Abstract
Atrial fibrillation (AF) is associated with significant morbidity and mortality related to stroke due to thromboembolism. Several novel oral anticoagulants (NOACs) have been developed that dose-dependently inhibit thrombin or activated factor X (factor Xa). These new agents offer potential advantages over vitamin K antagonists, however, several limitations exist. We will review the four large randomized trials comparing the efficacy and safety of new oral anticoagulants with warfarin for stroke prevention in patients with AF as well as assess “real world” data and discuss the limitations of the new agents.
Keywords: Anticoagulants, atrial fibrillation (AF), hemorrhage, stroke, warfarin
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with a lifetime risk exceeding 20% by 80 years of age (1). It is associated with significant morbidity and mortality related to stroke due to thromboembolism (2). Until 2009, warfarin and other vitamin K antagonists were the only class of oral anticoagulants available. Although these drugs are highly effective in the prevention of thromboembolism, their use is limited by the need for regular monitoring and the possibility of food and drug interactions. These limitations result in poor patient compliance and likely contribute to the underuse of vitamin K antagonists for stroke prevention (3,4).
Thus, a need arose for new anticoagulant agents that are effective, safe, and convenient to use. Several novel oral anticoagulants (NOACs) have been developed that dose-dependently inhibit thrombin or activated factor X (factor Xa) (5-8). The predictable anticoagulant effects of the NOACs enable the administration of fixed doses without the need for routine coagulation monitoring, thereby simplifying treatment. These new agents offer additional potential advantages over vitamin K antagonists, such as rapid onset and off set of action, absence of an effect of dietary vitamin K intake on their activity, and fewer drug interactions. Individually, the NOACs are at least as safe and effective as warfarin for prevention of stroke and systemic embolism in patients with AF (5-8). Despite this data, uptake has been slow and the majority of patients started on an anticoagulation for AF in the United States are still started on warfarin, largely by primary care physicians. But are the novel anticoagulants better than warfarin for patients with AF? We will review the four large randomized trials comparing the efficacy and safety of new oral anticoagulants with warfarin for stroke prevention in patients with AF as well as assess “real world” data and discuss the limitations of the new agents.
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly converted to the active direct thrombin inhibitor dabigatran (9). This conversion is carried out by a serum esterase that is independent of cytochrome P-450. It is administered in a fixed dose without laboratory monitoring. The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) was a randomized trial designed to compare two fixed doses of dabigatran, each administered in a blinded manner, with open-label use of warfarin [target international normalized ratio (INR), 2.0 to 3.0] in patients who had AF and were at increased risk for stroke (5). In this noninferiority trial, 18,113 patients were randomized. The median duration of the follow-up period was 2.0 years. The primary outcome was stroke or systemic embolism.
Both dabigatran doses were found to be noninferior to warfarin with respect to the primary efficacy outcome of stroke or systemic embolism. In addition, the 150-mg dose of dabigatran was found to be superior to warfarin with respect to stroke or systemic embolism, and the 110-mg dose was superior to warfarin with respect to major bleeding.
Rates of intracranial hemorrhage were significantly lower with both doses of dabigatran as compared to warfarin. Despite the overall lower rates of bleeding at other sites, there was an increase in the rate of gastrointestinal bleeding with the higher dabigatran dose. A low gastric pH is required to enhance absorption of dabigatran. Therefore, dabigatran capsules contain dabigatran-coated pellets with a tartaric acid core. This acidity may partly explain the increased incidence of dyspeptic symptoms with both dabigatran doses and the increased risk of gastrointestinal bleeding with the 150-mg dose (5).
The rate of myocardial infarction (MI) was also noted to be higher with both doses of dabigatran than with warfarin. Rates of MI were 0.82% and 0.81% with 110 and 150 mg of dabigatran, respectively, and 0.64% with warfarin (10). While this did not reach statistical significance, the explanation offered by the authors is that warfarin may provide better protection against coronary ischemic events than dabigatran, as warfarin is known to reduce the risk of MI (11). Whether thrombin inhibition contributes to the risk of MI is unclear. However, in a “real world” study published from the Danish Registry of Medicinal Product Statistics, the incidence of MI was lower with both dabigatran doses compared to warfarin (12).
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor. The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) was a multicenter, randomized, double-blind, double-dummy, event-driven trial (6). A total of 14,264 patients were randomized to receive either rivaroxaban (20 mg daily or 15 mg daily in patients with a creatinine clearance of 30 to 49 mL per minute) or dose-adjusted warfarin (target INR 2.0-3.0). These patients were at moderate-to-high risk for stroke. Elevated risk was indicated by a history of stroke, transient ischemic attack, or systemic embolism or at least two of the following risk factors: heart failure or a left ventricular ejection fraction of 35% or less, hypertension, an age of 75 years or more, or the presence of diabetes mellitus (i.e., a CHADS2 score of 2 or more, on a scale ranging from 1 to 6). The mean CHADS2 score was 3.47. The primary efficacy end point was the composite of stroke (ischemic or hemorrhagic) and systemic embolism. The principal safety end point was a composite of major and nonmajor clinically relevant bleeding events. The median follow-up period was 1.9 years.
Rivaroxaban was found to be noninferior to warfarin for the prevention of stroke or systemic embolism. There was no significant difference between rivaroxaban and warfarin with respect to rates of major or nonmajor clinically relevant bleeding. Bleeding that proved fatal or involved a critical anatomical site occurred less frequently in the rivaroxaban group, mainly because of lower rates of hemorrhagic stroke and other intracranial bleeding. In contrast, bleeding from gastrointestinal sites occurred more frequently in the rivaroxaban group, as did bleeding that led to a drop in the hemoglobin level or bleeding that required transfusion.
Apixiban
Apixaban is a direct oral factor Xa inhibitor with rapid absorption, a 12-hour half-life, and 25% renal excretion (13). The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial was a double-blind, double-dummy design randomizing patients to treatment with apixaban or dose-adjusted warfarin (target INR 2.0-3.0) (7). Apixiban 5 mg was administered twice daily; 2.5-mg doses were used in a subset of patients with two or more of the following criteria: an age of at least 80 years, a body weight of no more than 60 kg, or a serum creatinine level of 1.5 mg per deciliter or more. The primary objective was to determine whether apixaban was noninferior to warfarin in reducing the rate of stroke (ischemic or hemorrhagic) or systemic embolism among patients with AF and at least one other risk factor for stroke. The primary safety outcome was major bleeding. Superiority of apixaban compared to warfarin with respect to the primary outcome and to the rates of major bleeding and death from any cause was a secondary outcome. A total of 18,201 subjects were enrolled and the median duration of follow-up was 1.8 years.
The use of apixaban, as compared with warfarin, significantly reduced the risk of stroke or systemic embolism by 21%, major bleeding by 31%, and death by 11%. As compared with warfarin, apixaban was associated with a reduction in the rate of gastrointestinal bleeding and with consistently lower bleeding rates across age groups. This was the first study to demonstrate superiority as compared to warfarin in preventing stroke or systemic embolism, causing less bleeding, and resulting in lower mortality.
Edoxaban
Edoxaban is an oral, reversible, direct factor Xa inhibitor with a linear and predictable pharmacokinetic profile and 62% oral bioavailability (14). It achieves maximum concentrations within 1 to 2 hours, and 50% is excreted by the kidney (15). The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial was a three-group, randomized, doubleblind, double-dummy trial comparing two dose regimens of edoxaban with warfarin (8). The high-dose edoxaban group received 60 mg, and the low-dose group 30 mg. For patients in either group, the dose was halved if any of the following characteristics were present at the time of randomization or during the study: estimated creatinine clearance of 30 to 50 mL per minute, a body weight of 60 kg or less, or the concomitant use of verapamil, quinidine or dronedarone. The primary efficacy end point was the time to the first stroke (ischemic or hemorrhagic) or systemic embolic event. The principal safety end point was major bleeding during treatment. A total of 21,105 patients underwent randomization and the median follow-up was 2.8 years.
In this trial, both edoxaban regimens were noninferior to well-managed warfarin (median time in the therapeutic range, 68.4% of the treatment period) for the prevention of stroke or systemic embolic event. The rate of ischemic stroke was similar with high-dose edoxaban and warfarin but was higher with the low-dose edoxaban regimen. The incidence of hemorrhagic stroke and the rate of death from cardiovascular causes were significantly lower with both edoxaban regimens than with warfarin. As compared with warfarin, edoxaban was associated with consistently lower, dose-related rates of all types of bleeding, including major bleeding, intracranial bleeding, and life-threatening bleeding. The single exception was gastrointestinal bleeding, which occurred more frequently with high-dose edoxaban but less frequently with low-dose edoxaban than it did with warfarin.
Discussion
These four large randomized clinical trials have a number of similar conclusions. A recent meta-analysis of these trials aimed to assess the relative benefit of the new oral anticoagulants in key subgroups as well as determine the effects of these drugs on important secondary outcomes (16). Their results showed that stroke and systemic embolic events were significantly reduced in patients receiving new oral anticoagulants. This benefit was mainly driven by substantial protection against hemorrhagic stroke, which was reduced by half. The reasons for the potential reduction in intracranial hemorrhage that was associated with these agents are not clear, but one possibility is the effect on a single target in the hemostatic system by the new antithrombotic agents versus the multiple targets by warfarin (17). For the prevention of ischemic stroke, the new oral anticoagulants had similar efficacy to warfarin, which itself is very effective in this regard and reduces ischemic stroke by two-thirds compared with placebo (18).
In general, the new oral anticoagulants had a favorable safety profile compared with warfarin; however, they were associated with an increase in gastrointestinal bleeding. They were also associated with a significant reduction in all cause-mortality compared with warfarin. Only apixaban and low-dose edoxaban were associated with significant reductions in all cause-mortality, yet the point estimates for the hazard ratios for all drugs (and doses) are very similar. The results of the meta-analysis support the premise that compared with warfarin, the new oral anticoagulants, as a class, reduce all-cause mortality by about 10% in the populations enrolled in the clinical trials.
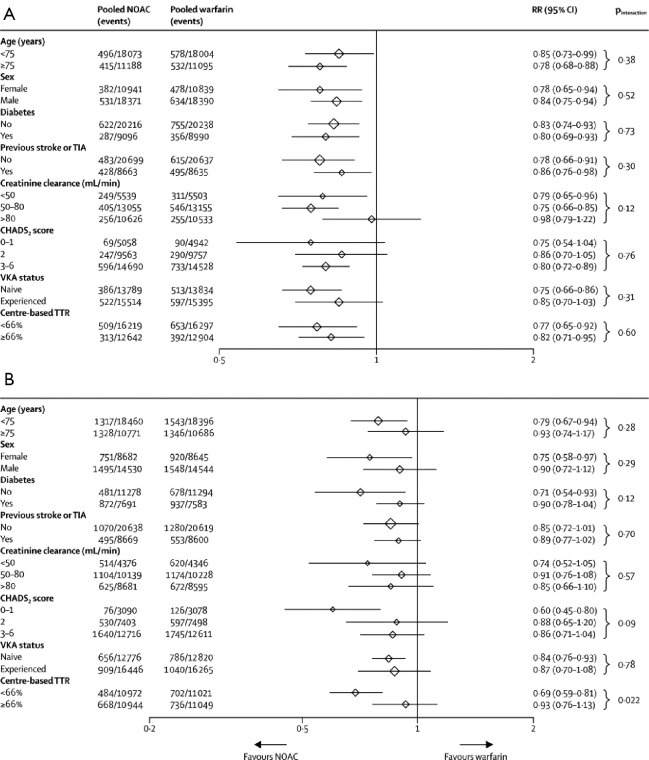
Perhaps more important is the ability of meta-analyses to enhance accuracy in assessment of the relative benefits of new oral anticoagulants in clinically relevant subgroups. Both risk of stroke and bleeding vary significantly across the range of patients with AF. Vulnerable populations, such as elderly people (aged ≥75 years) (19), patients with a previous history of stroke (20,21), and those with renal dysfunction (22,23), have an increased risk of both ischemic and bleeding events. Inclusion of these individuals in trials is variable and they are often underrepresented. Consequently, each trial alone can only offer partial reassurance that the overall balance of efficacy and safety is preserved in these high-risk groups. In this meta-analysis, the benefit of new oral anticoagulants compared with warfarin in reducing stroke or systemic embolic events was consistent across all subgroups examined (Figure 1). The safety of new oral anticoagulants compared with warfarin was generally consistent for the reduction of major bleeding across subgroups, with the exception of a significant interaction for time in therapeutic range (Figure 1). They noted a greater relative reduction in bleeding with new oral anticoagulants at centers that achieved a time in therapeutic range of less than 66% than at those achieving a time in therapeutic range of 66% or more.
Figure 1.
Stroke or systemic embolic events subgroups (A) and major bleeding subgroups (B). Data are n/N, unless otherwise indicated. No data available from RE-LY for the following major bleeding subgroups: sex, creatinine clearance, diabetes, and CHADS2 score. For ROCKET AF no major bleeding data available in the TTR and diabetes subgroup and major and non-major clinically relevant bleeding was used for subgroups of age, sex, CHADS2 score, and creatinine clearance. NOAC, new oral anticoagulant; RR, risk ratio; TIA, transient ischemic attack; VKA, vitamin K antagonist; TTR, time in therapeutic range. [Reproduced with permission from (16)].
After the introduction of NOACs into clinical practice, various reports of major, trauma-related, and fatal bleeding events were published, leading to cautionary recommendations from some regulatory authorities (24-27). Concerns were raised about an excess of bleeding events or MI among patients treated with NOACs compared to warfarin. A recently published cohort study assessed the efficacy and safety in an “everyday clinical practice” population of patients with AF treated with dabigatran etexilate after its post-approval availability in Denmark, compared with patients treated with warfarin (12). Rates of stroke, systemic embolism and major bleeding were similar with dabigatran (both doses) compared with warfarin. Mortality, intracranial bleeding, pulmonary embolism, and MI were lower with dabigatran, compared with warfarin. They found no evidence of an excess of bleeding events or MI among dabigatran-treated patients in this propensity-matched comparison against warfarin, even in the subgroup with ≥1-year follow-up, thus validating the clinical trial data in “everyday clinical practice”.
Despite the favorable data several limitations of the use of NOACs remain. As previously mentioned, populations such as the elderly and those with renal impairment are underrepresented in these studies and while the meta-analysis data is encouraging their use in these populations must be carefully considered. There is also a lack of information regarding the use of NOACs in pregnant women, pediatric patients and those with valvular disease. These areas require further investigation.
Another important concern that these clinical trials do not address is the absence of antidotes to rapidly reverse the anticoagulant effects of the NOACs in the case of life-threatening hemorrhage or surgery. Although no specific antidote for edoxaban is currently available, hemostatic agents such as prothrombin complex concentrate (PPSB-HT), activated prothrombin complex concentrate (Feiba), and recombinant factor VIIa (rFVIIa) have been shown to reverse its anticoagulant effect (28). Additional studies are currently underway to further investigate reversal agents for the individual NOACs (29,30). This limitation combined with the fact that there is no measurement to quantify the intensity of anticoagulation make their use in the perioperative period concerning.
Another limitation to the use of the NOACs is cost. Warfarin is expected to be markedly less expensive than the newer agents even after the costs associated with regular INR monitoring are considered. However, one analysis has suggested that dabigatran, as compared with warfarin, could be cost-effective in patients with AF (31). Additional data on cost-effectiveness are necessary and will likely further influence clinical decision making. For all of these reasons, although NOACs are attractive alternatives, it is likely that warfarin will continue to be used worldwide in many patients with AF.
So our answer to the question, “are the novel anticoagulants better than warfarin for patients with atrial fibrillation?” is a complex one. The NOACs overcome the need for routine blood monitoring, and the trial results have been encouraging overall and across important subgroups. Across four large studies with different populations of patients with AF, the direct thrombin and factor Xa inhibitors have been shown to have a more favorable bleeding profile than warfarin and are at least as efficacious. While it is difficult to understand why a practitioner would start warfarin in a new patient without a contraindication to a NOAC, switching to a newer agent may not be necessary for the patient in whom the INR has been well controlled with warfarin. In addition, although the newer anticoagulants have a more rapid onset and termination of anticoagulant action than does warfarin, agents to reverse the effect of the drugs are still under development and are not routinely available. So while a new era of anticoagulation is emerging, the decision to use a novel agent versus warfarin must be an individual one.
Acknowledgements
Disclosure: Peter Kowey has served as a consultant for Boehringer-Ingelheim, Bristol-Myers Squibb, Pfizer, Merck, Daiichi-Sankyo, Johnson & Johnson, and Portola. The authors declare no conflict of interest
References
- 1.Magnani JW, Rienstra M, Lin H, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation 2011;124:1982-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003;349:1019-26. [DOI] [PubMed] [Google Scholar]
- 3.Birman-Deych E, Radford MJ, Nilasena DS, et al. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke 2006;37:1070-4. [DOI] [PubMed] [Google Scholar]
- 4.Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689-96. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J 2009;157:805-10, 810.e1-2. [DOI] [PubMed]
- 10.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med 2010;363:1875-6. [DOI] [PubMed] [Google Scholar]
- 11.Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002;347:969-74. [DOI] [PubMed] [Google Scholar]
- 12.Larsen TB, Rasmussen LH, Skjøth F, et al. Efficacy and safety of dabigatran etexilate and warfarin in "real-world" patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol 2013;61:2264-73. [DOI] [PubMed] [Google Scholar]
- 13.Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009;37:74-81. [DOI] [PubMed] [Google Scholar]
- 14.Matsushima N, Lee F, Sato T, et al. Bioavailability and safety of the factor Xa inhibitor edoxaban and the effects of quinidine in healthy subjects. Clin Pharm Drug Dev 2013;2:358-66. [DOI] [PubMed] [Google Scholar]
- 15.Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol 2010;50:743-53. [DOI] [PubMed] [Google Scholar]
- 16.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363-72. [DOI] [PubMed] [Google Scholar]
- 18.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. [DOI] [PubMed] [Google Scholar]
- 19.Hughes M, Lip GY, Guideline Development Group, National Clinical Guideline for Management of Atrial Fibrillation in Primary and Secondary Care, National Institute for Health and Clinical Excellence . Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost 2008;99:295-304. [DOI] [PubMed] [Google Scholar]
- 20.Stroke Risk in Atrial Fibrillation Working Group . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology 2007;69:546-54. [DOI] [PubMed] [Google Scholar]
- 21.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100. [DOI] [PubMed] [Google Scholar]
- 22.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009;119:1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224-32. [DOI] [PubMed] [Google Scholar]
- 24.Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012;125:669-76. [DOI] [PubMed] [Google Scholar]
- 25.Truumees E, Gaudu T, Dieterichs C, et al. Epidural hematoma and intraoperative hemorrhage in a spine trauma patient on Pradaxa (dabigatran). Spine (Phila Pa 1976) 2012;37:E863-5. [DOI] [PubMed] [Google Scholar]
- 26.Legrand M, Mateo J, Aribaud A, et al. The use of dabigatran in elderly patients. Arch Intern Med 2011;171:1285-6. [DOI] [PubMed] [Google Scholar]
- 27.Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother 2012;46:e10. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda T, Honda Y, Kamisato C, et al. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost 2012;107:253-9. [DOI] [PubMed] [Google Scholar]
- 29.Available online: http://clinicaltrials.gov/ct2/show/NCT01688830
- 30.Available online: https://clinicaltrials.gov/ct2/show/NCT01955720
- 31.Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1-11. [DOI] [PubMed] [Google Scholar]