatment. Solid lines indicate frequency distributions for the dormancy-reducing alleles (Fsd7-1, Fsd1-2, or Fsd12) in each of the germinated subpopulations. Note: the expected allelic frequency for the genetic equilibrium status is p = 0.5 and frequencies for the dormancy-enhancing alleles (1-p) are not shown.
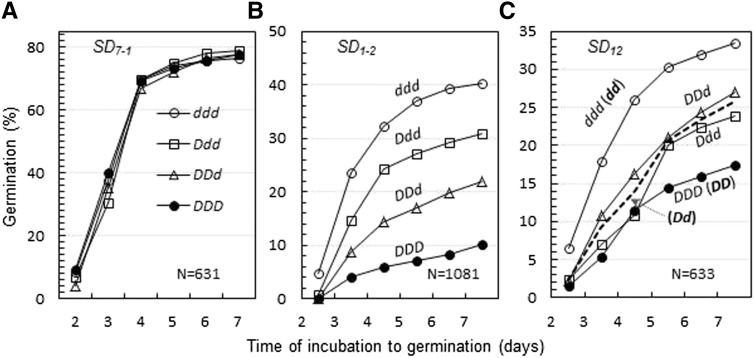
Ex. #3 yielded 77% germinated seeds and both germinated and nongerminated seeds were genotyped. Endosperm genotypic frequencies in the two subpopulations fit the expectation (Table 3), which confirmed the observations in Ex. #1 and 2. In addition, the four genotypes of seeds were same in germination distribution pattern (Figure 3A), indicating that they were identical in the degree of seed dormancy. Because SD7-1 had neither endospermic nor embryonic effect on germination in the three experiments, the dormancy gene should express in the maternal tissue(s) during seed development.
Figure 3.
Cumulative germination distributions for four endosperm genotypes of seeds. Seeds were sampled from populations segregating for the seed dormancy locus SD7-1 (A), SD1-2 (B), or SD12 (C). N was the number of seeds used for the germination experiment. Both germinated and nongerminated seeds were determined for endosperm genotypes, which are indicated by combinations of dormancy-enhancing (D) and/or -reducing (d) alleles. Embryo genotypes for SD12 are listed in the parentheses (C).
SD1-2 was associated with an endospermic effect on germination
Three germination experiments were conducted for 1-(Ex. #1 & 3) and 10-(Ex. #2) DAR seeds derived from the hybrid F1_SD1-2 (Table 1). Germination rate was 25% for Ex. #1 and 55% for Ex. #2 at the seventh day after imbibition, and germinated seeds were genotyped with the markers RM315 or RM3602. In the germinated subpopulation of Ex. #1, endosperm genotypic frequencies dramatically deviated from the expectation, with Fsd1-2sd1-2s1-2 (0.38) >FSD1-2sd1-2sd1-2 (0.30) > FSD1-2SD1-2sd1-2 (0.20) > FSD1-2SD1-2SD1-2 (0.13) (Table 3), and the frequency of the dormancy-reducing allele Fsd1-2 varied from 0.81 to 0.64 during the germination period from days 2 to 7 (Figure 2B). The genotypic segregation pattern and greater Fsd1-2 estimates in the germinated subpopulation indicate that SD1-2 expressed in the offspring tissue. However, endosperm genotypic frequencies fit the 0.25:0.25:0.25:0.25 expectation (Table 3) and Fsd1-2 distributed around 0.5 during the germination period (Figure 2B) in the germinated subpopulation of Ex. #2. Results from Ex. #2 suggested that the inhibitory effect of SD1-2 on germination was released during the after-ripening period of 10 d and seed dormancy left in the sample could be due to the other factors in the genetic background.
Ex. #3 (24%) was a repeat of Ex. #1 and both germinated and nongerminated seeds were genotyped for endosperms. Segregation distortion was observed in the germinated (Fsd1-2 = 0.65) and nongerminated (Fsd1-2 = 0.45) subpopulations, but not in their joined population (Fsd1-2 = 0.50, Table 3). In the germinated subpopulation, patterns for both genotypic (Fsd1-2sd1-2s1-2 > FSD1-2sd1-2sd1-2 > FSD1-2SD1-2sd1-2 > FSD1-2SD1-2SD1-2) and allelic (Figure 2B) frequency distributions were similar to those observed in Ex. #1. In contrast, the nongerminated subpopulation displayed an opposite genotypic frequency distribution pattern (Fsd1-2sd1-2s1-2 < FSD1-2sd1-2sd1-2 < FSD1-2SD1-2sd1-2 < FSD1-2SD1-2SD1-2), with the allelic frequency in favor of the dormancy-enhancing allele (FSD1-2 = 0.55). In addition, the four endosperm genotypes differed from each other in germination velocity, with sd1-2sd1-2sd1-2 > SD1-2sd1-2sd1-2 > SD1-2SD1-2sd1-2 > SD1-2SD1-2SD1-2 (Figure 3B). The distinct genotypic difference indicates that SD1-2 regulates germinability through the endosperm tissue.
It is noted that gamete genotypic frequencies for sd1-2, which were estimated based on germinated subpopulations of Ex. #1 and 3, were greater (P < 0.025) in the female (Fsd1-2’’ = 0.68−0.69) than in the male (Fsd1-2’ = 0.57−0.60) (Table 3). This result indicates that endosperm genotypes of the early germinated seeds consisted of more sd1-2 allele donated from the female than from the male gametes. The difference between Fsd1-2’’ and Fsd1-2’ has nothing to do with allelic differentiation in gamete fertility or preferential fertilization, because gamete genotypic frequencies in the joined population of Ex. #3 fit the Mendelian expectation (Table 3). In fact, the difference between Fsd1-2’’ and Fsd1-2’ is determined by frequencies for the two heterozygous endosperm genotypes (refer to formula in Table 2), which are different in the copy number (or dose) of the dormancy-reducing or -enhancing allele. In both of the germinated subpopulations, the frequency was greater for SD1-2sd1-2sd1-2 (~0.3), which has two germination-promoting alleles from the female gametes, than for SD1-2SD1-2sd1-2 (~0.2), which has one germination-promoting allele from the male gametes (Table 3). Therefore, the observed difference between Fsd1-2’’ and Fsd1-2’ can be accounted for by a dosage effect of the sd1-2 (SD1-2) allele in endosperms on germination promotion (inhibition).
It was estimated based on model (4) for endosperm genotypes that the SD1-2 locus consisted of only an additive effect on the time to germination (0.31 to 0.47 d) in the germinated subpopulations of Ex. #1 and 3 (Table 4).
Table 4. Summary of estimated gene component effects of the SD1-2 or SD12 locus on the time period of incubation required for individual seeds to complete germination in germinated subpopulationsa.
| Locus (Subpopulation) | Additive Effect, d | SE, d | t-Value | Probability | Model |
|---|---|---|---|---|---|
| SD1-2 (SD1-2 Ex. #1) | 0.47 | 0.08 | 5.76 | <0.0001 | Endosperm (4) |
| SD1-2 (SD1-2 Ex. #2) | 0.31 | 0.08 | 4.13 | <0.0001 | Endosperm (4) |
| SD12 (SD12 Ex. #1) | 0.22 | 0.11 | 2.08 | 0.0383 | Embryo (3) |
| SD12 (SD12 Ex. #2) | 0.51 | 0.14 | 3.64 | 0.0003 | Embryo (3) |
| SD12 (SD12 Ex. #3) | 0.53 | 0.22 | 2.41 | 0.0168 | Embryo (3) |
| SD12 (SD7-1 & SD12 Ex. #1) | 0.73 | 0.14 | 5.20 | <0.0001 | Embryo (3) |
Refer to Table 3 for additional information on the subpopulations. Gene component effects were estimated using the cited additive-dominance model for endosperm or embryo genotypes. Estimates for the component dominance effect are not listed as they are not significant at P = 0.05.
SD12 was associated with an embryonic effect on germination and a genetic differentiation in gamete preferential fertilization
Three germination experiments were conducted for 7-(Ex. #1), 10-(Ex. #3), or 14-(Ex. #2) DAR seeds derived from the hybrid F1_SD12 (Table 1). Germination rate was 20% for Ex. #1 and 36% for Ex. #2 at the seventh day after imbibition, and the germinated seeds were genotyped with the marker SD12m13. In germinated subpopulations of the two experiments, endosperm genotypic frequencies dramatically deviated from the expectation (Table 3) and the frequency of the dormancy-reducing allele Fsd12 varied from 0.68 at day 3 to 0.60 at day 7 (Figure 2C). The Fsd12 estimates reduced gradually with the increase in incubation time in these two experiments, indicating that the genotypic variation partly contributed to the germination heterogeneity and the dormancy gene expressed in the offspring tissue.
Germination rate was 26% for Ex. #3 at the seventh day after imbibition and both germinated and nongerminated (50%) seeds were genotyped. Segregation distortion for endosperm genotypes was observed in the germinated but not in the nongerminated subpopulation (Table 3) and the Fsd12 distribution pattern during the germination period was similar to those in the first two experiments (Figure 2C). In addition, the four endosperm genotypes of seeds displayed three germination distribution patterns, with the two heterozygotes (SD12sd12sd12 and SD12SD12sd12) similar in germination velocity (Figure 3C). The association between germination distribution patterns and embryo genotypes is an indication that SD12 is involved in embryo dormancy. It was estimated based on model (3) for embryo genotypes that the SD12 locus consisted of only an additive effect on the time to germination (0.22−0.53 d) in the germinated subpopulations of Ex. #1, 2, and 3 (Table 4).
It is noted that gamete genotypic frequencies for the dormancy-reducing allele sd12 in the joined population of Ex. #3 deviated from 0.5 for the male (Fsd12’ = 0.588) but not for the female (Fsd12’’ = 0.50) gametes (Table 3). The deviation suggests that there could be a SDL in the SD12-containing region affecting the fertilization of male gametes. To prove the hypothesis, a random sample of 484 F2 seeds was genotyped (Ex. #4). Four endosperm genotypes in this sample also deviated from the expected equal frequency, with the allelic frequency in favor of sd12 in the male (Fsd12’ = 0.57), but not in the female (Fsd12’’ = 0.52) gametes (Table 3). The genetic disequilibrium observed in the sizable random sample clearly indicates that the SD12 locus was associated with a genetic differentiation in fertilization capability and male gametes with the dormancy-enhancing allele SD12 tended to be less competitive in fertilization. The associated effect on gamete preferential fertilization contributed only part to the genetic disequilibrium in the germinated subpopulations, because the Fsd12’ estimates (0.61-0.67) in the above-stated three experiments were numerically greater than that (0.57) in the random sample.
Selection for early or late germination broke the genetic equilibrium for SD12, but not for SD7-1, in seed populations segregating for both loci
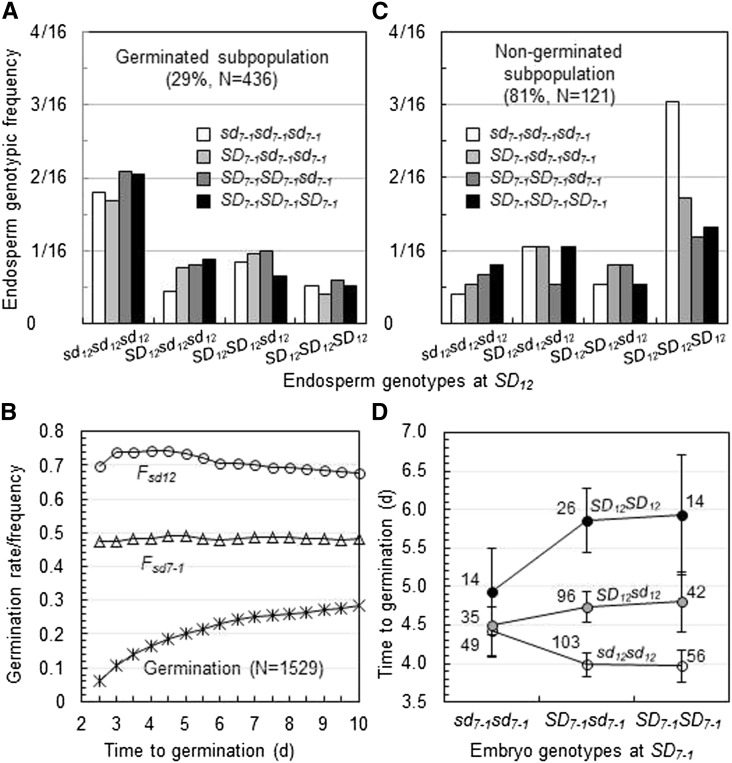
Two germination experiments were conducted for 14-(Ex. #1) or 35-(Ex. #2) DAR seeds derived from the dihybrid F1_SD7-1SD12 (Table 1). Germination rate for the 14-DAR seed samples was 29%, which was lower than those for the 10- and 14-DAR seed samples segregating only for SD7-1 (77%) and SD12 (36%), respectively. The reduced germination rate indicates that pyramiding of the dormancy-enhancing alleles at SD7-1 and SD12 lengthened the dormancy duration. Germinated seeds in Ex. #1 were genotyped for endosperms with the markers RID12 and SD12m13. In the germinated subpopulation, the joint frequency distribution for the two loci was dramatically biased in favor of the four genotypes homozygous (sd12sd12sd12) for the dormancy-reducing allele at SD12, the two (SD12SD12sd12 and SD12sd12sd12) groups of genotypes heterozygous for the SD12 locus were similar in frequency (Figure 4A), and allelic frequency distributions displayed two patterns, i.e., Fsd12 varied from 0.74 to 0.68 whereas Fsd7-1 was constant around 0.5 during the germination period (Figure 4B), which were similar to those observed in the germinated subpopulations segregating only for the SD7-1 or SD12 locus (Figure 2, A and C). These results indicate that SD12 played a major role in regulating germinability through the embryo, not endosperm tissue in the digenic system.
Figure 4.
Genotypic or allelic frequency distributions in germinated and nongerminated subpopulations segregating for SD7-1 and SD12. (A) and (C) Joint frequency distributions for endosperm genotypes of the two loci. The triploid genotypes for each of the two loci are represented by combinations of the dormancy-enhancing (upper case) and/or -reducing (low case) alleles. Refer to Table 3 (SD7-1 and SD12 Ex. #1 and 2) for single-locus information of the germinated (A) and nongerminated (C) subpopulations. (B) Germination and allelic frequency distributions. N was the number of seeds used for the germination experiment. Fsd7-1 and Fsd12 are frequencies for the dormancy-reducing alleles at SD7-1 and SD12, respectively, in the germinated subpopulation. (D) Genotypic differences in the time to germination. Data shown are means (circles), SEs (bars), and sample sizes (numbers) for the nine embryo genotypes of seeds.
It appeared that SD7-1 also interacted with SD12 in embryos to influence germination. For example, the variation in the time to germination among the three SD12 genotypes was smaller in the sd7-1sd7-1 than in the SD7-1sd7-1 or SD7-1SD7-1 backgrounds in Ex. #1 (Figure 4D). However, the two-way analysis of variance based on model (5) revealed that only the main effect of SD12 was significant (see Supporting Information, Table S1). The main effect was contributed by the additive component of the SD12 locus, based on model (3) for embryo genotypes (Table 4).
Germination rate was 81% for Ex. #2 and nongerminated seeds were identified for endosperm genotypes at SD7-1 and SD12. A relatively high selection strength (as indicated by the germination rate) for nongerminated seeds in this experiment is expected to suppress less dormant genotypes in the subpopulation. As expected, the joint frequency distribution for SD7-1 and SD12 was biased in favor of the four genotypes that are homozygous (SD12SD12SD12) for the dormancy-enhancing allele at SD12, in particular the digenic genotype (sd7-1sd7-1sd7-1SD12SD12SD12) (Figure 4C). Segregation distortion was detected for SD12 but not for SD7-1, with the Fsd12 estimate (0.35) lower than the Mendelian expectation (0.5) (Table 3). The results from the nongerminated subpopulation support that the SD12-controlled embryo dormancy played a major role in inhibiting germination and also suggest that SD7-1 may influence embryo dormancy as well in the digenic system, as implied by the observation in the germinated subpopulation of Ex. #1 (Figure 4D).
In addition, a numerical difference in genotypic frequency for the SD12 locus between male and female gametes, as estimated based on the nongerminated subpopulation, was observed (Fsd12’ < Fsd12’’ or FSD12’ > FSD12’’), which was opposite to the observation in the germinated subpopulation of Ex. #1 (Fsd12’ > Fsd12’’) (Table 3). The observations in the digenic system also confirmed that the associated effect of SD12 on male gamete preferential fertilization contributed only part to the genetic disequilibrium in the subpopulations segregating for the major seed dormancy locus.
Discussion
Applications of the endosperm genotype-based genetic approach
An endosperm genotype-based genetic approach was developed to determine whether previously mapped seed dormancy genes/QTL regulate germination through the embryo, endosperm or maternal tissues. This approach involves techniques: 1) partially after-ripening a segregating population of seeds to separate relatively less dormant from more dormant genotypes by germination testing, 2) sampling endospermic DNAs from individual germinated and nongerminated seeds, and 3) identifying all endosperm genotypes using regular PCR-based codominant markers. After DNA sampling, the newly germinated seeds can be selected to develop progeny lines. Thus, the techniques, similar to those reported for pregermination screening of genotypes (Chunwongse et al. 1993; Kang et al. 1998), can be also used for early selection of QTL alleles in breeding programs. Information collected using this approach includes: 1) endosperm and embryo genotypic frequencies for tested loci in germinated and nongerminated subpopulations (Table 3); 2) association between incubation times required for individual seeds to complete germination and allelic frequencies in the germinated subpopulation (the association was more informative before the seventh day, as shown in Figures 2, B and C); 3) genotypic differences for the degree of dormancy in a segregating population of seeds (Figure 4); 4) the dosage effect for a dormancy gene with an additive effect on germination in endosperms; and 5) genotypic frequencies for both male and female gametes that were involved in the double fertilization to form the seed sample. SDLs were frequently reported for mapping populations (Xu et al. 1997; Faris et al. 1998); the endosperm genotype-based genetic approach also can be used to characterize SDLs and artificial mutants for functions in gamete development and fertilization.
The genetic approach demonstrated in rice can be extended to the other cereal crops to characterize tissue-specific functions of mapped genes/QTL controlling seed dormancy and some other seed traits. Before DNA markers became prevalent for QTL mapping, Robertson (1952) combined morphological markers with an A-B (normal-supernumerary chromosome) translocation system to determine the tissue-specific function of the maize viviparous-5 mutant. The mutant distinguishes itself from the wild type by white endosperm and albino embryonic leaves and was concluded to regulate germination through the embryo, not the endosperm tissue. The A-B system was used in the reported research to help separate the four endosperm genotypes, because the morphological markers are dominant and have only two phenotypes for a locus. Nowadays, many codominant DNA markers can be selected to genotype endosperms in many species. We also tried a quantitative real-time PCR method to genotype endosperms with a dominant DNA marker in the SD1-2 region (data not shown) and found out that genotyping with regular PCR for a codominant marker was more reliable and cost-effective.
Compared with the endosperm genotype-based genetic approach, the embryo genotype-based genetic approach (Gu et al. 2008) provides less information, but it is relatively easy to conduct and can be used to distinguish an embryo from a maternal tissue effect on germination for a dormancy gene. If a seed dormancy gene functions in both maternal and offspring tissues, only the inhibitory effect on germination expressed in the offspring tissue(s) can be detected by the endosperm or embryo genotype-based genetic approach, because F2 seeds from single F1 plants do not segregate for the maternal tissue genotype (Table 2). For this case, a joint maternal-offspring model (e.g., Zhang et al. 2004) could be used to estimate effects of a seed dormancy gene on germination through different component tissues.
Genetic controls for types of seed dormancy
Natural variation for such a complex, adaptive trait as seed dormancy involves multiple genetic controls in different seed component tissues. This research provided evidence that SD12, SD1-2, and SD7-1 are involved in the genetic controls of seed dormancy through the embryo, endosperm, and maternal tissues, respectively. SD12 has the largest effect on seed dormancy in rice and its allele from SS18-2 delays germination (Gu et al. 2004). The major effect of SD12 on germination was contributed mainly by the additive component in embryos (Table 4) and contributed to the germination heterogeneity of seeds on the heterozygous (SD12sd12) plants. Therefore, marker-assisted selection for SD12 alleles can be conducted before (or after) germination using the endosperm (or embryo) genotype-based genetic approach, and phenotypic selection for an early or late germinated subpopulation from a segregating population of partially after-ripened seeds can be used to enrich the dormancy-reducing or -enhancing allele. SD12 also was associated with the preferential fertilization of male gametes on the heterozygous plants. The associated effect is defined as the preferential fertilization, not gamete fertility, because plants homozygous or heterozygous for the SD12 dormancy-enhancing allele had a normal seed setting rate (>95%) in this and previous research (Gu et al. 2004, 2008). Both the major effect on embryo dormancy and the association with gamete preferential fertilization must have restrained the distribution of the dormancy-enhancing allele during rice domestication. This also explains why the SD12 locus was not detected in the other mapping populations reported so far. Further research will elucidate if the association arises from a pleiotropic effect of SD12 or a gene linked to SD12.
SD1-2 could be the first naturally occurring gene that has been unambiguously associated with endosperm-imposed dormancy. Two analytic strategies were used to recognize SD1-2’s endospermic effect on germination: 1) a distinct difference in germination rate among the four endosperm genotypes in a segregating population of partially after-ripened seeds (Figure 3B), and 2) a significant difference in genotypic frequency between female and male gametes estimated based on the germinated subpopulation (Fsd1-2’’ > Fsd1-2’ in Table 3). It appeared that the inhibitory effect of SD1-2 on germination lasts only for several days after seed maturation or harvest. The previous research delimited SD1-2 to a short genomic region encompassing the gene Sd1 (or GA20ox-2) (Table 1; Ye et al. 2013). GA20ox-2 encodes GA20-OXIDASE2 catalyzing the second-to-last steps of the gibberellin (GA) biosynthesis and the loss-of-functional mutation (sd1) reduces the GA level in vegetative tissues and plant height (Ashikari et al. 2002; Monna et al. 2002; Spielmeyer et al. 2002). The dormancy-enhancing (SD1-2) and -reducing (sd1-2) alleles at the SD1-2 QTL couple with the GA functional and loss-of-functional alleles, respectively, and SD1-2’s effect on inhibiting germination could be compensated by GA application (Ye et al. 2013). The endosperm aleuronic cell layer is known to plays a central role in GA signaling to synthesize hydrolytic enzymes for cell wall weakening and food reserve mobilization during and post germination (Steber 2007). It is likely that GA20ox-2 is the QTL underlying gene. Research is being conducted to prove the hypothesis and to identify mechanisms for dormancy development and germination by SD1-2 expressed in endosperms.
SD7-1 was confirmed to control maternal tissue-imposed dormancy using the perfect isogenic system (ILSD7-1 is heterozygous only for a 2-kb SD7-1 intragenic segment, Table 1). The SD7-1 functional allele was isolated from the “red” weedy rice line SS18-2 and encodes a transcription factor that promotes the abscisic acid biosynthesis in early development seeds to induce primary dormancy and also activates the flavonoid biosynthesis pathway in the lower epidermal cell layer of the pericarp tissue to produce red pigments (Gu et al. 2011). Because an endospermic or embryonic effect on germination was not detected in the isogenic background (Figures 2A and Figure 3A), the SD7-1-regulated dormancy-inducing events must also occur in the maternal tissue. SD7-1 and SD12 control seed dormancy through different tissues, but their effects on delaying germination is cumulative, because it took a longer after-ripening period for seeds from the digenic system (F1_SD7-1SD12 plants) to reach similar germination rates than seeds from the monogenic systems (F1_SD7-1 and F1_SD12 plants). Questions to be addressed include how genes functioning in different tissues work in the same genetic system to lengthen the dormancy duration, and if a gene controlling coat-imposed dormancy is also involved in embryo dormancy, and vice versa.
Supplementary Material
Acknowledgments
Funding for this research was supported by grants from Natural Science Foundation (IOS 1021382 & 0641376) and United States Department of Agriculture-National Research Initiative (2008-35301-19058) and -National Institute of Food and Agriculture (2013-03572), and by South Dakota Agricultural Extension Station and Drought Center.
Footnotes
Supporting information is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.114.015362/-/DC1
Communicating editor: J. B. Holland
Literature Cited
- Alonso-Blanco C., Bentsink L., Hanhart C. J., Vries H. B. E., Koornneef M., 2003. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164: 711–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. A., Sorrells M. E., Tanksley S. D., 1993. RFLP analysis of genomic regions associated with resistance to pre-harvest sprouting in wheat. Crop Sci. 33: 453–459. [Google Scholar]
- Argyris J., Truco M. J., Ochoa O., Knapp S. J., Still D. W., et al. , 2005. Quantitative trait loci associated with seed and seedling traits in Lactuca. Theor. Appl. Genet. 111: 1365–1376. [DOI] [PubMed] [Google Scholar]
- Ashikari M., Sasaki A., Ueguchi-Tanaka M., Itoh H., Nishimura A., et al. , 2002. Loss-of-function of a rice gibberellin biosynthetic gene, GA20oxidase (GA20ox-2), led to the rice ‘green revolution’. Breed. Sci. 52: 143–150. [Google Scholar]
- Blaker K. M., Chaparro J. X., Beckman T. G., 2013. Identification of QTLs controlling seed dormancy in peach (Prunus persica). Tree Genet. Genomes 9: 659–668. [Google Scholar]
- Chunwongse J., Martin G. B., Tanksley S. D., 1993. Pre-germination genotypic screening using PCR amplification of half-seeds. Theor. Appl. Genet. 86: 694–698. [DOI] [PubMed] [Google Scholar]
- Faris J. D., Laddomada B., Gill B. S., 1998. Molecular mapping of segregation distortion loci in Aegilops tauschii. Genetics 149: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennimore S. A., Nyquist W. E., Shaner G. E., Doerge R. W., Foley M. E., 1999. A genetic model and molecular markers for wild oat (Avena fatua L.) seed dormancy. Theor. Appl. Genet. 99: 711–718. [DOI] [PubMed] [Google Scholar]
- Flintham J. E., 2000. Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci. Res. 10: 43–50. [Google Scholar]
- Foley M. E., 1992. Effect of soluble sugars and gibberellic acid in breaking dormancy of excised wild oat (Avena fatua) embryos. Weed Sci. 40: 208–214. [Google Scholar]
- Fujino K., Sekiguchi H., Matsuda Y., Sugimoto K., Ono K., et al. , 2008. Molecular identification of a major quantitative trait locus, qLTG3–1, controlling low-temperature germinability in rice. Proc. Natl. Acad. Sci. USA 105: 12623–12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S. D., Heesacker A. F., Freeman C. A., Argyris J., Bradford K., et al. , 2005. The self-incompatibility locus (S) and quantitative trait loci for self-pollination and seed dormancy in sunflower. Theor. Appl. Genet. 111: 619–629. [DOI] [PubMed] [Google Scholar]
- Gu X.-Y., Kianian S. F., Foley M. E., 2004. Multiple loci and epistases control genetic variation for seed dormancy in weedy rice (Oryza sativa). Genetics 166: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.-Y., Turnipseed E. B., Foley M. E., 2008. The qSD12 locus controls offspring tissue-imposed seed dormancy in rice. Genetics 179: 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.-Y., Liu T., Feng J., Suttle J. C., Gibbons J., 2010. The qSD12 underlying gene promotes abscisic acid accumulation in early developing seeds to induce primary dormancy in rice. Plant Mol. Biol. 73: 97–104. [DOI] [PubMed] [Google Scholar]
- Gu X.-Y., Foley M. E., Horvath D. P., Anderson J. V., Feng J., et al. , 2011. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 189: 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. W., Cho Y. G., Yoon U. H., Eun M. Y., 1998. A rapid DNA extraction method for RFLP and PCR analysis from a single dry seed. Plant Mol. Biol. Rep. 16: 1–9. [Google Scholar]
- Lee K. P., Piskurewicz U., Turecková V., Strnad M., Lopez-Molina L., 2010. A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc. Natl. Acad. Sci. USA 107: 19108–19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijavetzky D., Martinez M. C., Carrari F., Hopp H. E., 2000. QTL analysis and mapping of pre-harvest sprouting resistance in sorghum. Euphytica 112: 125–135. [Google Scholar]
- Lin S. Y., Sasaki T., Yano M., 1998a Mapping quantitative trait loci controlling seed dormancy and heading date in rice, Oryza sativa L., using backcross inbred lines. Theor. Appl. Genet. 96: 997–1003. [Google Scholar]
- Lyttle T. W., 1991. Segregation distorters. Annu. Rev. Genet. 25: 511–517. [DOI] [PubMed] [Google Scholar]
- Masojć P., Banek-Tabor A., Milczarski P., Twardowska M., 2007. QTLs for resistance to preharvest sprouting in rye (Secale cereale L.). J. Appl. Genet. 48: 211–217. [DOI] [PubMed] [Google Scholar]
- Mo H., 1988. Genetic expression for endosperm traits, pp. 478–487 in Proceedings of the Second International Conference on Quantitative Genetics, Sinauer Associates, Sunderland, MA. [Google Scholar]
- Monna L., Kitazawa N., Yoshino R., Suzuki J., Masuda H., et al. , 2002. Positional cloning of rice semidwarfing gene, sd-1: rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 9: 11–17. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Moffatt J. M., Sears R. G., Paulsen G. M., 1989. Seed dormancy and responses of caryopses, embryos, and calli to abscisic acid in wheat. Plant Physiol. 90: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V. G., Ponnaiya B. W. X., Raman V. S., 1965. Studies on seed dormancy in certain short-duration rice varieties. India J. Agric. Sci. 35: 234–246. [Google Scholar]
- Nakamura S., Abe F., Kawahigashi H., Nakazono K., Tagiri A., et al. , 2011. A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell 23: 3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll J. S., Dyck P. L., Czarnecki E., 1982. Expression of RL4137 type of dormancy in F1 seeds of reciprocal crosses in common wheat. Can. J. Plant Sci. 2: 153–187. [Google Scholar]
- Robertson D. S., 1952. The genotype of the endosperm and embryo as it influences vivipary in maize. Genetics 38: 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute , 2011. Base SAS 9.3 Procedures Guide, SAS Institute Inc., Cary, NC. [Google Scholar]
- Schatzki J., Schoo B., Ecke W., Herrfurth C., Feussner I., et al. , 2013. Mapping of QTL for seed dormancy in a winter oilseed rape doubled haploid population. Theor. Appl. Genet. 126: 2405–2415. [DOI] [PubMed] [Google Scholar]
- Spielmeyer W., Ellis M. H., Chandler P. M., 2002. Semidwarf (sd-1), ‘‘green revolution’’ rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 99: 9043–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber C. M., 2007. De-repression of seed germination by GA signaling, pp. 248–263 in Seed Development, Dormancy and Germination, edited by Bradford K., Nonogaki H. Blackwell Publishing, London, UK. [Google Scholar]
- Sugimoto K., Takeuchi Y., Ebana K., Miyao A., Hirochika H., et al. , 2010. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 107: 5792–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., 1963. Studies on the dormancy of wild rice seeds. Part 2. Role of seed coat, embryo and endosperm in dormant seeds, pp. 75–85 in The 241st Report of the Institute for Agricultural Research, Tohoku University, Sendai, Japan. [Google Scholar]
- Ullrich S. E., Hayes P. M., Dyer W. E., Blake T. K., Clancy J. A., 1993. Quantitative trait locus analysis of seed dormancy in ’Steptoe’ barley, pp. 136–145 in Pre-harvest Sprouting in Cereals, edited by Walker-Simmons M. K., Ried J. L. American Association of Cereal Chemists, St. Paul, MN. [Google Scholar]
- Wang M., Heimovaara-Dijkstra S., Duijn B. V., 1995. Modulation of germination of embryos isolated from dormant and nondormant barley grains by manipulation of endogenous abscisic acid. Planta 195: 586–592. [Google Scholar]
- Xu Y., Zhu L., Xiao J., Huang N., McCouch S. R., 1997. Chromosomal regions associated with segregation distortion of molecular markers in F2, backcross, doubled haploid, and recombinant inbred populations in rice (Oryza sativa L.). Mol. Gen. Genet. 253: 535–545. [DOI] [PubMed] [Google Scholar]
- Ye H., Beighley D. H., Feng J., Gu X.-Y., 2013. Genetic and physiological characterization of two clusters of quantitative trait loci associated with seed dormancy and plant height in rice. G3 (Bethesda) 3: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang M. C. K., Gallo-Meagher M., Wang X. L., Larkins B. A., et al. , 2004. A model for estimating joint maternal-offspring effects on seed development in autogamous plants. Physiol. Genomics 19: 262–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.