Abstract
Ablations of atrial fibrillation (AF) have become more widely performed, and the strategy about long-term usage of oral anticoagulants (OACs) after catheter ablation is an important issue, especially for patients without obvious evidences of recurrences. The annual rate of thromboembolic (TE) event after catheter ablation was less than 1%. CHADS2 and CHA2DS2-VASc scores could be used to identify patients at the risk of TE events after ablations who should continue OACs regardless of the status of recurrence. Despite the improvement in understanding of AF and advancement of technology in catheter ablation, the long-term successful rates of paroxysmal and non-paroxysmal AF are around 50% and 30%, respectively. Patients with a high CHADS2 score are at a high risk of recurrence which could continuously occur after the catheter ablation without reaching a plateau. Among the patients with a CHADS2 score of ≥3, 26.9% of the recurrences happened 2 years post catheter ablation. Compared to the episodes of AF before catheter ablation, the AF episodes after ablation procedures are less symptomatic and shorter in duration. Therefore, it may not be safe to stop OACs for patients with a high risk score since the AF episodes are difficult to be detected after ablation procedures, but remain dangerous. In conclusion, the decision about the long-term strategy of OACs should be based on patients’ baseline clinical risk scores, such as CHADS2 and CHA2DS2-VASc scores, rather than the status of recurrence.
Keywords: Atrial fibrillation (AF), oral anticoagulants (OACs), catheter ablation, CHADS2 score, CHA2DS2-VASc score
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia which is associated with marked morbidity, mortality, and socioeconomic burden (1,2). Catheter ablation targeting the pulmonary veins (PVs) has been reported to be a potential method for treating AF since the late 1990s (3,4). As the techniques and technologies have improved, catheter ablation of AF has become the standard and an effective therapy for patients with symptomatic and drug-refractory AF and its popularity continues to escalate (5). According to the 2010 AF management guideline of European Society of Cardiology (ESC), catheter ablation of drug-refractory persistent and long-standing persistent AF were class IIa and IIb recommendations, respectively (6). Catheter ablation was class I recommendation for drug-refractory paroxysmal AF in the updated 2012 ESC guideline, and could be considered as the first-line therapy (class IIa recommendation) (7). The survey in Australia showed that the provision of catheter-based AF ablation services has increased exponentially over the past decade, with the annual growth rate (30.9%) exceeding that of percutaneous coronary interventions (5.1%) (8). Since AF ablations become more widely performed, the strategy about long-term usage of oral anticoagulants (OACs) after catheter ablation is an important issue, especially for patients without obvious evidences of AF recurrences.
There are several issues should be clarified before the physicians decide to stop OACs after AF ablation procedures: (I) how to predict the occurrences of thromboembolic (TE) events after AF ablations? A useful and effective risk stratification system can help physicians identify high-risk patients who should keep OACs; (II) what is the recurrence-free rate after AF ablation during a long-term follow up? For patients with a high risk of recurrence, long-term use of OACs should be considered since recurrence can still occur several years later after “successful” ablations; (III) is it easy to detect AF recurrence after AF ablations? If it was difficult to detect AF episodes after ablations, it may be too late to restart OACs for high-risk patients.
Prediction of TE events for AF patients receiving ablation procedures
Several studies have reported the rates of TE events after AF ablations (9-13). Although these studies differed in patients’ CHADS2 scores, strategy of the use of anti-thrombotic agents and ablation procedures, the annual rate of systemic thromboembolisms was lower than 1% in these investigations. In a recent study performed by Lin et al., AF patients with rhythm control achieved by ablation strategy have a better long-term survival compared to patients receiving medical treatments, irrespective of recurrence state (14). This observation may suggest that the catheter ablation would modify the risk of adverse events carried by AF, and may therefore change its natural course. Accordingly, the scoring systems in predicting adverse events which were derived from AF patients without ablations had better to be further validated to confirm its usefulness in those who received catheter ablations. Currently, several scoring systems were available for stroke risk stratifications in AF, including CHADS2 (15), CHA2DS2-VASc (16), R2CHADS2 (17) and ATRIA (18) scores, which were constituted of different clinical risk factors (Table 1). Among these four scoring systems, the CHADS2, CHA2DS2-VASc and R2CHADS2 schemes have been validated in previous studies focusing on ablation cohorts (Table 1) (13,19,20). In our previous study which enrolled 565 AF patients receiving catheter ablation, the CHADS2 and CHA2DS2-VASc scores were significant predictors of TE events independent from AF recurrence or not (13). The risk of TE events was significantly higher for patients with a CHA2DS2-VASc score of ≥2, which could be used to further stratify patients with CHADS2 scores of 0 or 1 into two groups with different event rates (7.1% vs. 1.1%, P=0.003) (13). Two recent studies showed that the predictive accuracies of R2CHADS2 and CHA2DS2-VASc scores assessed by C-statistics were similar in predicting post-ablation TE events (19,20). Besides, the CHA2DS2-VASc score can differentiate TE risk in the low-risk strata based on R2CHADS2 score and may be superior in the subgroup with AF recurrences (19). These findings suggested that the strategy of long-term stroke prevention should be determined based on patients’ baseline risk scores, rather than the status of recurrence. Among the three scoring systems (CHADS2, CHA2DS2-VASc and R2CHADS2) which have been validated in the ablation cohort, CHA2DS2-VASc may be a preferred scoring scheme.
Table 1. Stroke risk predicting systems in AF*.
| Scoring scheme, year | Score range | Age | HTN | DM | CHF | Stroke/TIA | Vascular diseases | Female sex | Renal dysfunction/proteinuria | Being verified in AF ablation cohort |
|---|---|---|---|---|---|---|---|---|---|---|
| CHADS2 [2001] (15) | 0-6 | 2 points for age ≥75 | + | + | + | + | – | – | – | Yes (13) |
| CHA2DS2-VASc [2010] (16) | 0-9 | 2 points for age ≥75; 1 point for age 65-74 | + | + | + | + | + | + | – | Yes (13) |
| R2CHADS2 [2013] (17) | 0-8 | 2 points for age ≥75 | + | + | + | + | – | – | + | Yes (19,20) |
| ATRIA [2013] (18) | 0-12 (for patients without prior stroke); 7-15 (for patients with prior stroke) | Extended range for score assignment (<65, 65-74, 75-84, ≥85) | + | + | + | Different rules of score calculation for patients with or without prior stroke | – | + | + | No |
*, the table was modified from (21); +, different rules of score calculation for patients with or without prior stroke. AF, atrial fibrillation; HTN, hypertension; DM, diabetes mellitus; CHF, congestive heart failure; TIA, transient ischemic attack.
Long-term successful rate after AF ablations
Although many ablation centers have reported the AF recurrence rates after catheter ablation, these reporting outcomes of AF ablation have predominantly limited follow-up of 1 to 2 years after the index ablation procedure and the data of long-term efficacy of AF ablations were limited. Since the natural course after the ablation may differ between paroxysmal and persistent AF, the ablation results should therefore be reported separately for paroxysmal and non-paroxysmal AF patients. Figure 1 summarized three reports of long-term ablation outcomes (>5 years) of paroxysmal AF with a sample size more than 150 subjects which showed very similar results. The off-drug single procedure successful rate was near 50% in these three well-experienced ablation centers (22-24). The long-term recurrence-free rate after single ablation procedure was even lower for non-paroxysmal AF patients ranging from 20.3% to 29.4% (25,26). Therefore, a considerable number of patients would suffer from AF recurrence during the long-term follow up and were still under the risk of AF-related TE events.
Figure 1.
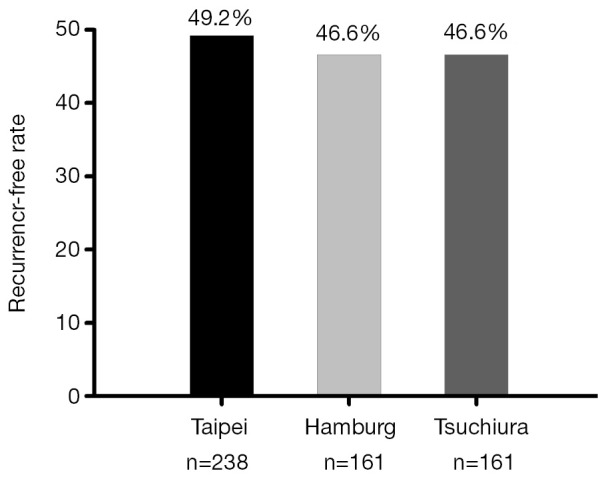
Long-term outcome of paroxysmal AF ablation of three reports with a patient number more than 150. The off-drug single procedure successful rate was near 50% in these three well-experienced ablation centers after a follow-up more than 5 years. AF, atrial fibrillation.
In our previous study which enrolled 247 paroxysmal AF patients, CHADS2 score was shown to be an independent risk factor of recurrences after catheter ablations (27). The similar finding has also been demonstrated in another study performed by Letsas et al. which enrolled 126 patients with paroxysmal AF (28). A high CHADS2 score (≥3) was also an important predictor of AF recurrence after catheter ablations for non-paroxysmal AF (25). Patients with different CHADS2 scores may also have different patterns of recurrences (23). For patients with a CHADS2 score of zero, there were less than 1% of these patients suffering from recurrences 2 years after the ablation procedure. On the contrary, the recurrence rate continuously increased after the catheter ablation without reaching a plateau in the patients with a high CHADS2 score (≥3). Among the patients with a CHADS2 score (≥3), 26.9% of the recurrences happened 2 years post catheter ablation (23). These findings suggest that patients with a high risk of stroke, represented by a high CHADS2 score, are also at a high risk of AF recurrence even the ablation procedures seem to be successful after 2 years of follow up. Therefore, if OACs were stopped based on the assumption that the AF did not recur in these patients with a high CHADS2 score who should theoretically receive OACs, these patients may be exposed to the risk of TE events because the possibility of very late recurrence of AF is high.
Detection of AF recurrence after AF ablations
It is known that AF is difficult to be detected due to its paroxysmal nature, and the detection of recurrent episodes may be more difficult in patients receiving AF ablations. In the study performed by Verma et al., 50 patients with symptomatic AF underwent implantation of an implantable cardiac monitor (ICM) with an automated AF detection algorithm 3 months before and 18 months after ablation (29). The ratio of asymptomatic to symptomatic AF episodes increased from 1.1 before to 3.7 after ablation. Besides, post-ablation AF episodes were significantly shorter, decreasing to a median of 6 (interquartile range, 5-40) from 22 (range, 10-202) minutes (29). These findings suggested that the AF episodes became asymptomatic and shorter after catheter ablations, and were therefore much more difficult to be detected using routine Holter monitoring in the daily practice. The Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) has proved that patients with subclinical atrial tachyarrhythmia, defined as an episode of rapid atrial rate (190 beats or more per minute), lasting more than 6 minutes, that was detected by the pacemaker or defibrillator, have a higher risk of TE events compared to those without (hazard ratio =2.49) (30). Therefore, asymptomatic AF episodes with short durations occurring in patients after ablations are subtle and difficult to be detected but remain dangerous. The strategy about stopping OACs first and restarting it when AF recurrences were detected may not be practical and not safe for patients with a high clinical risk score.
Current evidences and limitations
Several investigators tried to answer the question that whether it is safe to stop OACs for patients receiving catheter ablations without evidences of recurrences. The study designs, results and main findings of these reports were summarized in Table 2 (9,12,31). Although these studies showed promising results suggesting that OACs may be stopped safely for patients without recurrences, limitations of these investigations should be paid attention (as shown in Table 2), and these results should be interpreted carefully. Since data of the prospective and randomized trial regarding this issue are lacking, we had better comply with the recommendations of the current guidelines. The 2012 ESC focused update of AF management guideline suggests that continuation of long-term OAC therapy post-ablation is recommended in all patients with a CHA2DS2-VASc score of ≥2, irrespective of apparent procedural success (7). The 2012 consensus document of Heart Rhythm Society suggests that discontinuation of systemic anticoagulation therapy post ablation is not recommended in patients who are at high risk of stroke as estimated by currently recommended schemes (CHADS2 or CHA2DS2-VASc) (32). Therefore, patients with a high risk of stroke should receive long-term treatment of OACs after catheter ablation unless further solid data supporting discontinuation of OACs are available.
Table 2. Summaries of the studies reporting the results of stopping OACs after AF ablations.
| Author [year] | Patient number and characteristics | Use of anticoagulants after catheter ablation | Event rate | Main findings | Limitations |
|---|---|---|---|---|---|
| Oral et al. [2006] (9) | Paroxysmal AF: 490 | Warfarin was used for 3 months after catheter ablation. Among 522 patients who remained in sinus rhythm, warfarin was discontinued in 79% of patients without risk factors and in 68% of patients with ≥1 risk factor of stroke after 3 months | Rate of TEs: 0.9% within 30 days of ablation procedure; 0.3% beyond 30 days after the procedure | Discontinuation of warfarin appears to be safe after successful ablation | The authors continued OATs for patients with an age of >65 years or a prior history of stroke/TIA after successful ablation |
| Chronic AF: 265 | |||||
| 55±11 years | |||||
| 56% had ≥1 risk factor for stroke | |||||
| Themistoclakis et al. [2010] (31) | A total of 3,355 patients (60% paroxysmal AF) | Off-OAC group: 2,692 patients discontinued OACs 3 to 6 months after ablation | During follow-up (mean 28±13 vs. 24±15 months), 2 (0.07%) off-OAC group patients and 3 (0.45%) on-OAC group patients had an ischemic stroke (P=0.06) A major hemorrhage was observed in 1 (0.04%) off-OAC group patient and 13 (2%) on-OAC group patients (P<0.0001) |
The risk-benefit ratio favored the suspension of OACs after successful AF ablation even in patients at moderate-high risk of TE | The authors kept their patients on OACs when any arrhythmic recurrences, left atrial dysfunction, or severe PV stenosis were observed |
| 57±11 years | On-OAC group: 663 patients remained on OACs after 3 to 6 months post ablation | ||||
| CHADS2 score: 0 in 53%, 1 in 29%, ≥2 in 18% | |||||
| Yagishita et al. [2011] (12) | A total of 524 patients (16% had a CHADS2 score of >2) underwent AF ablation and were followed up for at least 24 months | Warfarin was discontinued in 400 (93%) of 429 patients without AF recurrence | None of the patients without AF recurrence suffered from TE events, where 3 of 95 patients (3%) with AF recurrence did (P<0.001) | Neither a TE nor hemorrhagic events occurred in patients who were AF-free and off warfarin | The authors continued warfarin in 29 patients without an AF recurrence due to the concern about undetected AF recurrences |
OACs, oral anticoagulants; AF, atrial fibrillation; TE, thromboembolic; TIA, transient ischemic attack; PV, pulmonary vein.
Conclusions
AF ablations have become more widely performed, and the strategy about long-term usage of OACs after catheter ablation is an important issue, especially for patients without obvious evidences of recurrences. CHADS2 and CHA2DS2-VASc scores could be used to identify patients at the risk of TE events after ablations who should continue OACs despite the status of recurrence. Patients with a high CHADS2 score are at a high risk of recurrence which could continuously occur after the catheter ablation without reaching a plateau. Therefore, it may not be safe to stop OACs for patients with a high risk score since the AF episodes are difficult to be detected after ablation procedures, but remain dangerous.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Wolf PA, Mitchell JB, Baker CS, et al. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998;158:229-34. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Blackshear JL, Shen WK, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 2001;37:371-8. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [DOI] [PubMed] [Google Scholar]
- 4.Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879-86. [DOI] [PubMed] [Google Scholar]
- 5.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32-8. [DOI] [PubMed] [Google Scholar]
- 6.European Heart Rhythm Association ; European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Walters TE, Halloran K, et al. Ten-year trends in the use of catheter ablation for treatment of atrial fibrillation vs. the use of coronary intervention for the treatment of ischaemic heart disease in Australia. Europace 2013;15:1702-9. [DOI] [PubMed] [Google Scholar]
- 9.Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006;114:759-65. [DOI] [PubMed] [Google Scholar]
- 10.Bunch TJ, Crandall BG, Weiss JP, et al. Warfarin is not needed in low-risk patients following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol 2009;20:988-93. [DOI] [PubMed] [Google Scholar]
- 11.Tao H, Ma C, Dong J, et al. Late thromboembolic events after circumferential pulmonary vein ablation of atrial fibrillation. J Interv Card Electrophysiol 2010;27:33-9. [DOI] [PubMed] [Google Scholar]
- 12.Yagishita A, Takahashi Y, Takahashi A, et al. Incidence of late thromboembolic events after catheter ablation of atrial fibrillation. Circ J 2011;75:2343-9. [DOI] [PubMed] [Google Scholar]
- 13.Chao TF, Lin YJ, Tsao HM, et al. CHADS(2) and CHA(2)DS(2)-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol 2011;58:2380-5. [DOI] [PubMed] [Google Scholar]
- 14.Lin YJ, Chao TF, Tsao HM, et al. Successful catheter ablation reduces the risk of cardiovascular events in atrial fibrillation patients with CHA2DS2-VASc risk score of 1 and higher. Europace 2013;15:676-84. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. [DOI] [PubMed] [Google Scholar]
- 17.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224-32. [DOI] [PubMed] [Google Scholar]
- 18.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornej J, Hindricks G, Kosiuk J, et al. Renal dysfunction, stroke risk scores (CHADS2, CHA2DS2-VASc, and R2CHADS2), and the risk of thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol 2013;6:868-74. [DOI] [PubMed] [Google Scholar]
- 20.Chao TF, Lin YJ, Chang SL, et al. R2CHADS2 score and thromboembolic events after catheter ablation of atrial fibrillation in comparison with the CHA2DS2-VASc score. Can J Cardiol 2014;30:405-12. [DOI] [PubMed] [Google Scholar]
- 21.Chao TF, Chen SA. Stroke risk predictor scoring systems in atrial fibrillation. Available online: http://www.jafib.com/published/published.php?id=998&is=current_issue&f=full [DOI] [PMC free article] [PubMed]
- 22.Ouyang F, Tilz R, Chun J, et al. Schmidt B,Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368-77. [DOI] [PubMed] [Google Scholar]
- 23.Chao TF, Ambrose K, Tsao HM, et al. Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm 2012;9:1185-91. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama T, Miyazaki S, Taniguchi H, et al. Six-year follow-up of catheter ablation in paroxysmal atrial fibrillation. Circ J 2013;77:2722-7. [DOI] [PubMed] [Google Scholar]
- 25.Chao TF, Tsao HM, Lin YJ, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol 2012;5:514-20. [DOI] [PubMed] [Google Scholar]
- 26.Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol 2012;60:1921-9. [DOI] [PubMed] [Google Scholar]
- 27.Chao TF, Cheng CC, Lin WS, et al. Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm 2011;8:1155-9. [DOI] [PubMed] [Google Scholar]
- 28.Letsas KP, Efremidis M, Giannopoulos G, et al. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace 2014;16:202-7. [DOI] [PubMed] [Google Scholar]
- 29.Verma A, Champagne J, Sapp J, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173:149-56. [DOI] [PubMed] [Google Scholar]
- 30.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120-9. [DOI] [PubMed] [Google Scholar]
- 31.Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010;55:735-43. [DOI] [PubMed] [Google Scholar]
- 32.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21. [DOI] [PubMed]


