Abstract
Background and objective
Bronchoscopic lung volume reduction coil (LVR-coil) treatment has been shown to be safe and clinically effective in patients with severe emphysema in the short term; however, long-term safety and effectiveness has not been evaluated. The aim of this study was to investigate the long-term safety and effectiveness of LVR-coil treatment in patients with severe emphysema.
Methods
Thirty-eight patients with severe emphysema (median age is 59 years, forced expiratory volume in 1 s is 27% predicted) who were treated in LVR-coil clinical trials were invited for a voluntary annual visit. Safety was evaluated by chest X-ray and recording of adverse events and by efficacy by pulmonary function testing, 6-min walk distance (6MWD) and questionnaires.
Results
Thirty-five patients visited the hospital 1 year, 27 patients 2 years and 22 patients 3 years following coil placement. No coil migrations were observed on X-rays. At 1-year follow-up, all clinical outcomes significantly improved compared with baseline. At 2 years, residual volume % pred, modified Medical Research Council (mMRC) and the SGRQ score were still significantly improved. At 3 years, a significant improvement in mMRC score remained, with 40% of the patients reaching the 6MWD minimal important difference, and 59% for the St George's Respiratory Questionnaire (SGRQ) minimal important difference.
Conclusions
Follow-up of the patients treated with LVR-coils in our pilot studies showed that the coil treatment is safe with no late pneumothoraces, coil migrations or unexpected adverse events. Clinical benefit gradually declines over time; at 3 years post-treatment, around 50% of the patients maintained improvement in 6MWD, SGRQ and mMRC.
Keywords: bronchoscopy and interventional technique, clinical respiratory medicine, coil, emphysema, long-term follow-up
Summary at a Glance
This is the first study to investigate the safety and efficacy of the lung volume reduction coil treatment in the long term. At 3-year follow-up, this treatment showed no long-term unexpected adverse and device-related events, with clinical benefit gradually declining over time.
Introduction
Bronchoscopic lung volume reduction (BLVR) is a new minimally invasive treatment option for patients with severe emphysema.1 BLVR with one-way endobronchial valves, a ‘blocking’ device, is an efficacious method in a selected group of patients with absence of collateral ventilation (CV).2,3 For the majority of patients with severe emphysema, a BLVR treatment that works independently of CV, a ‘non-blocking’ device, must be used. One of the currently investigated non-blocking devices is the lung volume reduction (LVR) coil (RePneu, PneumRx, Inc., Mountain View, CA, USA). This nitinol coil is bronchoscopically delivered in both lungs in either upper or lower lobe heterogeneous emphysema or homogeneous emphysema,4,5 thereby compressing diseased parenchyma and radially suspending airways after placement in the lung.
To date, five studies investigating LVR-coil treatment have been published.4–8 Four non-randomized studies (n = 10, 11, 16 and 60 patients)4,6–8 and one randomized study (24 controls and 23 treated patients)5 showed that the procedure is feasible, safe and well tolerated. Significant improvements in quality of life, exercise capacity and pulmonary function were observed.4,5,7,8 Most studies had relatively short follow-up times: 3 months,5,6 6 months4,8 and one study up to 12 months after treatment.7 To our knowledge, no study investigated a longer follow-up time after LVR-coil treatment. This longer follow-up time is needed to document both safety and effectiveness of the procedure. In our hospital, we performed two pilot studies investigating bronchoscopic LVR-coil therapy, with treatments in 2009 and 2010.
The aim of this study is to investigate the safety and effectiveness of LVR treatment with coils 1, 2 and 3 years post-treatment in patients with severe emphysema who participated in pilot trials.
Methods
Study population
Between April 2009 and November 2010, 38 patients were treated with the LVR-coil at our institution, in one of two pilot studies (NCT012209084 and NCT013288997). The inclusion and exclusion criteria for both can be found in Table S1. Both studies were approved by the University Medical Center Groningen Medical Ethics Committee, and all participants signed informed consents.
LVR-coil treatment
The LVR-coil procedure has been described before.4,6 In brief, the coils (RePneu, PneumRx Inc.) are made of shape-memory nitinol wire, range in length from 70 to 200 mm to accommodate airways of different sizes and are designed to compress the lung parenchyma. The coils were bronchoscopically placed under general anaesthesia in two sequential procedures using fluoroscopy.
Study design
The follow-up period of both studies were 64 and 12 months7 after the second treatment. After completing and exiting the study, patients were invited for a voluntary annual follow-up visit. Patients performed pulmonary function measurements, 6-min walk test (6MWT) and chest X-ray and completed questionnaires. Patients also had a consultation with a physician who reported the patient's health status during the past year.
Measurements
Spirometry, bodyplethysmography and the 6MWT were performed using European Respiratory Society/American Thoracic Society (ATS) guidelines.9–11 Health-related quality of life was measured by the St George's Respiratory Questionnaire (SGRQ)12 and dyspnoea severity by the modified Medical Research Council (mMRC) dyspnoea scale.13
Safety was measured by recording all adverse events reported by the patients during the yearly follow-up visits. The first X-ray after the treatment and the last performed X-ray at final follow-up visit for all participants were assessed for presence of coil migration (defined as displacement of the original post-treatment coil position in the segment), atelectasis and consolidation of tissue around the coils.
Pre-treatment decline in forced expiratory volume in 1 s
All available spirometry results of the pre-treatment years were collected from the patient's own hospital, serving as a reference of the expected decline in lung function of our patients.
Lung transplantation
Two patients underwent a lung transplantation: one patient at 1 year and the second patient at 4 years post-treatment. Both patients gave permission for histopathological examination of the explant. The lung tissue was processed according to routine clinical guidelines for confirmation of disease diagnosis and assessment of any potential concurrent disease. Haematoxylin and eosin stains were made on lung sections after careful removal of the nitinol coils, and representative sections were photographed and unedited used for presentation in this study.
Statistical analysis
Due to non-normally distributed data, Wilcoxon signed rank tests were performed to compare the clinical characteristics at 1-, 2- and 3-year follow-up against baseline and to compare if baseline characteristics differed between responders and non-responders at 3-year follow-up. For the responder analyses, we counted the number of patients who reached the earlier established minimal important difference (MID) for forced expiratory volume in 1 s (FEV1) (100 mL14 and 10%), RV (400 mL15), 6-min walk distance (6MWD) (26 m16), and the SGRQ (4 points17). The annual change in post-bronchodilator FEV1 before the treatment was derived from the slope of the regression line for each patient's individual FEV1 values measured at their own hospital. We only calculated the annual change in FEV1 of patients when at least three FEV1 values were available. Paired sample t-tests were performed to compare the difference in the decline in FEV1 before and after the treatment. P-values < 0.05 were considered statistically significant. IBM-SPSS Statistics (v20) was used for statistical analysis (IBM, Armonk, NY, USA).
Results
Patients
The baseline characteristics of the 38 patients are shown in Table 1. One year after the treatment, 35 patients performed follow-up measurements, at 2 years 27 patients and at 3 years 22 patients (Fig. 1).
Table 1.
Patient characteristics at baseline (n = 38)
| Female, n (%) | 28 (74) |
| Age, years | 59.2 ± 7.7 |
| BMI, kg/m2 | 24.9 (18.6–35.4) |
| Diagnosis emphysema, years | 8.9 ± 3.5 |
| Pack-years, years | 34.7 ± 11.2 |
| Heterogeneous emphysema, n (%) | 35 (92) |
| FEV1, % predicted | 27 (16–42) |
| GOLD stage, n (%) | |
| III | 13 (34) |
| IV | 25 (66) |
| FVC, % predicted | 81.5 ± 15.3 |
| RV, % predicted | 228 (155–341) |
| RV/TLC, ratio | 0.61 (0.50–0.74) |
| mMRC score, n (%) | 3.0 (2.0–4.0) |
| 6MWD, meter | 326 ± 94 |
| SGRQ total score, points | 63.2 (36.9–83.0) |
Data are presented as number (%), mean ± standard deviation or median (range).
6MWD, 6-min walk distance; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council; RV, residual volume; SGRQ, St George's Respiratory Questionnaire; TLC, total lung capacity.
Figure 1.
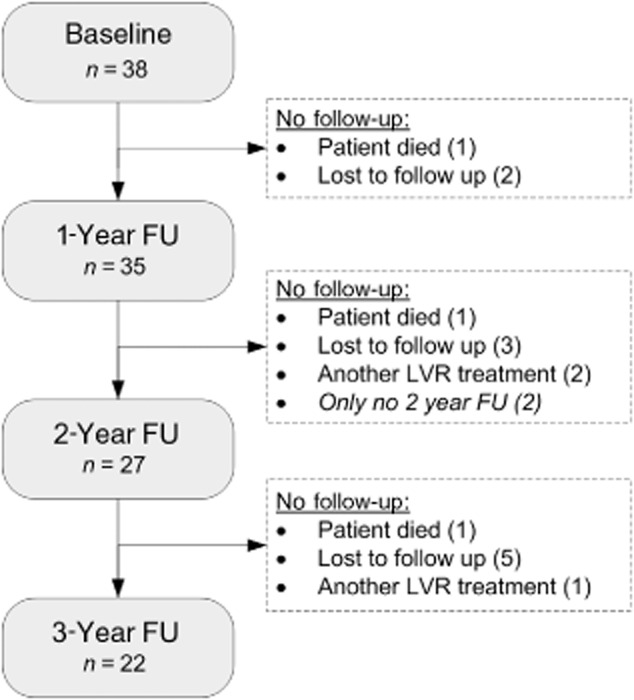
Flowchart of study participants. FU, follow-up; LVR, lung volume reduction.
Safety
The adverse events are shown in Table 2. Six patients (16%) died during the 3-year follow-up independent of the treatment. The causes of death are reported in Table 2. Two patients had a pneumothorax directly after the coil procedure; however, no long-term pneumothoraces occurred. Of the patients, 74% reported a very mild haemoptysis just post-procedure; only one patient reported spontaneous settling of more severe haemoptysis at 3-year follow-up. On the follow-up chest X-rays, we observed no coil migrations, a segmental atelectasis was visible in 3 patients (8%) and consolidation of tissue around some of the coils in 11 patients (29%) (see Fig. 2 for the first X-ray post-procedure and the follow-up X-ray at 3-year follow-up of two example patients).
Table 2.
Number of reported adverse events
| Baseline to 1-year FU (n = 35) | 1-Year to 2-year FU (n = 27) | 2-Year to 3-year FU (n = 22) | |
|---|---|---|---|
| Death (%)† | 1 (3) | 3 (8) | 2 (6) |
| Pneumothorax, yes (%) | 2 (6) | 0 (0) | 0 (0) |
| Pneumonia, yes (%) | 16 (46) | 2 (7) | 1 (5) |
| Hospitalization due to COPD exacerbation, yes (%) | 18 (51) | 10 (37) | 8 (36) |
| Haemoptysis, yes (%) | 0 (0) | 0 (0) | 1 (5) |
Percentages of patients who died were calculated based on the total number of patients at baseline.
Data are presented as number of patients (%). Causes of death (n = 6, time post-treatment): 1: 20 months (right upper lobe only); pneumonia of the left lung with pseudomonas sepsis. 2: 10 months (right upper lobe only); end-stage COPD, complicated by a osteoporotic Th6 fracture causing immobilization and severe pain. 3: 16 months (bilateral upper lobe); end-stage COPD with cor pulmonale. 4: 16 months (bilateral upper lobe); sudden cardiac death not further specified. 5: 38 months (bilateral upper lobe); myocardial infarction. 6: 35 months (bilateral upper lobe); end-stage COPD.
COPD, chronic obstructive pulmonary disease; FU, follow-up.
Figure 2.
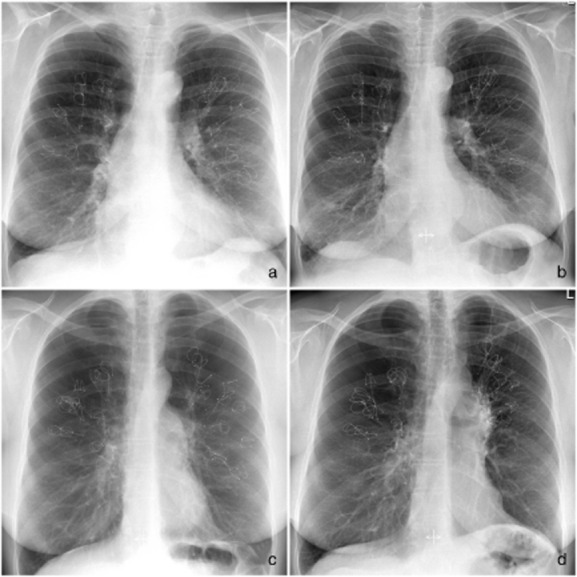
The first X-ray after the procedure and last available follow-up X-ray of two example patients. (a) Directly after the procedure in patient 1. (b) Three years after the procedure in patient 1 without any changes. (c) Directly after the procedure in patient 2. (d) Three years after the procedure in patient 2, showing some ‘crowding’ of the coils in the left-upper lobe resulting in volume reduction and a better left hemi-diaphragm position.
Effectiveness
At 1-year follow-up, forced vital capacity, RV, RV/total lung capacity, mMRC, 6MWD and SGRQ total score were all significantly improved compared with baseline. At 2-year follow-up, RV, mMRC and the SGRQ total score were significantly improved when compared with baseline. At 3-year follow-up, only the mMRC was significantly improved compared with baseline. The other clinical characteristics were not significantly changed at 3 years compared with baseline (Table 3).
Table 3.
Change in clinical characteristics at 1-, 2- and 3-year follow-up
| Δ 1 Year FU to baseline |
P-value | Δ 2 Year FU to baseline |
P-value | Δ 3 Year FU to baseline |
P-value | |
|---|---|---|---|---|---|---|
| n = 35 | n = 27 | n = 22 | ||||
| FEV1, L | 0.2 (−0.2 to 0.45) | 0.171 | −0.04 (−0.26 to 0.36) | 0.809 | −0.05 (−0.39 to 0.39) | 0.664 |
| FEV1, % predicted | 1 (−6 to 20) | 0.080 | −1.0 (−9.0 to 17.0) | 0.949 | 0 (−14 to 19) | 0.747 |
| FVC, L | 0.04 (−0.39 to 1.13) | 0.060 | −0.02 (−0.85 to 1.11) | 0.597 | 0.04 (−0.56 to 0.91) | 0.723 |
| FVC, % predicted | 3 (−12 to 44) | 0.014 | 1.0 (−25 to 44) | 0.741 | 6 (−18 to 38) | 0.169 |
| RV, L | −0.32 (−1.88 to 0.68) | <0.001 | −0.14 (−1.57 to 0.92) | 0.093 | 0.07 (−1.67 to 1.41) | 0.629 |
| RV, % predicted | −21.0 (−91.0 to 32.0) | <0.001 | −10.0 (−83 to 43) | 0.012 | −2 (−89 to 57) | 0.509 |
| RV/TLC, ratio | −3.55 (−21.3 to 5.7) | <0.001 | −0.23 (−18.6 to 10.3) | 0.428 | 1.49 (−19.0 to 12.5) | 0.664 |
| mMRC, score | 0 (−3 to 2) | 0.007 | 0.0 (−3.0 to 1.0) | 0.007 | −0.5 (−3 to 1) | 0.039 |
| 6MWD, m | 31.0 (−110 to 185) | 0.010 | −12.0 (−140 to 238) | 0.696 | −31.5 (−120 to 177) | 0.970 |
| SGRQ, total score | −4.2 (−44.0 to 13.1) | 0.005 | −8.0 (−39.9 to 20.4) | 0.032 | −7.2 (−29.6 to 21.2) | 0.101 |
Data are presented as median (range) (change between follow-up and baseline) and P-values. Baseline and follow-up measurements were compared with Wilxocon signed-rank test (significant P-values are shown in bold).
6MWD, 6-min walk distance; FEV1, forced expiratory volume in 1 s; FU, follow-up; FVC, forced vital capacity; mMRC, modified Medical Research Council; RV, residual volume; SGRQ, St George's Respiratory Questionnaire; TLC, total lung capacity.
The number of patients reaching the MID for FEV1 ranged from 20–30% (absolute change) to 30–40% (relative change) throughout the 1- to 3-year follow-up. The number of patients reaching the MID for RV decreased during the 1- to 3-year follow-up, from 51% to 19%. The number of patients reaching the MID for 6MWD decreased during the 1- to 3-year follow-up from 57% to 40%. The number of patients reaching the MID for SGRQ ranged from 50% to 60% throughout the 1- to 3-year follow-up (Table 4). No differences were found in baseline characteristics between patients who reached the MID for SGRQ or 6MWD at 3-year follow-up compared to patients who did not reach the MID.
Table 4.
Responder analysis
| 6-Month FU (n = 35) | 1-Year FU (n = 35) | 2-Year FU (n = 27) | 3-Year FU (n = 22) | |
|---|---|---|---|---|
| Δ FEV1 ≥ 100 mL (%) | 11 (31) | 8 (23) | 5 (19) | 7 (33)‡ |
| Δ FEV1 ≥ 10% (%) | 17 (49) | 11 (31) | 9 (33) | 8 (38)‡ |
| Δ RV ≤ 400 mL (%) | 18 (51) | 14 (40) | 8 (30) | 4 (19)‡ |
| Δ 6MWD ≥ 26 m (%) | 20 (57) | 20 (57) | 7 (27)† | 8 (40)§ |
| Δ SGRQ ≤ 4 points (%) | 22 (63) | 18 (51) | 17 (63) | 13 (59) |
n = 26;
n = 21;
n = 20.
Data are presented as n (%).
Δ, delta compared with baseline; 6MWD, 6-min walk distance; FEV1, forced expiratory volume in 1 s; FU, follow-up; FVC, forced vital capacity; MID, minimal important difference; mMRC, modified Medical Research Council; RV, residual volume; SGRQ, St George's Respiratory Questionnaire; TLC, total lung capacity.
Pre-treatment decline in FEV1
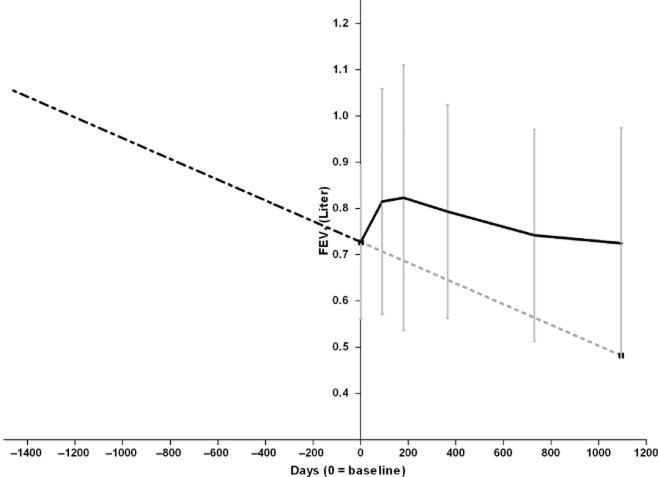
At least three previously performed FEV1 measurements were available for 30/38 patients (79%). The median number of available measurements was 9 (range 3–23) and the median number of days for the first available measurement before treatment was 1989 days (range: 292–4376). The mean decline in FEV1 before the LVR-coil treatment was −0.082 L/year (standard deviation: 0.073). This was significantly different compared with the mean decline in FEV1 during study participation (mean decline: −0.036 L/year, P = 0.018). The decline in FEV1 after more than 6 months of follow-up did not significantly differ compared with the decline before the treatment (mean decline: −0.060 L/year, P = 0.45) (Fig. 3).
Figure 3.
Decline in forced expiratory volume in 1 s (FEV1) before and after the LVR-coil treatment. Baseline and post-treatment FEV1 shown as mean (±standard deviation). ‘ ’: before treatment; ‘
’: before treatment; ‘ ’: trend line; ‘
’: trend line; ‘ ’: during study participation.
’: during study participation.
Lung transplant explant evaluation
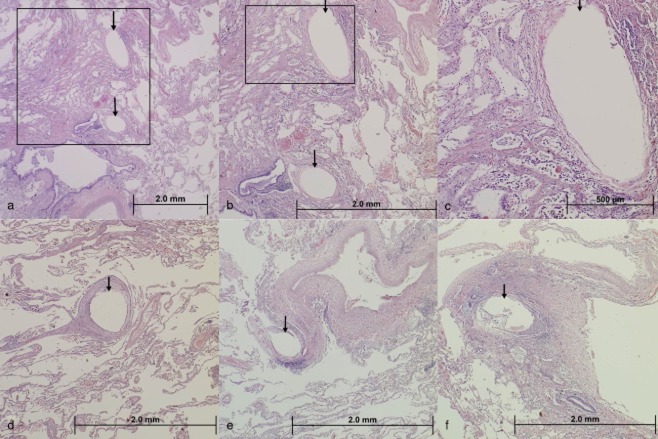
On gross macroscopic evaluation of the lung explants, the coils could be identified in the main segmental and sub-segmental airways. No vascular disruptions were noticed, nor were there any abscess formations in the coiled regions. Histopathological examination revealed in both patients, besides presence of emphysematous tissue, a thin, compressed capsule of tissue around the imprints of the airways with a slight inflammatory reaction. It was unclear whether these changes represent pre-existing pathology in these patients or if this is associated with device placement. In the 1-year specimen, the presence of interstitial fibrosis of alveolar septa with the device ‘capsule’ and the surrounding alveolar parenchyma was visible. In the 4-year specimen, the device imprint in the airways was surrounded by a well-organized fibrous capsule comprised of compressed, concentric rings of stroma, and this was also found in the alveolar parenchyma, where the device imprint was in an area of more dense fibrous tissue. No abundant inflammatory reaction or infection was found in either explant (see Fig. 4a–f).
Figure 4.
Histology of transplanted lungs of two patients (photomicrograph, haematoxylin and eosin stain). (a) Low power magnification of lung tissue demonstrating two device imprints (arrows) in the alveolar parenchyma. (b) Higher magnification of the boxed area in image (a) demonstrating the two device imprints in tissue. At this magnification, it is evident that there is a thin, compressed capsule of tissue around the imprints with no other significant inflammatory reaction present. This image also demonstrates the presence of interstitial fibrosis of alveolar septa along the left hand side of the image. (c) Higher magnification of the boxed area in image (b) demonstrating a closer view of the device capsule and the surrounding alveolar parenchyma. (d) Low power magnification of a single device imprint in the alveolar parenchyma (arrow). The imprint is surrounded by a well-organized fibrous capsule comprised of compressed, concentric rings of stroma. Pre-existing emphysema (enlarged alveolar spaces) is also evident in this image. (e) Low power magnification of a single device imprint (arrow) in the alveolar parenchyma adjacent to a pulmonary vein. (f) Low power magnification of a single device imprint in an area of more dense fibrous tissue. The device capsule contains a mild degree of inflammation. (a–c) Patient 1 year after LVR-coil treatment; (d–f) patient 4 years after LVR-coil treatment.
Discussion
This was the first study that investigated the long-term safety and effectiveness of bronchoscopic LVR treatment with nitinol coils. In this trial, we followed our first pilot study patients over the years and showed that the treatment is safe in the long term. After 1 year, the treatment was found to be clinically effective compared with baseline, with a median gradual decline of the clinical benefits over time, with 3-year follow-up approaching similar parameters to the pre-treatment baseline for the overall group and with a responder rate of 59% of the patients reaching MID for SGRQ and 40% for 6MWD at 3 years.
In the 3-year follow-up of our pilot studies, patients showed that the LVR-coil treatment was safe in the long term. We witnessed no late pneumothoraces, no coil migrations, no major haemoptysis, no major infectious complications or unexpected adverse device events and no treatment-related deaths. The 3-year survival in our group (84%) is in line with survival reports in the literature for comparable patient populations. Lange et al. reported a 74.2% 3-year survival,18 and a 55–65% 3-year survival is reported when using Collaborative Cohorts to Assess Multicomponent Indices of COPD in Spain, Global Initiative for Chronic Obstructive Lung Disease, or ATS/Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease severity criteria.19 Evaluation of post-lung transplant-explanted lung tissue showed that the proximal and mid portions of the coils can still be found in the segmental and sub-segmental airways, encapsulated by some fibrotic/organizing reaction, with occasionally the most distal part of the coils being encapsulated in the surrounding lung tissue, but with no signs of serious inflammatory or infectious reactions. These findings indicate that there is tendency of the airways and lung tissue to slowly organize around the coils, which might be due to local tissue stress, compression and micromovements of the coils.
The treatment was beneficial for a large group of patients after 1 year, with overall mean clinical parameters returning to baseline values at 3 years. Unfortunately, we did not have a control group in which we could investigate the natural decline of clinical parameters. However, the National Emphysema Treatment Trial (NETT) study20 that investigated lung volume reduction surgery (LVRS) in severe emphysema patients with a median follow-up of 4.3 years reported that clinical parameters like SGRQ declined in both the treatment and control group.20 To estimate the natural rate of functional decline in our patients, we collected all available pre-treatment spirometries. We found that the rate of decline did not change after the LVR-coil treatment but that treatment increased FEV1 to the extent that return to pre-treatment baseline levels occurred only after approximately 3 years (Fig. 3). That the rate of decline did not change is unsurprising; two other studies investigating LVRS also showed that the rate of decline after surgery was comparable with the rate of decline before surgery.21,22
We believe it is as important to evaluate clinical significance as it is with statistical significance of outcomes from treatment. Therefore, we also investigated whether patients reached the MID for FEV1, RV, 6MWD and SGRQ at each time point. However, a confounding factor is that most MIDs were calculated for short-term changes, ranging from 115 to 616 months post-intervention. A long-term MID (for example 3 years) could be lower than an MID for the short term. Therefore, the MIDs used in our analyses could underestimate the number of meaningful responders at 3 years. Unfortunately, this is not known and would be interesting to investigate. We did not find any predictive factors to identify responders at 3-year follow-up. However, our sample size was too small to be able to evaluate this in detail. Current ongoing large randomized controlled trials (NCT01608490 and NCT01822795) will possibly give more insight in the best responder profile for this treatment.
Long-term follow-up after BLVR with coils has not been investigated before. A few other studies investigating other LVR techniques included at least 12 months follow-up. The NETT study20 found that 20% of the patients improved more than 8 points on the SGRQ total score 3 years after LVRS (patients who died or were lost to follow-up were considered not improved). When we apply the same rules for improvement, 31% of our patients (n = 11) improved more than 8 points after 3 year. As in our study, the NETT study also found a larger improvement in the quality of life in the long term than in exercise capacity. Another study investigated the effect of lung sealant therapy for emphysema in 16 patients 2 years after the initial treatment.23 They found a much higher number of patients who reached the MID for FEV1 2 years after the treatment, which is 50% compared with 19% in our population. Not much literature to date has been published on longer-term follow-up data for bronchoscopic LVR devices. Three small cohort studies investigated long-term follow-up of endobronchial valve treatment. Venuta et al.24 showed promising results after 3 and 5 years follow-up. Unfortunately, patient loss to follow-up was not taken into account, and paired statistical analyses were not used, making the result difficult to interpret. A retrospective study by Kotecha et al.25 showed that 6 out of 16 patients (38%) had sustained long-term improvements in FEV1 (Δ > 0), which is comparable with our study (at 2-year follow-up:11/27 (31%)). Furthermore, Hopkinson et al.26 showed that the occurrence of atelectasis following endobronchial valve treatment was associated with prolonged survival at 6 years follow-up.
The major disadvantage of our study is the non-controlled design and possible selection bias of patients who volunteered for yearly follow-up visits after participating in one of our pilot studies. Although a large number of patients did visit our hospital yearly, the results at 2- and 3-year follow-up should be interpreted with caution as patients with worse response could be presumed less likely to return for follow-up. It would be useful to investigate the long-term efficacy and safety of the LVR-coil treatment in a randomized controlled intervention study with long-term follow-up. Currently, a large (n = 315) randomized controlled trial with 5-year follow-up is enrolling patients and will give additional insight into the long-term effectiveness and safety of coil treatment (Lung Volume Reduction Coil Treatment in Patients With Emphysema Study:NCT01608490).
In conclusion, follow-up of our very first pilot patients showed that LVR-coil treatment is safe in the long term, with no late pneumothoraces, coil migrations or unexpected adverse events. Clinical benefit gradually declines over time; at 3 years post-treatment, around 50% of the patients maintained improvement in 6MWD, SGRQ and mMRC.
Acknowledgments
We would like to acknowledge Dr T. Spangler (VDx Veterinary Diagnostics, Davis, USA) for the histopathologically evaluation of the explanted lung tissue. Furthermore, we would like to thank the pulmonary function technicians of the University Medical Center Groningen for their dedicated pulmonary function testing.
Glossary
- 6MWD
6-min walk distance
- 6MWT
6-min walk test
- ATS
American Thoracic Society
- BLVR
bronchoscopic lung volume reduction
- CV
collateral ventilation
- FEV1
forced expiratory volume in 1 s
- LVR
lung volume reduction
- LVRS
lung volume reduction surgery
- MID
minimal important difference
- mMRC
modified Medical Research Council
- NETT
National Emphysema Treatment Trial
- SGRQ
St George's Respiratory Questionnaire
Supplementary Information
Additional Supplementary Information can be accessed via the html version of this article at the publisher's web-site:
In- and exclusion criteria.
References
- 1.Mineshita M, Slebos DJ. Bronchoscopic interventions for chronic obstructive pulmonary disease. Respirology. 2014;19:1126–1137. doi: 10.1111/resp.12362. [DOI] [PubMed] [Google Scholar]
- 2.Herth FJ, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, Schmidt B, Slebos DJ. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur. Respir. J. 2013;41:302–308. doi: 10.1183/09031936.00015312. [DOI] [PubMed] [Google Scholar]
- 3.Shah PL, Herth FJ. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax. 2014;69:280–286. doi: 10.1136/thoraxjnl-2013-203743. [DOI] [PubMed] [Google Scholar]
- 4.Slebos DJ, Klooster K, Ernst A, Herth FJ, Kerstjens HA. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest. 2012;142:574–582. doi: 10.1378/chest.11-0730. [DOI] [PubMed] [Google Scholar]
- 5.Shah P, Zoumot Z, Singh S, Bicknell SR, Ross ET, Quiring J, Hopkinson NS, Kemp SV RESET Trial Study Group. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir. Med. 2013;1:233–240. doi: 10.1016/S2213-2600(13)70047-X. [DOI] [PubMed] [Google Scholar]
- 6.Herth FJ, Eberhard R, Gompelmann D, Slebos DJ, Ernst A. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther. Adv. Respir. Dis. 2010;4:225–231. doi: 10.1177/1753465810368553. [DOI] [PubMed] [Google Scholar]
- 7.Deslee G, Klooster K, Hetzel M, Stanzel F, Kessler R, Marquette CH, Witt C, Blaas S, Gesierich W, Herth FJ, et al. Lung volume reduction coil treatment for patients with severe emphysema, a European multicenter feasibility trial. Thorax. 69:980–986. doi: 10.1136/thoraxjnl-2014-205221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klooster K, ten Hacken N, Franz I, Kerstjens HA, van Rixvoort EM, Slebos DJ. Lung volume reduction coil treatment in COPD patients with homogeneous emphysema: a prospective feasibility trial. Respiration. 2014;88:116–125. doi: 10.1159/000362522. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 11.Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003;167:1287. doi: 10.1164/ajrccm.167.9.950. [DOI] [PubMed] [Google Scholar]
- 12.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir. Med. 1991;85(Suppl. B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33–7. [DOI] [PubMed] [Google Scholar]
- 13.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 15.Hartman JE, Ten Hacken NH, Klooster K, Boezen HM, de Greef MH, Slebos DJ. The minimal important difference for residual volume in patients with severe emphysema. Eur. Respir. J. 40:1137–1141. doi: 10.1183/09031936.00219111. [DOI] [PubMed] [Google Scholar]
- 16.Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F NETT Research Group. The minimal important difference of exercise tests in severe COPD. Eur. Respir. J. 2012;37:784–790. doi: 10.1183/09031936.00063810. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones PW. St: George's Respiratory Questionnaire. MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 18.Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, Nordestgaard BG. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am. J. Respir. Crit. Care Med. 2012;186:975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 19.Almagro P, Martinez-Camblor P, Soriano JB, Marin JM, Alfageme I, Casanova C, Esteban C, Soler-Cataluna JJ, de Torres JP, Celli BR, et al. Finding the best thresholds of FEV1 and dyspnea to predict 5-year survival in COPD patients: the COCOMICS study. PLoS ONE. 2014;9:e89866. doi: 10.1371/journal.pone.0089866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann. Thorac. Surg. 2006;82:431–443. doi: 10.1016/j.athoracsur.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 21.Gelb AF, McKenna RJ, Jr, Brenner M, Epstein JD, Zamel N. Lung function 5 yr after lung volume reduction surgery for emphysema. Am. J. Respir. Crit. Care Med. 2001;163:1562–1566. doi: 10.1164/ajrccm.163.7.2009048. [DOI] [PubMed] [Google Scholar]
- 22.Travaline JM, Gaughan JP, Furukawa S, Criner GJ. Effect of bilateral lung volume reduction surgery on FEV1 decline in severe emphysema. COPD. 2005;2:203–208. [PubMed] [Google Scholar]
- 23.Kramer MR, Refaely Y, Maimon N, Rosengarten D, Fruchter O. Two-year follow-up in patients treated with emphysematous lung sealant for advanced emphysema. Chest. 2013;144:1677–1680. doi: 10.1378/chest.13-0446. [DOI] [PubMed] [Google Scholar]
- 24.Venuta F, Anile M, Diso D, Carillo C, De Giacomo T, Andrilli D, Fraioli F, Rendina EA, Coloni GF. Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur. Respir. J. 2012;39:1084–1089. doi: 10.1183/09031936.00071311. [DOI] [PubMed] [Google Scholar]
- 25.Kotecha S, Westall GP, Holsworth L, Pham A, Williams TJ, Snell GI. Long-term outcomes from bronchoscopic lung volume reduction using a bronchial prosthesis. Respirology. 2011;16:167–173. doi: 10.1111/j.1440-1843.2010.01896.x. [DOI] [PubMed] [Google Scholar]
- 26.Hopkinson NS, Kemp SV, Toma TP, Hansell DM, Geddes DM, Shah PL, Polkey MI. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur. Respir. J. 2011;37:1346–1351. doi: 10.1183/09031936.00100110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In- and exclusion criteria.