Abstract
Recurrence of papillary thyroid cancer (PTC) after optimized surgery requires a full understanding of the disease, especially as it has changed in the last 15 years, what comprises optimized surgery, and the different types and implications of disease relapse that can be encountered. PTC has evolved to tumors that are much smaller than previously seen, largely due to various high quality imaging studies obtained for different reasons, but serendipitously identifying thyroid nodules that prove to be papillary thyroid microcarcinomas (PTMC). With rare exception, these cancers are cured by conservative surgery without additional therapy, and seldom result in recurrent disease. PTC is highly curable in 85% of cases because of its rather innocent biologic behavior. Therefore, the shift in emphasis from disease survival to recurrence is appropriate. As a result of three technologic advances—high-resolution ultrasound (US), recombinant TSH, and highly sensitive thyroglobulin (Tg)—disease relapse can be discovered when it is subclinical. Endocrinologists who largely control administration of radioactive iodine have used it to ablate barely detectable or even biochemically apparent disease, hoping to reduce recurrence and perhaps improve survival. Surgeons, in response to this new intense postoperative surveillance that has uncovered very small volume disease, have responded by utilizing US preoperatively to image this disease, and incorporated varying degrees of lymphadenectomy into their initial treatment algorithm. Bilateral thyroid resection—either total or near-total thyroidectomy—remains the standard for PTC >1 cm, although recent data has re-emphasized the value of unilateral lobectomy in treating even some PTC measuring 1-4 cm. Therapeutic lymphadenectomy has universal approval, but when lymph nodes in the central neck are not worrisome to the surgeon’s intraoperative assessment, although that judgment in incorrect up to 50%, whether they should be excised has reached a central point of controversy. Disease relapse can occur individually or in combination of three different forms: lymph node metastasis (LNM), true soft tissue local recurrence, and distant disease. The latter two are worrisome for potentially life-threatening consequences whereas nodal metastases are often persistent from the initial operation, and mostly comprise a biologic nuisance rather than virulent disease. A moderate surgical approach of bilateral thyroid resection, with usual central neck nodal clearance, and lateral internal jugular lymphadenectomy for node-positive disease can be performed safely, and with about a 5% recurrence rate.
Keywords: Papillary thyroid cancer (PTC), recurrence, surgery, lymph nodes
Introduction
Even though this eight-word title is seemingly simple and straightforward, the three major topics—papillary thyroid cancer (PTC), optimized surgery, and recurrence—are actually complex and sometimes controversial. In contrast to most malignancies, where the focus and endpoint is survival, the endpoint here is recurrence, which I think is entirely appropriate. To comprehend fully this overall subject requires appreciation of the multitude of factors of each topic, recognizing that virtually no controlled trials have been conducted.
PTC—context of the disease
In the United States an explosive rise in the frequency of diagnosing PTC had been seen, resulting in a nearly 800% increase over the prior 35 years, according to SEER registry data (1). Similar increases have been verified in Europe and Japan.
Papillary thyroid microcarcinoma (PTMC)
Nearly 50% of the increase proved to be PTC of 1 cm or smaller, and 87% were ≤2 cm. However, thyroid cancer mortality has remained flat, implying that these small cancers would not likely have progressed to be life-threatening. This dramatic rise in subclinical disease has been attributed largely to more frequent and widespread use of imaging of the head and neck for unrelated investigations, with the unanticipated discovery of these incidental cancers. The largest increase had been found in patients of 45 years and older, to the point that the most frequent diagnosis of PTC currently in the United States is a “papillary thyroid microcarcinoma” (PTMC) of ≤1 cm in a patient of 45 years or older (2). One of the conclusions of this study by Hughes et al. was that “the minimal clinical significance of microcarcinomas may mean that treatment of these tumors will provide minimal to no benefit in terms of survival, recurrence, or risk of progression to locally advanced disease in individual patients or the population as a whole”. Actually, the frequency of small PTC has been known for years, derived from autopsy studies ranging from 5.6% to 35.6% (3). So common were these PTMC that the authors concluded they could “be regarded as a normal finding”. Moreover, because these tumors seemed to remain small or even to regress in the vast majority of cases, these authors suggested that “in practice, detection of (PTMC) without regional metastases should not lead to any treatment.” A similar autopsy study of over 1,000 cases found 6.2% PTMC, and determined that “no surgical or other therapeutic consequence should be followed whenever such a small carcinomas is found incidentally in a gland removed for reasons other than tumor” (4).
The innocent biologic behavior of these PTMC when identified clinically is amply verified by a bold and unique study by Ito (5,6), effectively demonstrating the natural history of these tumors. Whereas 1,055 PTMC patients were operated, a selected group of 340 patients were observed without surgery with a mean of 74 months follow-up. Only 2 patients of all these patients had distant metastasis at diagnosis, and only 2 (0.14%) patients died of cancer, 79 and 94 months after initial operation. With a threshold of 3 mm for meaningful enlargement of the PTMC in the observation group, 5- and 10-year enlargement occurred in only 6.7% and 16%, respectively. Eventually, 1/3 of the observed patients underwent thyroidectomy with no subsequent recurrences, indicating that even with signs of disease progression in an observed patient, surgical treatment remains highly successful and “not too late”.
Several large series have been published regarding PTMC. Hay (7) investigated 900 patients with PTMC from Mayo Clinic from 1945-2004, with a mean follow-up of 17.2 years. Despite tumors being multifocal in 23%, associated with lymph node metastases (LNM) in 31%, 3 (0.3%) with distant metastases at diagnosis, the 20- and 40-year tumor recurrence rates were only 6% and 8%, respectively. None of the 892 patients with initial complete resection developed metastatic spread during 20 postoperative years. Chow (8) reported the cause-specific survival, locoregional metastasis failure-free survival, and distant metastasis failure-free survival rates at 10 years to be 100%, 92%, and 97%. Of 281 PTMC patients treated at Gustave-Roussy Institute, only 3.9% had locoregional recurrence, and a single patient had lung metastasis (9). Finally, a meta-analysis substantiated these large series with review of 9,313 patients (10), only 35 cases (0.37%) had distant metastases at diagnosis, and cancer-specific death occurred in only 0.34% (32 of 9,379 patients).
To summarize regarding PTMC, even though the label of cancer is applied to these small tumors, with rare exception, they are biologically innocent. Even though spread to lymph nodes at surgical resection occurs in about 30% (7,8), these metastasis are usually quite small and recurrence is seldom seen. It would seem only rational that in the near future, the interventional resources utilized to treat PTMC will be scaled back to fit the almost inert biologic behavior of these tumors.
PTC—biology of disease
Next, to consider PTC excluding tumors of 1 cm or smaller, over the prior three decades prognostic scoring systems have been developed that reliably predict the risk of disease-related mortality. MACIS (11) (metastasis, age, completeness of surgery, gross local invasion, and tumor size) has emerged as the best and most widely accepted system directed specifically for PTC. Universally used and accepted, however, is the TNM system which has specific criteria for most malignancies. Separate risk groups can be identified based on a combination of clinical and pathology criteria, consistently demonstrating that about 85% of patients with PTC have a <5% risk of disease-specific death over at least 20 years (11,12). The factors within the TNM and MACIS systems for predicting mortality rely on markers of more virulent biology (tumor size, invasiveness, distant spread, even age correlates with more virulent disease as does the ability of the surgeon to remove completely the disease). It is well to remember Blake Cady’s keen insight into tumor biology and the interplay with surgical intervention. To paraphrase, “Biology is King; patient selection is Queen. Technical details of surgical procedures are the Princes and Princesses of the realm who frequently try to overthrow the powerful forces of the King and Queen, usually to no long-term avail…technical wizardry cannot overcome biological restraints.” (13). One of the unique characteristics of PTC is the relative lack of influence on mortality of LNM. This has been consistently verified in multiple prognostic scoring systems, derived from multivariate analysis. Only when the lymph nodes are large and invasive of surrounding soft tissue with extranodal spread—a biologic characteristic mirroring the aggressiveness of the primary tumor—do the lymph nodes signify worse prognosis (14,15). In fact, prior to about 2003, LNM attracted only modest attention. To add routine radical surgery for lymph node disease, in the face of the widely held opinion that LNM did not reduce survival, was further discouraged as it was associated with alarming results. Mazzaferri reported that extensive lymph node dissections were associated with hypoparathyroidism in 20% and an overall complication rate of 44%; yet recurrences were not prevented with a recurrence rate of 2% per year, and a 20% complication rate with further reoperation (16).
Disease relapse testing—fear
With the turn of the new millennium, it seemed that the goal of endocrinologists was to eradicate all detectable and potentially all molecular evidence of PTC disease. Intertwined in the development of this attitude were two new extraordinarily sensitive measures to detect miniscule disease: high-resolution ultrasound (US), and stimulated thyroglobulin (Tg). US could identify potentially abnormal LNM of only a few millimeters, and verify disease presence with the addition of US-directed fine needle aspiration. Studies on the use of Tg determined that a stimulated (rh-TSH) Tg of only 2 ng/mL should prompt further investigation (even though seldom could structural disease be found even with US until the Tg was ≥4 ng/mL). Armed with this information, the use of 131I for therapeutic ablation of this trace disease became standard of care. The economic burden of these practices produced an overall probability of medical bankruptcy of 5%, and a risk almost 3.5 time higher than controls—a rate exceeded only by lung cancer (17). Moreover, thyroid cancer had the highest bankruptcy rate of all cancers at 1 year after the diagnosis.
Fear of dire consequences from LNMs was generated when contradictory evidence emerged. A population-based, case-control study of over 5,000 patients in Sweden with PTC found that LNM were associated with a 2.5-fold increased mortality (18). In another study, LNM were associated with a 4-fold increase in local recurrence, and a 2.5-fold increase in cause-specific mortality (19). Serum Tg level, in addition to its role as a very sensitive marker of disease, was reported to be predictive of disease-free remission and death (20). Mazzaferri offered the perspective that up to 25% of patients with PTC of 1.5 cm or smaller will have persistent or recurrent disease, whereas the combination of total thyroidectomy and radioactive iodine (RAI) has the potential to reduce this risk to zero (21). Kloos and Mazzaferri further warned, “Although mortality rates for DTC are low, tumor recurrence rates are high and may portend death from thyroid cancer.” (22).
Shift of emphasis: disease-specific mortality to relapse
Reassuringly, however, in a meta-analysis of 23 studies regarding the use of RAI for PTC, the 10-year disease-specific mortality remained extremely low at about 1.7% (23). With the new fixation of concern on lymph node relapse, the new ability to uncover even tiny amounts of disease during the course of compulsive postoperative disease surveillance, consequent liberal use of RAI to ablate this modest disease, the fear on the part of physicians and patients alike that even miniscule amounts of cancer was extremely worrisome and required intervention, surgeons felt the obvious impetus to minimize any potential disease relapse at the time of surgery. Disease relapse occurs in three important forms: distant disease, “true” local recurrence (soft tissue disease not within lymph nodes), and lymph nodes. While the first two are evidence of biologically aggressive disease that may eventually be life-threatening, they are distinctly uncommon in most consecutive series of PTC—consistent with the known very low disease-specific mortality of PTC. However, about 90% of PTC disease relapse is LNM. These LNM are the usual culprits identified during postoperative surveillance, and, therefore, have been the focus of considerable study and debate over the prior 15 years.
Lymph node metastasis (LNM)
LNM occur early and often in PTC, initially located in compartment VI (C-VI; central neck compartment bordered laterally by the carotid arteries, superiorly by the hyoid bone, and inferiorly at or just below the sternal notch). They are often small, escaping the detection by the surgeon in up to 50% (24). Noguchi authored what is now a classic study involving systematic node dissection in 57 patients (25). The key findings were (I) LNM were present in 90% of patients, 57% of which were <3 mm; (II) the LNM occurred initially in C-VI followed soon by spread to compartments III and IV (low and mid internal jugular lymph nodes from the base of the neck at and slightly below the clavicle extending superiorly to the level of the hyoid bone); and (III) LNM were misjudged in 80% to be negative by the surgeon. Rather stunning, however, was a subsequent study by the same group involving 300 patients who were operated without systematic lymph node dissection, but without reported recurrences (26). Similar studies have confirmed that while LNMs may be present in 60% of patients operated even with PTMCs, if systematic node dissection is not performed, recurrence may be rare, even less than 1% in one study (27). Carried to the extreme, Qubain (28) studied 80 patients who underwent nodal dissection with all nodes being negative for metastasis by H & E staining. The overall rate of positive lymph nodes by cytokeratin immunohistochemistry was 53%, with >90% positive nodes in the central compartment. Nevertheless, all patients were alive at the time of follow-up, implying that the node positivity was not life-threatening. More recent efforts to characterize LNM principally by size has been undertaken by Randolph (29), and, consistent with breast cancer research, to constitute a clinical threat, the metastasis must be at least 0.2 mm in size (the size threshold thought to be necessary for neovascularity, not just individual tumor cells surviving by diffusion of nutrients). However, the actual size and virulence thresholds for LNM of PTC to relapse clinically remain to be fully elucidated.
Because the burden of disease within lymph nodes associated with PTC that is of clinical significance is not fully understood, and no randomized clinical trials are available nor likely to be performed to answer this question, debate over this topic has taken center stage among endocrine surgeons. Macroscopic LNM are unanimously accepted as indication for node dissection, but node clearance, especially in C-VI when intraoperatively evident LNMs are not apparent, is controversial.
Optimized surgery for PTC
Surgery for PTC can be divided into two components: thyroidectomy, and lymphadenectomy. Based on the preponderance of data from large, relevant retrospective studies, the American Thyroid Association (ATA) has enumerated a treatment strategy with (30):
-
Several reasonable overall goals:
Remove the tumor and metastatic nodes as well as locally involved structures;
Minimize morbidity;
Allow staging that facilitates further management and follow-up;
Minimize disease recurrence, both local and distant.
-
Specific to surgical goals, excerpts from Recommendation 15 state:
“To remove the primary tumor, disease that has extended beyond the thyroid capsule and involved cervical lymph nodes. Completeness of surgical resection is an important determinant of outcome, while residual metastatic lymph nodes represent the most common site of disease persistence/recurrence.”
“To minimize the risk of disease recurrence and metastatic spread. Adequate surgery is the most important treatment variable influencing prognosis…”
-
The specific tactics to achieve the goals are also enumerated:
For PTC >1 cm, total or near-total thyroidectomy is indicated. Lobectomy is sufficient for <1 cm PTC if low risk, unifocal, intrathyroid, with no metastatic lymph nodes or prior radiation;
Therapeutic clearance of C-VI or lateral neck LNM is indicated;
Prophylactic clearance of C-VI may be performed;
No C-VI dissection may be appropriate for small, T1-2 PTC.
An important corollary to the above guidelines is that “these recommendations should be interpreted in light of available surgical expertise”.
Controversies—extent of thyroidectomy
Extending over at least three decades was the debate over the extent of the thyroidectomy. Publication of a sentinel study in 2007 by Bilimoria (31) based on the NCDB database showed that recurrence was slightly but statistically higher (9.8% vs. 7.7%) and survival was slightly lower (97.1% vs. 98.4%) in lobectomy patients vs. bilateral resection. However, lobectomy had been shown to be equivalent to near-total (NTTx) or total thyroidectomy (TTx) in survival in T1-2 N0 patients in publications from Memorial Sloan Kettering Cancer Center (32) and our institution (33). The controversy has been re-kindled by two recent publications for PTC even as large as 4 cm (34,35). The Japanese study reviewed over 1,000 patients who underwent lobectomy for tumors up to 4 cm in size, with follow-up of 17.6 years (without addition of RAI), in the absence of clinically positive lymph nodes or extrathyroidal extension, no patient under 45 years of age died. The American study re-analyzed 61,775 patients from the NCDB, and overall survival was similar in patients undergoing total thyroidectomy versus lobectomy for tumors 1-4 cm in size. Because the overwhelmingly large number of patients treated with bilateral resection, the general consensus today that bilateral resection is preferred, and the complexity of using RAI or Tg follow-up in lobectomy patients, it is reasonable to accept bilateral resection—either NTTx or TTx—as the optimal extent of thyroidectomy for PTC >1 cm.
Extent of lymphadenectomy
As alluded to previously, the increasing extent of lymphadenectomy was at least initially driven by the frequency of relapse of LNM coincident with the introduction of and rapid acceptance of new technologies for detecting this disease: high-resolution US, recombinant TSH, and Tg. With the ability to detect postoperatively, tiny, subclinical disease, surgeons naturally responded with more aggressive methods to detect and remove these nodes. It rapidly became clear that US was far more sensitive for detecting even macroscopic lymph nodes than clinical examination, so preoperative US became routine (36,37).
Whereas a number of studies suggested a very low relapse rate even in the absence of more thorough lymphadenectomy, a major study from Memorial Sloan Kettering by Tuttle (38) elucidated a more realistic picture of the behavior of PTC. Studying 588 patients who had undergone total thyroidectomy, appropriate lymph node dissection and routine RAI, they utilized the ATA Risk of Recurrence Classification to estimate the chance of recurrence of these patients according to their pathology and subsequent radioactive scans (Table 1).
Table 1. Frequency of PTC recurrence according to ATA recurrence risk classification (biochemical and structural).
| Risk classification frequency by % of patient group | Structural disease, % | Elevated Tg, % | Total recurrence, % |
|---|---|---|---|
| Low, 25% | 3 | 11 | 14 |
| Intermediate, 50% | 21 | 22 | 43 |
| High, 25% | 68 | 18 | 86 |
PTC, papillary thyroid cancer; ATA, American Thyroid Association; Tg, thyroglobulin.
Low risk: no local or distant metastasis; no local invasion or aggressive histology; R0 surgical resection; no 131I outside of the thyroid bed;
Intermediate risk: microinvasion, 131I or LNM present outside the thyroid bed; aggressive histology or vascular invasion could be present;
High risk: macroinvasion, incomplete resection—gross residual, or distant metastases were present.
Almost 25% of the low and intermediate groups developed structural disease recurrence, and >50% had either biochemical or structural recurrence despite excellent surgical and RAI treatments. Yet following additional treatments, at the time of final follow-up with a median of 7 years, 67% were free of disease, persistence/recurrence was present in 28% (only 1-2% being true local recurrence—soft tissue disease), and only 5% had died of disease, of whom 26 of 28 initially had stage IV disease. This study confirms that, despite high quality local treatments structural recurrence in PTC occurs in 25% and that frequency is doubled if biochemical criteria for recurrence are included. However, in the absence of distant disease at the time of diagnosis, PTC is rarely lethal (39,40).
Value and rationale for C-VI lymph node dissection
With the current practice of intense postoperative surveillance searching for even miniscule disease, the efforts to achieve thorough lymph node dissection are worth serious consideration. Because therapeutic lymph node dissection is virtually unanimously supported, the focus of debate has revolved around what is termed “prophylactic” dissection—removing nodes even when not grossly abnormal in the judgment of the surgeon. Also, until recently, few in Western countries have supported lateral jugular lymph node dissection (compartments variably including II-V). Therefore, C-VI “prophylactic” node dissection has attracted considerable attention and investigation. The reasons to undertake routine C-VI lymph node dissection include:
Preoperative US in the initial cervical exploration is nearly blind to the detection of LNM in C-VI (in contradistinction to lateral neck LNM) (36,37);
Surgeons cannot reliably differentiate innocent from LNM in many cases;
LNMs occur in up to 50% of patients operated on for PTC (41);
Missed LNM are typically found along the recurrent laryngeal nerve (RLN) in the trachea-esophageal groove, a potentially dangerous location if reoperation becomes necessary;
Dissection would logically lead to reductions in relapse and consequently reoperation;
C-VI dissection can be accomplished safely, although this is a major statement of contention;
Disease staging could be changed for patients over 45 years, from stage I to stage III, with potential for additional treatment implications;
RAI is unreliably effective in “cleaning up” residual macroscopic LNM.
In support of above statements, Sywak from a highly respected surgical group in Sydney, Australia reviewed 447 clinically node-negative PTC patients, with 391 having undergone only TTx, whereas the remaining 56 underwent TTx plus ipsilateral central neck lymph node dissection (CLND) (42). The CLND group had a statistically lower Tg than those with TTx only. An undetectable Tg was achieved by 72% with CLND as opposed to only 43% with TTx alone. Worth added consideration is the fact that at least biochemically, fewer than half of the TTx patients were actually cured in expert surgical hands. Three additional, recently published powerful studies support CLND, with sufficient numbers of patients to reach statistical differences. All followed a similar study design: comparison of TTx plus CLND vs. TTx only; the PTCs were >1 cm; retrospective analysis; and all patients were clinically node negative. A multi-institutional study of 606 patients showed recurrence-free survival to be statistically greater when CLND was added (Figure 1) (43). Stimulated Tg was also lower as was evidence of any recurrence. Similarly, disease-specific survival was superior in the CLND group in a second study of 640 patients (Figure 2) (44). And finally, in a third study by Hartl (45), the rate of C-VI reoperation and Tg were lower with CLND as was overall reintervention. A meta-analysis of prophylactic CLND confirmed a 35% lower recurrence with CLND, but at a cost of 26% higher rate of temporary hypocalcemia (46).
Figure 1.
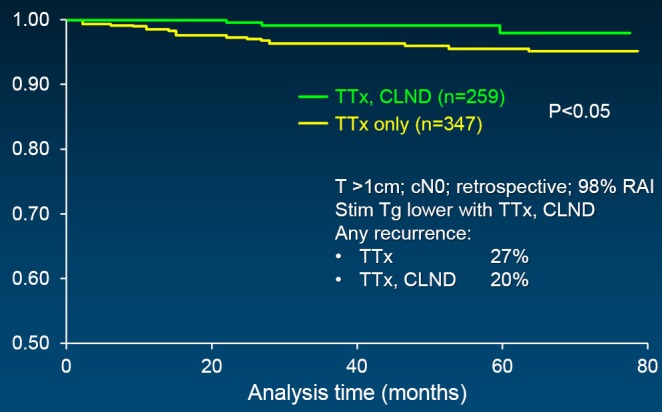
Compartment VI recurrence-free survival; multi-institutional study (N=606). CLND, central neck lymph node dissection; RAI, radioactive iodine ablation; Tg, thyroglobulin; TTx, total thyroidectomy. Reprinted with permission (43).
Figure 2.
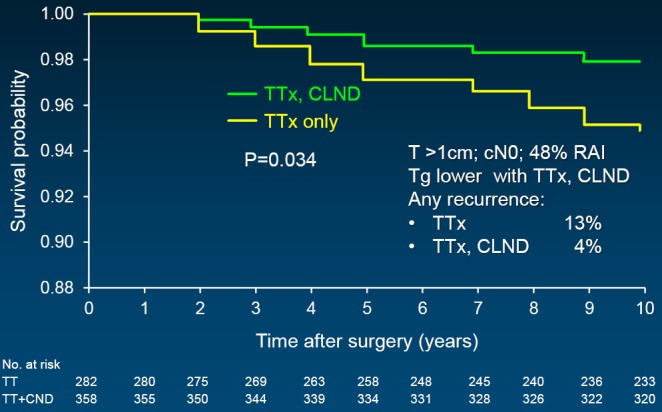
Disease-specific survival (N=640). CLND, central neck lymph node dissection; RAI, radioactive iodine ablation; Tg, thyroglobulin; TTx, total thyroidectomy. Reprinted with permission (44).
Surgical approaches—variations
As noted previously, the most widely accepted approach to PTC, size >1 cm, is bilateral thyroidectomy, either NTTx or Tx. So the differences in overall surgical management are focused on the central and lateral compartment lymph nodes. Essentially all agree that preoperative US is crucial, and clinically positive lymph nodes should be dissected in a compartment-oriented (rather than “node-picking”) approach. Those surgeons who support a more conservative approach to C-VI CLND raise significant concern about risk to the RLN and at least temporary hypoparathyroidism. Additionally, they cite studies that DFS, OS and even actual LNM relapse are quite uncommon in the absence of elective or prophylactic CLND. They also find support from prominent endocrinologists who have stated, “As long as postoperative RAI is planned, dissection of nonpalpable lymph nodes is probably not essential.” (47).
A moderate approach, favored by surgeons at Mayo Clinic, is to add routine C-VI CLND, and add lateral jugular node dissection (typically C-III and C-IV with possible addition of C-II as indicated) if US or palpable positive nodes identified.
More aggressive approaches have been advocated not only including routine C-VI dissection, but routine C-III and C-IV ± C-II-V on the side of the tumor (48). With a yield of 42% positive nodes in the lateral dissection, they have been criticized for the inadequacy of the preoperative US and the morbidity of lateral neck dissection particularly in the node-negative patients. Even more aggressive, not only routine C-VI dissection, but routine bilateral lateral jugular node dissection (C-III-IV ± II) has been reported (49).
Disease recurrence
Definition of terms
Relapse of PTC can occur in three forms: distant metastasis, “true” local recurrence, and disease within lymph nodes (LNM). It is estimated that 90% of disease relapse in PTC is LNM, and because of the usual indolent nature of the disease, and because relapse is typically identified within the first 3-4 years (50), this most likely represents disease persistence with possible enlargement rather than true recurrence. Although LNM and local recurrence are often lumped together, they represent very different entities both biologically and with respect to surgical re-excision. LNMs most typically are well-defined and most often—in the absence of significant extranodal spread—can be excised precisely without need for either resection or damage to surrounding structures. In contrast, true local recurrence (newly formed disease found within soft tissue, often densely attached or invading these structures) makes surgical resection more difficult and potentially involving sacrifice of important structures such as RLN, tracheal cartilage, etc. This is a reflection of more virulent disease that mirrors the initial aggressive thyroid cancer. Distinguishing LNM from local recurrence may be extremely difficult preoperatively, and sometime even intraoperatively when lymph nodes have been totally replaced with disease that also had gross extranodal disease. Modern day surveillance usually discovers residual disease much earlier than in prior decades, and the implications of contemporary disease discovery is vastly different than in times past. Many years ago, disease recurrence became evident by new symptoms such as hoarseness or new enlarging mass. It was this type of disease that Gagel was referring to when he stated that 40-50% of patients who die of thyroid carcinoma do so because of recurrent disease in the central compartment of the neck, and a high percentage of patients with recurrence in the thyroid bed (as high as 50%) will die of their carcinoma (51). It is well to recall the study by Tuttle (38) of 588 patient, only 5% had died of disease of whom 26 of 28 initially had stage IV disease.
Our reoperative experience with PTC at Mayo Clinic (52) included 410 patients who were operated from 1999-2008. This encompassed a widely heterogeneous group of patients, ranging over 9 decades in age, nearly 75% having been given an average of 200 mCi of RAI, and having undergone multiple previous neck operations to a maximum of seven involving both the central and the lateral neck. These patients all had structural disease, identifiable by either palpation and/or imaging. We were gratified that nearly three-quarters of our patients at last follow-up had no structural evidence of disease. Strikingly, however, 25% of the reoperative patients had either died of disease (11%) or were alive with structurally persistent disease (14%). This is a highly selected group containing a disproportionate number of very high-risk patients, not reflective of the usual population of PTC patients initially coming to surgical intervention.
Recurrence of PTC after optimized surgery
Having considered in detail the disease PTC, aspects of optimized surgery for this disease, and different forms and implications of disease recurrence, a coherent management plan can be synthesized.
For the dramatic rise in diagnosis of small PTMCs, restraint in the surgical approach should be the rule rather than the exception. Lobectomy only, unless there is disease on the contralateral side, should be amply sufficient. The addition of unilateral lymph node dissection is optional, but should not threaten the RLN. With this approach, hypoparathyroidism is positively avoided. Use of RAI is not justified. Ultimately, progress could be made in this country to avoid operation altogether except for cases with threatening characteristics.
In the current climate of nearly routine use of RAI, compulsive use of postoperative surveillance, and aggressive intervention for even subclinical disease, total thyroidectomy for PTC >1 cm still must remain standard of care. The future should narrow the need for bilateral resection as moderation in the compulsion for both diagnosis and intervention for subclinical PTC disease is embraced. More aggressive surgical measures will remain important for biologically aggressive disease.
As has occurred with other malignancies, notably breast cancer, recognition that not every molecule of disease needs to be ablated to allow long-term, disease-free survival. As molecular thumbprinting of PTC matures, directed therapy will be developed as opposed to the blanket RAI adjuvant therapy currently employed. Clinically important disease will be targeted, and indolent, biologically inert microscopic disease will be tolerated—both at the time of initial diagnosis and surgery, and postoperatively. This process is still in its infancy, but suspicious lymph nodes found by US postoperatively that are <8-10 mm are being intentionally observed rather than subjected to US-FNA. Once a diagnosis of “cancer” is pronounced, the patient understandably is worried, wants some form of intervention and clearance of disease.
Initial CLND for C-VI remains appropriately controversial. Mandated surgical clearance of indeterminate or innocent C-VI nodes that might threaten normal parathyroid function or the integrity of the RLN is unacceptable. However, to clear the C-VI nodes and reliably maintain blood supply and function of the superior parathyroid glands is almost always possible in capable surgical hands. Because it is presently impossible to predict which apparently innocent but actually metastatically involved C-VI lymph nodes will prove to be worrisome enough to at least be biopsied, proved positive, and ultimately lead to additional intervention, it seems that CLND is justified when it can be performed safely. A risk-stratified approach recommended by the European Society of Endocrine Surgeons seems very reasonable (53).
Prophylactic lateral jugular lymph node dissection has to this point been unacceptable in the United States as the sensitivity and reliability of preoperative US, when carefully performed by experienced sonographers, has been a quite acceptable alternative.
As evidence in support of this approach, the results are presented of the Mayo Clinic moderate surgical approach (41) including preoperative US for detection and mapping of LNM NTTx or TTx; routine C-VI CLND, and lateral internal jugular lymph node dissection when indicated by either positive nodes detected by palpation or US. From 1999-2006, 420 patients were treated with this comprehensive approach, and excluded only the few patients who were found intraoperatively to be unresectable. Tumors were multicentric in 40%, averaged 1.7 cm in size, were bilateral in 30%, demonstrated extrathyroidal extension in 17%, were associated with C-VI LNM in 51% and lateral LNMs in 20%, and had MACIS low-risk prognostic scores in 84%. RAI was used in 40% of patients. Relapse of LNM occurred in previously operated fields in 5% of patients; 3% had true local recurrence or distant metastasis, with complications limited to 1.2% hypoparathyroidism and only a single patient suffered unintentional RLN paralysis. Only a single patient had died as a direct result of PTC at last follow-up.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [DOI] [PubMed] [Google Scholar]
- 2.Hughes DT, Haymart MR, Miller BS, et al. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 2011;21:231-6. [DOI] [PubMed] [Google Scholar]
- 3.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer 1985;56:531-8. [DOI] [PubMed] [Google Scholar]
- 4.Lang W, Borrusch H, Bauer L.Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol 1988;90:72-6. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34:28-35. [DOI] [PubMed] [Google Scholar]
- 7.Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery 2008;144:980-7; discussion 987-8. [DOI] [PubMed] [Google Scholar]
- 8.Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer 2003;98:31-40. [DOI] [PubMed] [Google Scholar]
- 9.Baudin E, Travagli JP, Ropers J, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer 1998;83:553-9. [DOI] [PubMed] [Google Scholar]
- 10.Roti E, degli Uberti EC, Bondanelli M, et al. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol 2008;159:659-73. [DOI] [PubMed] [Google Scholar]
- 11.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993;114:1050-7; discussion 1057-8. [PubMed] [Google Scholar]
- 12.Cady B, Rossi R.An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988;104:947-53. [PubMed] [Google Scholar]
- 13.Cady B.Basic principles in surgical oncologyu. Presidential Address. Arch Surgery 1978;132:338-46. [DOI] [PubMed] [Google Scholar]
- 14.Hay ID, Bergstralh EJ, Grant CS, et al. Impact of primary surgery on outcome in 300 patients with pathologic tumor-node-metastasis stage III papillary thyroid carcinoma treated at one institution from 1940 through 1989. Surgery 1999;126:1173-81; discussion 1181-2. [DOI] [PubMed] [Google Scholar]
- 15.Voutilainen PE, Multanen MM, Leppäniemi AK, et al. Prognosis after lymph node recurrence in papillary thyroid carcinoma depends on age. Thyroid 2001;11:953-7. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferri EL, Young RL, Oertel JE, et al. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore) 1977;56:171-96. [PubMed] [Google Scholar]
- 17.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren E, Ljunghall S, Akerström G, et al. Case-control study on symptoms and signs of "asymptomatic" primary hyperparathyroidism. Surgery 1998;124:980-5; discussion 985-6. [PubMed] [Google Scholar]
- 19.Loh KC, Greenspan FS, Gee L, et al. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab 1997;82:3553-62. [DOI] [PubMed] [Google Scholar]
- 20.Heemstra KA, Liu YY, Stokkel M, et al. Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2007;66:58-64. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract 2007;13:498-512. [DOI] [PubMed] [Google Scholar]
- 22.Kloos RT, Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab 2005;90:5047-57. [DOI] [PubMed] [Google Scholar]
- 23.Sawka AM, Thephamongkhol K, Brouwers M, et al. Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab 2004;89:3668-76. [DOI] [PubMed] [Google Scholar]
- 24.Machens A, Hinze R, Thomusch O, et al. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 2002;26:22-8. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi S, Noguchi A, Murakami N.Papillary carcinoma of the thyroid. I. Developing pattern of metastasis. Cancer 1970;26:1053-60. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi S, Murakami N.The value of lymph-node dissection in patients with differentiated thyroid cancer. Surg Clin North Am 1987;67:251-61. [DOI] [PubMed] [Google Scholar]
- 27.Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 2003;237:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qubain SW, Nakano S, Baba M, et al. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery 2002;131:249-56. [DOI] [PubMed] [Google Scholar]
- 29.Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [DOI] [PubMed] [Google Scholar]
- 30.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer , Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [DOI] [PubMed] [Google Scholar]
- 31.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375-81; discussion 381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery 2012;151:571-9. [DOI] [PubMed] [Google Scholar]
- 33.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 1987;102:1088-95. [PubMed] [Google Scholar]
- 34.Matsuzu K, Sugino K, Masudo K, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg 2014;38:68-79. [DOI] [PubMed] [Google Scholar]
- 35.Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg 2014;260:601-5; discussion 605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 2003;134:946-54; discussion 954-5. [DOI] [PubMed] [Google Scholar]
- 37.Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg 2006;141:489-94; discussion 494-6. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010;20:1341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young S, Harari A, Smooke-Praw S, et al. Effect of reoperation on outcomes in papillary thyroid cancer. Surgery 2013;154:1354-61; discussion 1361-2. [DOI] [PubMed] [Google Scholar]
- 40.Nixon IJ, Wang LY, Palmer FL, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery 2014;156:137-46. [DOI] [PubMed] [Google Scholar]
- 41.Grant CS, Stulak JM, Thompson GB, et al. Risks and adequacy of an optimized surgical approach to the primary surgical management of papillary thyroid carcinoma treated during 1999-2006. World J Surg 2010;34:1239-46. [DOI] [PubMed] [Google Scholar]
- 42.Sywak M, Cornford L, Roach P, et al. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 2006;140:1000-5; discussion 1005-7. [DOI] [PubMed] [Google Scholar]
- 43.Popadich A, Levin O, Lee JC, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 2011;150:1048-57. [DOI] [PubMed] [Google Scholar]
- 44.Barczyński M, Konturek A, Stopa M, et al. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg 2013;100:410-8. [DOI] [PubMed] [Google Scholar]
- 45.Hartl DM, Mamelle E, Borget I, et al. Influence of prophylactic neck dissection on rate of retreatment for papillary thyroid carcinoma. World J Surg 2013;37:1951-8. [DOI] [PubMed] [Google Scholar]
- 46.Lang BH, Ng SH, Lau LL, et al. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid 2013;23:1087-98. [DOI] [PubMed] [Google Scholar]
- 47.Pearce EN, Braverman LE. Papillary thyroid microcarcinoma outcomes and implications for treatment. J Clin Endocrinol Metab 2004;89:3710-2. [DOI] [PubMed] [Google Scholar]
- 48.Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab 2009;94:1162-7. [DOI] [PubMed] [Google Scholar]
- 49.Ducoudray R, Trésallet C, Godiris-Petit G, et al. Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg 2013;37:1584-91. [DOI] [PubMed] [Google Scholar]
- 50.Durante C, Montesano T, Torlontano M, et al. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab 2013;98:636-42. [DOI] [PubMed] [Google Scholar]
- 51.Gagel RF, Goepfert H, Callender DL. Changing concepts in the pathogenesis and management of thyroid carcinoma. CA Cancer J Clin 1996;46:261-83. [DOI] [PubMed] [Google Scholar]
- 52.Onkendi EO, McKenzie TJ, Richards ML, et al. Reoperative experience with papillary thyroid cancer. World J Surg, 2014;38:645-52. [DOI] [PubMed] [Google Scholar]
- 53.Sancho JJ, Lennard TW, Paunovic I, et al. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 2014;399:155-63. [DOI] [PubMed] [Google Scholar]


