Abstract
Acute and chronic parathyroid insufficiency syndromes are the most common complication after total thyroidectomy. Permanent hypoparathyroidism imposes an important medical burden on patient lifestyle due to the need for lifetime medication, regular visits and significant long-term costs. Its true prevalence has been underestimated due to lack of clear definitions, inadequate follow-up and conflicts of interest when reporting individual patient series. The aim of this review is to propose precise definitions for the different syndromes associated to parathyroid failure based on the follow-up and management of patients developing hypocalcemia (<8 mg/dL at 24 hours) after first-time total thyroidectomy for cancer or goiter at our unit. Short and long-term post-thyroidectomy parathyroid failure presents as three different metabolic syndromes: (I) postoperative hypocalcemia is defined as a s-Ca <8 mg/dL (<2 mmol/L) within 24 hours after surgery requiring calcium/vit D replacement therapy at the time of hospital discharge; (II) protracted hypoparathyroidism as a subnormal iPTH concentration (<13 pg/mL) and/or need for calcium/vit D replacement at 4-6 weeks; and (III) permanent hypoparathyroidism as a subnormal iPTH concentration (<13 pg/mL) and/or need for calcium/vit D replacement 1 year after total thyroidectomy. Each of these syndromes has its own pattern of recovery and should be approached with different therapeutic strategies.
Keywords: Definitions, hypocalcemia, hypoparathyroidism, parathyroid splinting, total thyroidectomy
Postoperative hypocalcemia is the most common complication after total thyroidectomy (1-4) and impacts negatively of patient’s quality of life due to the need for lifetime medication, regular visits and significant long-term costs. A recent study carried out a survey of 374 patients with permanent hypoparathyroidism (>80% post-thyroidectomy). Of those polled, 75% experienced more than 10 symptoms despite appropriate treatment, nearly 80% had visited emergency department and or required hospital stay and 85% reported disabilities to perform household activities (5).
The impact of postoperative parathyroid failure
The estimated prevalence of postoperative hypocalcemia and permanent hypoparathyroidism according to a recent review and meta-analysis (6) varies from 19% to 38% and from 0% and 3% respectively. Its true prevalence, however, is probably underestimated for reasons shown in Table 1.
Table 1. Reasons for the underestimation of the prevalence hypocalcemia and hypoparathyroidism.
| Lack of clear definitions |
| Conflicts of interest |
| Variety of laboratory ranges for normocalcemia and reference values |
| Timing of blood sampling in the postoperative period |
| Wide range in thyroid procedures included in the analysis |
| Different case mix |
| Small series |
| Missing data in national audits |
| Different policies for calcium and vitamin D supplements |
| Short/incomplete follow-up |
| Follow-up not performed by the surgical team but by referring physicians |
National registries and large multicenter studies have shown that the rates of permanent hypoparathyroidism are much higher than those reported from single institutions, its prevalence ranging from 6% to 12% (Table 2). The Fourth National Audit of the British Association of Endocrine and Thyroid Surgeons (11) reported a 12.1% rate of permanent hypoparathyroidism after total thyroidectomy, while the Scandinavian Quality Register for Thyroid and Parathyroid Surgery (13) records a 6.4%. A multicenter German study showed a prevalence of 9% (8), quite similar to that observed in thyroid cancer patients in the USA (7). Furthermore, in some of these registries, the true rate of permanent hypoparathyroidism may be underestimated due to failure to follow-up all patients. For example, in the 2012 BAETS registry (11), 25% of patients do not have long term data on calcium and vitamin D replacement.
Table 2. Prevalence and definitions of hypocalcemia and permanent hypoparathyroidism in national registries and large multicenter studies.
| Study | Year | No. of procedures | Postoperative hypocalcemia prevalence | Definition | Value | Timing of blood sampling | Permanent hypoparathyroidism prevalence | Definition | Time at diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Hundahl et al. (7) (The ACS Commission on Cancer) | 2000 | 1,926 TT | Not available | Unspecified | Unspecified | Not available | 12.4% | Unspecified | Not available |
| Thomusch et al. (8) | 2003 | 5,846 BT | 24% | In ward calcium/vit D treatment | Unspecified | Not available | 9% | Undetectable PTH vitamin D or calcium supplementation | 6 months after surgery |
| Swedish register (9) | 2008 | 1,648 BT | 9.9% | Ca and/or vit D supplementation at discharge | Unspecified | Day 1 | 4.4% | Vit D and/or calcium supplementation | 6 months after surgery |
| Swedish register (10) (Graves’ disease) | 2012 | 956 TT | 13.6% | Ca and/or vit D supplementation at discharge | 4.3% | Vit D supplementation | 6 months after surgery | ||
| BAETS Fourth National Audit Report (11) | 2012 | 3,788 TT | 27.4% | Serum calcium | <2.1 mmol/L | Day 1 | 12.1% | Calcium/vit D supplementation | 6 months after surgery |
| Duclos et al. (12) | 2012 | 2,669 TT | Not available | Serum calcium | < 2 mmol/L | 48 h | 2.6% | Calcium/vit D supplementation | 6 months after surgery |
TT, total thyroidectomy; BT, bilateral thyroidectomy.
As can be seen in Table 2, there are substantial differences in the definitions of hypoparathyroidism and, especially postoperative hypocalcemia. Approximately, half of the reported studies define postoperative hypocalcemia as the need for calcium or vitamin D supplements whereas the rest define it according to low s-Ca concentrations, usually within 24 hours after surgery. The majority of these studies define permanent hypoparathyroidism as the need for calcium and or vitamin D supplements at 6 months after surgery. In addition, some of these analyses include conservative bilateral procedures and not only total thyroidectomies (8-12).
Some authors (14-16) have highlighted the lack of standardised definitions for postoperative hypocalcemia and hypoparathyroidism after total thyroidectomy. A review of 19 publications (14) showed that 26% of the studies failed to provide appropriate definitions for hypocalcemia, transient and permanent hypoparathyroidism. When provided, there was inconsistency in the biochemical definition of hypocalcemia (cut-off points ranging from 1.8 to 2.12 mmol/L). Mehanna et al. (16) applied different definitions reported in the literature to their cohort of thyroidectomy patients and demonstrated how the rate of hypocalcemia ranged from 0% to 46% depending on the definition used.
Not only do surgeons underestimate the prevalence of postoperative parathyroid failure, but also the impact of long-term hypoparathyroidism on patients’ well-being (5,17). Besides recurrent symptoms of hypocalcemia, patients with permanent hypoparathyroidism are at risk of developing renal failure, basal ganglia calcifications, neuropsychiatric derangements and infections (18,19).
Etiology and pathophysiology of iatrogenic hypoparathyroidism
The general consensus is that the main cause of hypocalcemia is an acute parathyroid insufficiency due to a reduction of the functioning parathyroid parenchyma (1,20). Impaired PTH secretion leads to postoperative hypocalcemia by inhibiting bone resorption and reducing 1,25-dihydroxyvitamin D synthesis in the kidney resulting in a reduced intestinal absorption of calcium (21).
An early postoperative decrease in serum iPTH concentrations associated with a s-Ca <8 mg/dL has been shown in several studies (22-24), and is consistent with the hypothesis that acute parathyroid insufficiency often occurs after total thyroidectomy. Barczyński et al. demonstrated a significantly lower iPTH serum concentrations and a higher drop of iPTH at skin closure and 4 hours after total thyroidectomy in patients developing hypocalcemia compared to normocalcemic patients (22). They concluded that iPTH levels <10 pg/mL at 4 hours after total thyroidectomy had the best precision to predict hypocalcemia (s-Ca <8 mg/dL) 24 hours after surgery, a positive predictive value of 90%. Grodski et al.’s review (23) found that post-thyroidectomy PTH levels accurately predict hypocalcemia, which is unlikely to occur if iPTH levels are normal, and can be used cautiously to discharge patients on the first postoperative day (24).
The reduction of the functioning parathyroid tissue is secondary to an intraoperative damage to the parathyroid glands caused by a combination of factors such as mechanical or thermal trauma, gland devascularization, obstruction of venous outflow, inadvertent parathyroid excision, and parathyroid autotransplantation. Fewer parathyroid glands identified during total thyroidectomy may result in gland injury and accidental parathyroidectomy (8,9). Few parathyroid glands kept in situ due to incidental parathyroidectomy (1,25-29) or autotransplantation (9,10,20,30,31) has been repeatedly reported to be a crucial factor leading to acute parathyroid insufficiency. This, however, has not been properly substantiated by biochemical tests until recently (32).
Other factors that may contribute to the development of post-thyroidectomy hypocalcemia include hemodilution, urinary calcium excretion facilitated by surgical stress, calcitonin release due to thyroid manipulation, vitamin D deficiency and hungry bone syndrome (14). Studies on PTH postoperative kinetics, however, cast little doubt about the major role of parathyroid failure in the pathogenesis of postoperative hypocalcemia.
Definitions of parathyroid failure syndromes at the Hospital del Mar
Postoperative hypocalcemia
There is consensus that the diagnosis and treatment of postoperative hypocalcemia must precede the development of symptoms and that PTH and/or s-Ca should be monitored after total thyroidectomy in order to start treatment before symptoms occur. Alternatively, some groups have proposed to give calcium and vitamin D supplements to all patients (preventive therapeutic strategy) and do not care much about biochemical parameters (33). In some registries, calcium and vitamin D replacement are used as surrogate variables for postoperative hypocalcemia (8-10).
The cut-off value and timing of blood sampling used to define postoperative hypocalcemia differs. Most authors (1,22,26,27,34) agree on the biochemical diagnosis hypocalcaemia as a total s-Ca concentrations <8 mg/dL or 2 mmol/L. Total s-Ca is cheap and easy to interpret and is preferable to ionized calcium concentrations which are highly dependent on blood sampling, transport and pH. A cut-off of 8 mg/dL (2 mmol/L) corrects for recumbency and mild hemodilution, and only exceptionally are symptoms of hypocalcemia observed above this value. Other authors (20,35) define hypocalcemia as a s-Ca <1.8 or 1.9 mmol/L but this risks to underestimate the diagnosis of hypocalcemia since patients may develop symptoms when s-Ca drops below 2 mmol/L. Finally, raising the cut-off up to 2.1 mmol/L (36) may lead to an overestimation of hypocalcaemia rates and overtreatment.
Timing of s-Ca measurement after thyroidectomy is critical because it has an impact on the prevalence of hypocalcemia rates: the closer the blood sampling is performed to surgery the lower the rates of hypocalcemia will be. On the other hand, if s-Ca is determined too late, patients may develop clinical symptoms before treatment is commenced.
For these reasons, we adhere to the more widespread proposal that postoperative hypocalcemia be defined as a s-Ca <8 mg/dL (2 mmol/L) 24 hours after total thyroidectomy (26) and that oral treatment with calcium and calcitriol be started if s-Ca drops below this value. This selective therapeutic strategy allows for patients to be discharged home early on the next day and minimizes overtreatment of the normocalcemic patients.
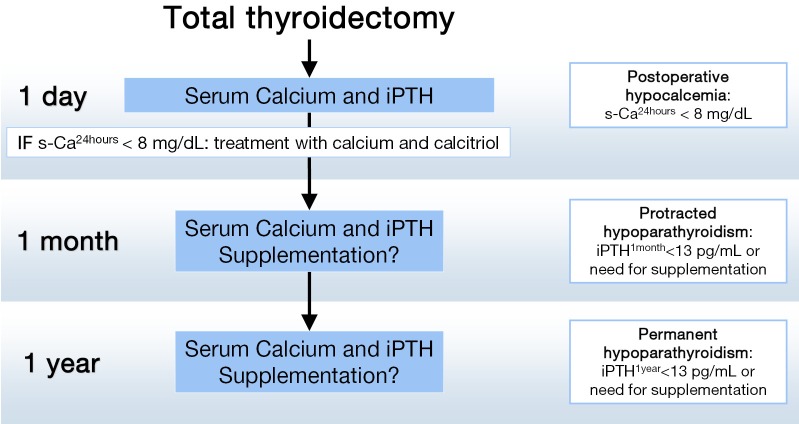
In addition, we recommend that a second blood test including iPTH be obtained the morning following surgery. This postoperative iPTH concentration is used as a reference value to check parathyroid function recovery during patient follow up. If PTH is low or undetectable, calcium and calcitriol supplements are continued when the patient is seen the next week in the outpatient clinic. All patients with postoperative hypocalcemia are followed in our unit with regular checks of s-Ca and iPTH until recovery or a final diagnosis of permanent hypoparathyroidism (26) is made (Figure 1).
Figure 1.
Time points for classification of patients developing hypocalcemia after total thyroidectomy at the Hospital del Mar.
Protracted hypoparathyroidism
Parathyroid function recovery can be expected in at least two thirds of patients with postoperative hypocalcemia within 1 month of thyroidectomy. Those who need treatment beyond this time period suffer from protracted hypoparathyroidism. Promberger et al. (37) proposed the concept of protracted hypoparathyroidism for those patients requiring replacement therapy 2 weeks after thyroidectomy. This period of time, however, may be a little too short to diagnose early parathyroid function recovery. Other authors assessed the hypoparathyroidism rates around 1 month after surgery. Hallgrimsson et al. (10), reported a 9.1% of protracted hypoparathyroidism assessing the need for vitamin D supplements at 6 weeks after surgery. Bergenfelz et al. (9) reported continuation of calcium, vitamin D or both in 7.8%, 2.6% and 7.3% of patients, respectively, 6 weeks after bilateral (not always total) thyroidectomy. In agreement with these two teams, we propose the following definition of protracted hypoparathyroidism: a subnormal iPTH concentration (<13 pg/mL) and/or need for calcium replacement with or without calcitriol at 4-6 weeks after thyroidectomy (26).
A proper definition of protracted hypoparathyroidism has clinical relevance as a predicting tool when informing patients. If the patient is hypoparathyroid 1 month after surgery, the probability of recovering the parathyroid function during the next 12 months is 75%. In addition, the chances of parathyroid function recovery after 1 month are better if iPTH is detectable (4-14 pg/mL) that when it is undetectable (26) (Figure 2).
Figure 2.
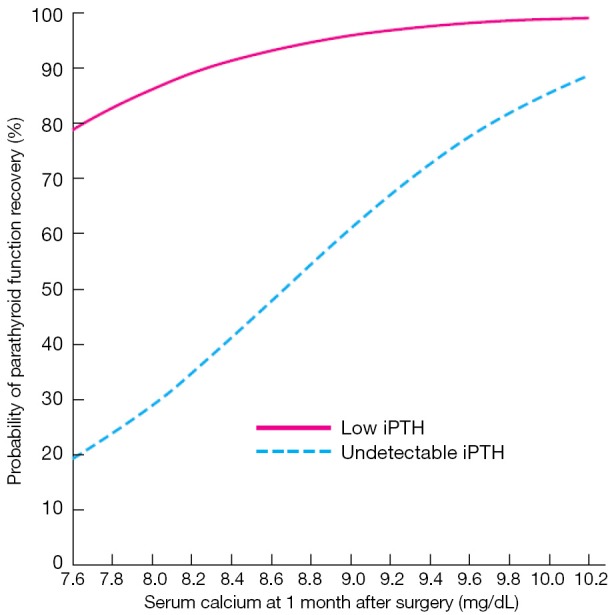
Probability of recovery of parathyroid function in patients with protracted hypoparathyroidism according to s-Ca and iPTH concentrations 1 month after total thyroidectomy. With permission from (26).
Permanent hypoparathyroidism
Permanent hypoparathyroidism is defined as the need for replacement therapy 6 months (21) or 1 year after thyroidectomy (1,26). We propose to use the 1-year deadline since according to our data about 20% of patients who recover from protracted hypoparathyroidism do so after 6 months. Subnormal iPTH concentration (<13 pg/mL) is the rule in these cases. A closer look at permanent hypoparathyroidism allows for a subclassification of this syndrome into three conditions:
Aparathyroidism (undetectable PTH, high phosphate);
Hypoparathyroidism (detectable but subnormal iPTH concentrations, normal phosphate);
Relative parathyroid insufficiency (normal iPTH levels but insufficient to maintain s-Ca within normal limits).
Broadly speaking, aparathyroidism always requires vitamin D supplementation, hypoparathyroidism can often be managed with calcium salts alone, and relative parathyroid insufficiency is seen in patients with associated conditions (treatment with biphosphonates, malabsorption, bowel resection, gastric bypass) impairing calcium absorption or resorption in whom there is an insufficient parathyroid functional reserve to respond to hypocalcemia. Interestingly, a study showed that the secretory response of parathyroid glands is impaired in some patients’ long term after with total thyroidectomy despite PTH levels are within normal limits (38). After a hypocalcemic stimulus with sodium bicarbonate infusion, PTH levels increased in total thyroidectomy patients but to a lesser degree compared with non-thyroidectomized patients.
The best vitamin D substitute for treatment of permanent hypoparathyroidism is controversial. We favor calcifediol because is cheap, non-nephrotoxic and can be usually started as one ampoule (10,000 UI, 266 mg) twice a week. Once diagnosed and stabilized, patients with permanent hypoparathyroidism should be controlled twice a year. s-Ca, P, iPTH levels, 25-hydroxyvitamin D and 1,25-hydroxyvitamin D (particularly if calcitriol is being administered) are determined to adjust replacement therapy and prevent hypo- and hypercalcemia.
Risk factors for postoperative parathyroid failure
Postoperative hypocalcemia
A recent meta-analysis (6) isolated as predictive factors for transient hypocalcemia biochemical parameters such as preoperative calcium levels, perioperative PTH and 25-hydroxyvitamin D levels and postoperative magnesium. Additionally, surgical factors found to be predictive were reoperation for recurrent goiter or for bleeding. Graves’ disease (8,39) and thyroid cancer (30) have been reported to be associated with higher rates of post-thyroidectomy hypocalcemia. Reported patient-related factors to hypocalcemia are younger age and female gender (8,39,40).
Lower perioperative levels or a larger decline in serum calcium (34,40,41), lower intraoperative or postoperative PTH levels (31,41,42), and also larger decline in intraoperative (22,41) and postoperative PTH (43-45) appear as biochemical factors associated with postoperative hypocalcemia as well as low preoperative vitamin D (34,46), low postoperative magnesium (47), high preoperative alkaline phosphatase and bone turnover markers (34,40) which is consistent with hungry bone syndrome.
With regard to surgical factors, increased risk of transient hypocalcaemia is mainly associated with the extent of surgery, central compartment node dissection (3,9,26,27), redo operations, reoperation for bleeding and wound infection (9,10). Some authors found that a lower hospital volume and therein, less experimented endocrine surgery team leads to an increase in hypocalcemia rates (10). Regarding surgical technique, few parathyroid glands maintained in situ due to inadvertent parathyroid excision (25-28) and/or autotransplantation (9,10,20,26,27,30,31,37) emerge as a major post-thyroidectomy hypocalcaemia risk factor. Gland injury and accidental parathyroid excision may be facilitated by failure to identify properly the parathyroid glands during total thyroidectomy (8,9). Hence, we usually look for parathyroid glands in their orthotopic position. Nevertheless, some authors have suggested that parathyroid gland identification has no influence on postoperative hypocalcemia (10,44) whereas others have considered it as a risk factor for hypocalcemia (31,48,49).
Protracted hypoparathyroidism
Protracted hypoparathyroidism is associated with weight of the resected thyroid gland, lymph node dissection, reoperation for bleeding, wound infection, esternotomy, few parathyroid glands identified and autotransplantation.
Multivariate analysis of our own data (32) revealed that protracted hypoparathyroidism was strongly associated with the number of parathyroid glands remaining in situ: 4-(excised + autografted). At this stage, the demographic and clinical variables as well as the extension of surgery were much less relevant. The prevalence of protracted hypoparathyroidism doubled in patients who received autotransplantation. There was a linear influence of the number of parathyroid glands remaining in situ on protracted hypoparathyroidism rates (32).
Permanent hypoparathyroidism
Despite attempts to predict permanent hypoparathyroidism on the basis of biochemical parameters at the time of hospital discharge the fact is that no single postoperative variable can be used to accurately predict it (50,51). A s-Ca level <1.88 mmol/L at 24 hours after surgery, identification of fewer parathyroid glands during the surgery (1,8) reoperation for bleeding (10), Graves’ disease and heavier thyroid specimens (52) have been identified as independent predictors (6). Parathyroid function recovery, however, is a dynamic event and cannot be predicted early after thyroidectomy. Our data (32) indicate that the best predictors of iPTH recovery in patients with protracted hypoparathyroidism are the number of parathyroid glands remaining in situ and the s-Ca concentration at one month after surgery (see below).
Whether parathyroid autotransplantation prevents permanent hypoparathyroidism is a very controversial issue. All authors admit that autotransplantation results in higher rates of postoperative hypocalcemia but some have proposed that in the long-term it prevents permanent hypoparathyroidism (4,53-57). Several studies, however, have found a strong association between autotransplantation and permanent hypoparathyroidism (1,20,26,30).
In our hands, parathyroid autotransplantation in the SCM muscle using the fragmented tissue technique proposed by Olson et al. (4) resulted in a threefold increase of permanent hypoparathyroidism: 3% in non-transplanted vs. 9% in transplanted patients. Interestingly, in patients with three glands remaining in situ, the rate of permanent hypoparathyroidism was the same whether the fourth gland was autotransplanted or was found in the specimen in the pathology lab.
Likelihood of recovery of parathyroid function
Postoperative hypocalcaemia is usually (>60-70%) a transient phenomenon and calcium supplements can be stopped within 1 month after surgery. If calcium and calcitriol supplements are still required after 1 month, the chance to develop permanent hypoparathyroidism is 25%. Clinical and surgical variables (age, gender, extension of surgery, diagnosis) do lose predictive significance and calcium and calcitriol dosage at hospital discharge, high s-Ca and low but detectable iPTH levels one month after surgery become the most relevant predictive variables (26). Higher s-Ca concentrations associated with higher calcium and calcitriol dosages at the time of hospital discharge have a positive effect on parathyroid function recovery. We have described this phenomenon as “parathyroid splinting”, meaning that the injured and ischemic parathyroid glands are allowed to rest in a normal-high s-Ca environment (Figure 2). Parathyroid splinting has a synergistic effect with the number of parathyroid glands remaining in situ to facilitate recovery of the parathyroid function (32).
Conclusions
The approach to post-thyroidectomy hypoparathyroidism may be facilitated by the understanding of the three different metabolic syndromes of parathyroid failure. Selective calcium/vit D replacement therapy of postoperative hypocalcemia at the time of hospital discharge is recommended. A detectable iPTH, all parathyroid glands remaining in situ and high levels of serum calcium one month after surgery increase the likelihood of recovery from protracted hypoparathyroidism. Permanent hypoparathyroidism can be managed according to iPTH levels. Aparathyroidism with undetectable iPTH requires vitamin D supplementation whereas hypoparathyroidism (detectable but subnormal iPTH) can often be managed with calcium salts alone. Associated conditions such as malabsorption, gastric bypass or treatment with biphosphonates may cause a relative parathyroid insufficiency.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 1998;22:718-24. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Fried MP. Assessment of the morbidity and complications of total thyroidectomy. Arch Otolaryngol Head Neck Surg 2002;128:389-92. [DOI] [PubMed] [Google Scholar]
- 3.Abboud B, Sargi Z, Akkam M, et al. Risk factors for postthyroidectomy hypocalcemia. J Am Coll Surg 2002;195:456-61. [DOI] [PubMed] [Google Scholar]
- 4.Olson JA, Jr, DeBenedetti MK, Baumann DS, et al. Parathyroid autotransplantation during thyroidectomy. Results of long-term follow-up. Ann Surg 1996;223:472-8; discussion 478-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadker N, Egan J, Sanders J, et al. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the paradox study. Endocr Pract 2014;20:671-9. [DOI] [PubMed] [Google Scholar]
- 6.Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [DOI] [PubMed] [Google Scholar]
- 7.Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 2000;89:202-17. [DOI] [PubMed] [Google Scholar]
- 8.Thomusch O, Machens A, Sekulla C, et al. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003;133:180-5. [DOI] [PubMed] [Google Scholar]
- 9.Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [DOI] [PubMed] [Google Scholar]
- 10.Hallgrimsson P, Nordenström E, Almquist M, et al. Risk factors for medically treated hypocalcemia after surgery for Graves' disease: a Swedish multicenter study of 1,157 patients. World J Surg 2012;36:1933-42. [DOI] [PubMed] [Google Scholar]
- 11.Available online: http://www.baets.org.uk/
- 12.Duclos A, Peix JL, Colin C, et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ 2012;344:d8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Available online: http://www.thyroid-parathyroidsurgery.com
- 14.Wu J, Harrison B.Hypocalcemia after Thyroidectomy: The Need for Improved Definitions. World J End Surg 2010; 2:17-20. [Google Scholar]
- 15.Balasubramanian SP. Iatrogenic/post-surgical hypoparathyroidism: where do we go from here? Endocrine 2014;47:357-9. [DOI] [PubMed] [Google Scholar]
- 16.Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2010;32:279-83. [DOI] [PubMed] [Google Scholar]
- 17.Cho NL, Moalem J, Chen L, et al. Surgeons and patients disagree on the potential consequences from hypoparathyroidism. Endocr Pract 2014;20:427-46. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Underbjerg L, Sikjaer T, Mosekilde L, et al. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res 2014;29:2504-10. [DOI] [PubMed] [Google Scholar]
- 20.Asari R, Passler C, Kaczirek K, et al. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg 2008;143:132-7; discussion 138. [DOI] [PubMed] [Google Scholar]
- 21.Shoback D.Clinical practice. Hypoparathyroidism. N Engl J Med 2008;359:391-403. [DOI] [PubMed] [Google Scholar]
- 22.Barczyński M, Cichoń S, Konturek A.Which criterion of intraoperative iPTH assay is the most accurate in prediction of true serum calcium levels after thyroid surgery? Langenbecks Arch Surg 2007;392:693-8. [DOI] [PubMed] [Google Scholar]
- 23.Grodski S, Serpell J.Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg 2008;32:1367-73. [DOI] [PubMed] [Google Scholar]
- 24.Payne RJ, Hier MP, Tamilia M, et al. Postoperative parathyroid hormone level as a predictor of post-thyroidectomy hypocalcemia. J Otolaryngol 2003;32:362-7. [DOI] [PubMed] [Google Scholar]
- 25.Glinoer D, Andry G, Chantrain G, et al. Clinical aspects of early and late hypocalcaemia afterthyroid surgery. Eur J Surg Oncol 2000;26:571-7. [DOI] [PubMed] [Google Scholar]
- 26.Sitges-Serra A, Ruiz S, Girvent M, et al. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687-95. [DOI] [PubMed] [Google Scholar]
- 27.Pereira JA, Jimeno J, Miquel J, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 2005;138:1095-100. [DOI] [PubMed] [Google Scholar]
- 28.McLeod IK, Arciero C, Noordzij JP, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid 2006;16:259-65. [DOI] [PubMed] [Google Scholar]
- 29.Paek SH, Lee YM, Min SY, et al. Risk factors of hypoparathyroidism following total thyroidectomy for thyroid cancer. World J Surg 2013;37:94-101. [DOI] [PubMed] [Google Scholar]
- 30.Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg 2007;245:604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang BH, Yih PC, Ng KK. A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg 2012;36:1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Wang TS, Cheung K, Roman SA, et al. To supplement or not to supplement: a cost-utility analysis of calcium and vitamin D repletion in patients after thyroidectomy. Ann Surg Oncol 2011;18:1293-9. [DOI] [PubMed] [Google Scholar]
- 34.Erbil Y, Barbaros U, Temel B, et al. The impact of age, vitamin D(3) level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am J Surg 2009;197:439-46. [DOI] [PubMed] [Google Scholar]
- 35.Wiseman JE, Mossanen M, Ituarte PH, et al. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg 2010;34:532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilhelm SM, McHenry CR. Total thyroidectomy is superior to subtotal thyroidectomy for management of Graves’ disease in the United States. World J Surg 2010;34:1261-4. [DOI] [PubMed] [Google Scholar]
- 37.Promberger R, Ott J, Kober F, et al. Intra- and postoperative parathyroid hormone-kinetics do not advocate for autotransplantation of discolored parathyroid glands during thyroidectomy. Thyroid 2010;20:1371-5. [DOI] [PubMed] [Google Scholar]
- 38.Anastasiou OE, Yavropoulou MP, Papavramidis TS, et al. Secretory capacity of the parathyroid glands after total thyroidectomy in normocalcemic subjects. J Clin Endocrinol Metab 2012;97:2341-6. [DOI] [PubMed] [Google Scholar]
- 39.Pesce CE, Shiue Z, Tsai HL, et al. Postoperative hypocalcemia after thyroidectomy for Graves' disease. Thyroid 2010;20:1279-83. [DOI] [PubMed] [Google Scholar]
- 40.Erbil Y, Bozbora A, Ozbey N, et al. Predictive value of age and serum parathormone and vitamin d3 levels for postoperative hypocalcemia after total thyroidectomy for nontoxic multinodular goiter. Arch Surg 2007;142:1182-7. [DOI] [PubMed] [Google Scholar]
- 41.Walsh SR, Kumar B, Coveney EC. Serum calcium slope predicts hypocalcaemia following thyroid surgery. Int J Surg 2007;5:41-4. [DOI] [PubMed] [Google Scholar]
- 42.Cavicchi O, Piccin O, Caliceti U, et al. Transient hypoparathyroidism following thyroidectomy: a prospective study and multivariate analysis of 604 consecutive patients. Otolaryngol Head Neck Surg 2007;137:654-8. [DOI] [PubMed] [Google Scholar]
- 43.Chapman DB, French CC, Leng X, et al. Parathyroid hormone early percent change: an individualized approach to predict postthyroidectomy hypocalcemia. Am J Otolaryngol 2012;33:216-20. [DOI] [PubMed] [Google Scholar]
- 44.Vanderlei FA, Vieira JG, Hojaij FC, et al. Parathyroid hormone: an early predictor of symptomatic hypocalcemia after total thyroidectomy. Arq Bras Endocrinol Metabol 2012;56:168-72. [DOI] [PubMed] [Google Scholar]
- 45.Lecerf P, Orry D, Perrodeau E, et al. Parathyroid hormone decline 4 hours after total thyroidectomy accurately predicts hypocalcemia. Surgery 2012;152:863-8. [DOI] [PubMed] [Google Scholar]
- 46.Kirkby-Bott J, Markogiannakis H, Skandarajah A, et al. Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J Surg 2011;35:324-30. [DOI] [PubMed] [Google Scholar]
- 47.Wilson RB, Erskine C, Crowe PJ. Hypomagnesemia and hypocalcemia after thyroidectomy: prospective study. World J Surg 2000;24:722-6. [DOI] [PubMed] [Google Scholar]
- 48.Pfleiderer AG, Ahmad N, Draper MR, et al. The timing of calcium measurements in helping to predict temporary and permanent hypocalcaemia in patients having completion and total thyroidectomies. Ann R Coll Surg Engl 2009;91:140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheahan P, Mehanna R, Basheeth N, et al. Is systematic identification of all four parathyroid glands necessary during total thyroidectomy?: a prospective study. Laryngoscope 2013;123:2324-8. [DOI] [PubMed] [Google Scholar]
- 50.Almquist M, Hallgrimsson P, Nordenström E, et al. Prediction of permanent hypoparathyroidism after total thyroidectomy. World J Surg 2014;38:2613-20. [DOI] [PubMed] [Google Scholar]
- 51.Julián MT, Balibrea JM, Granada ML, et al. Intact parathyroid hormone measurement at 24 hours after thyroid surgery as predictor of parathyroid function at long term. Am J Surg 2013;206:783-9. [DOI] [PubMed] [Google Scholar]
- 52.Thomusch O, Machens A, Sekulla C, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg 2000;24:1335-41. [DOI] [PubMed] [Google Scholar]
- 53.Gourgiotis S, Moustafellos P, Dimopoulos N, et al. Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbecks Arch Surg 2006;391:557-60. [DOI] [PubMed] [Google Scholar]
- 54.Ondik MP, McGinn J, Ruggiero F, et al. Unintentional parathyroidectomy and hypoparathyroidism in secondary central compartment surgery for thyroid cancer. Head Neck 2010;32:462-6. [DOI] [PubMed] [Google Scholar]
- 55.Sakorafas GH, Stafyla V, Bramis C, et al. Incidental parathyroidectomy during thyroid surgery: an underappreciated complication of thyroidectomy. World J Surg 2005;29:1539-43. [DOI] [PubMed] [Google Scholar]
- 56.Almquist M, Hallgrimsson P, Nordenström E, et al. Prediction of permanent hypoparathyroidism after total thyroidectomy. World J Surg 2014;38:2613-20. [DOI] [PubMed] [Google Scholar]
- 57.Lo CY, Tam SC. Parathyroid autotransplantation during thyroidectomy: documentation of graft function. Arch Surg 2001;136:1381-5. [DOI] [PubMed] [Google Scholar]