Abstract
Recent research and publication of various landmark trials have led to the approval and subsequent use of novel oral anticoagulants (NOACs) in clinical practice. The use of these newer agents for anticoagulation offers several benefits such as greater specificity, relatively rapid onset and offset of action and a predictable pharmacological profile as compared to warfarin. With the increasing use of these agents, several key issues ranging from appropriate selection to management of complications and considerations for concurrent procedures (cardioversion and catheter ablation) have also emerged. The timing of interruption of anticoagulants prior to catheter ablation and re-initiation after the procedure to minimize the peri-procedural thromboembolism risk without increasing the bleeding risk is of key relevance in electrophysiology practice. The use of NOACs in patients undergoing catheter ablation and cardioversion also requires special considerations based on the pharmacological properties of the individual agent and the presence of comorbidities such as renal and or hepatic impairment. In this review we aim to discuss the practical considerations with the use of NOACs in the setting of cardioversion and catheter ablation based on the currently available data.
Keywords: Novel oral anticoagulants (NOACs), atrial fibrillation (AF), cardioversion, catheter ablation
Introduction
Atrial fibrillation (AF) poses a significant challenge from a public health perspective. AF is associated with a 5-fold increased risk of stroke; it is also observed that strokes in patients with AF are associated with a greater disability and mortality as compared to the patients in sinus rhythm (SR) (1-3). The incidence of AF is expected to increase with the ageing population and therefore is intuitive to believe that there will be a parallel increase in the incidence of stroke and systemic embolism (4). Oral anticoagulation therapy with warfarin has been the mainstay for the prevention of stroke and systemic embolism in the patients with AF; however the limitations associated with the use of warfarin have posed a significant challenge for its use in clinical practice. Limitations of warfarin particularly, inter and intra-individual variations in therapeutic levels, drug-drug interactions and frequent need of internationalized therapeutic ratio (INR) testing have led to a significant research effort in the development of novel oral anticoagulants (NOACs). The publication of various landmark trials has led to the approval of dabigatran, rivaroxaban and apixaban for thromboprophylaxis in the patients with AF. With the increased use of the NOACs in routine clinical practice, several practical issues ranging from appropriate patient selection, dose adjustments in the setting of renal impairment and considerations for cardioversion and catheter ablation have emerged. Cardioversion is associated with a modest but definite risk of thrombotic complications in the patients with AF. Catheter ablation for AF is also associated with a peri-procedural thromboembolic and bleeding risk. The use of NOACs in the setting of cardioversion and catheter ablation should involve an approach based on the published data, patient comorbidities and procedural factors.
Practical considerations for the use of NOACs in clinical practice
According to the consensus based on the currently published recommendations by the European Society of Cardiology (ESC) and the American Heart Association (AHA), the use of one of the NOACs should be considered in most patients with AF as an alternative to an adjusted-dose vitamin K antagonist (VKA) (5,6). After the approval of dabigatran, the American College of Cardiology Foundation (ACCF)/AHA/Heart Rhythm Society (HRS) task force released an update on the prevention of stroke and systemic embolism in the patients with non-valvular AF. This update recommended that in AF patients without coexisting hemodynamically significant valvular disease or presence of a prosthetic valve, dabigatran can be considered as a useful alternative to warfarin for thromboprophylaxis (class I, level of evidence: B) (Tables 1,2). Due to the heterogeneity of various landmark clinical trials and lack of data on head-to-head comparison of the NOACs it is difficult to recommend one agent over the other.
Table 1. Latest ACCF/AHA/HRS recommendations compared with ESC, ACCP, and CCS recommendations for the use of dabigatran in patients with non-valvular AF.
| NOAC | ACCF/AHA/HRS | ESC | ACCP | CCS |
|---|---|---|---|---|
| Dabigatran | An alternative to warfarin in patients without prosthetic heart valves or hemodynamically significant valvular disease, renal failure, or advanced liver disease (impaired baseline clotting function); 150 mg twice daily in patients with CrCl >30 mL/min; 75 mg twice daily in patients with CrCl 15-30 mL/min (class I; level of evidence B) | NOACs in preference to warfarin (class IIa: level of evidence A) Dabigatran 150 mg bid for most patients; 110 mg bid for patients >80 years old, concomitant use of interacting drugs (e.g., verapamil), HAS-BLED score ≥3, or in patients with CrCl 30-49 mL/min (class IIa; level of evidence B) |
150 mg bid rather than VKA, except for patients with AF and mitral stenosis, stent, or CHADS2 ≥1 who experience ACS (grade 2B) | NOACs in preference to VKAs Dabigatran 150 mg bid preferable to 110 mg bid, except in certain patients (e.g., patients with low body weight, decreased renal function, or at increased risk of major bleeding) |
ACCF, American College of Cardiology Foundation; AHA, American Heart Association; HRS, Heart Rhythm Society; ESC, European Society of Cardiology; ACCP, American College of Chest Physicians; CCS, Canadian Cardiovascular Society; NOAC, novel oral anticoagulant; VKA, vitamin K antagonist.
Table 2. ESC recommendations for the use of NOACs in patients with non-valvular AF for prevention of stroke and systemic embolism.
| Recommendations for prevention of thromboembolism in non-valvular AF-NOACs | Classψ | Levelɸ |
|---|---|---|
| When adjusted-dose VKA (INR 2-3) cannot be used in a patient with AF where an OAC is recommended, due to difficulties in keeping within the therapeutic anticoagulation, experiencing side effects of VKAs, or inability to attend or undertake INR monitoring, one of the NOACs, either: | I | B |
| A direct thrombin inhibitor (dabigatran); or | ||
| An oral factor Xa inhibitor (e.g., rivaroxaban, apixaban) is recommended | ||
| Where OAC is recommended, one of the NOACs, either: | IIa | A |
| A direct thrombin inhibitor (dabigatran); or | ||
| An oral factor Xa inhibitor (e.g., rivaroxaban, apixaban) should be considered rather than adjusted-dose VKA (INR 2-3) for most patients with non-valvular AF, based on their net clinical benefit | ||
| Where dabigatran is prescribed, a dose of 150 mg bid should be considered for most patients in preference to 110 mg bid with the latter dose recommended in: | IIa | B |
| Elderly patients, age >80 yrs | ||
| Concomitant use of interacting drugs (e.g., verapamil) | ||
| High bleeding risk (HAS-BLED score ≥3) | ||
| Moderate renal impairment (CrCl 30-49 mL/min) | ||
| Where rivaroxaban is being considered, a dose of 20 mg o.d. should be considered for most patients in preference to 15 mg o.d., with the latter dose recommended in | IIa | C |
| High bleeding risk (HAS-BLED score ≥3) | ||
| Moderate renal impairment (CrCl 30-49 mL/min) | ||
| Baseline and subsequent regular assessment of renal function (by CrCl) is recommended in patients following the initiation of any NOAC, which should be done but more frequently in those with moderate renal impairment where CrCl should be assessed 2-3 times per year | IIa | B |
| NOACs are not recommended in patients with severe renal impairment (CrCl <30 mL/min) | III | A |
ΨClassification/grading of recommendation: class I, evidence and/or general agreement that a given treatment is beneficial, useful and effective; class II, conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure; class IIa, weight of the evidence/opinion is in favor of usefulness/efficacy; class IIb, usefulness/efficacy is less well established by evidence/opinion; class III, evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases could be harmful. ɸLevel/quality of evidence: A (high), data derived from multiple randomized clinical trials or meta-analyses; B (moderate), data derived from a single randomized clinical trial or large nonrandomized studies; C (low), consensus of opinion of experts and/or small studies, retrospective studies, registries. ESC, European Society of Cardiology; NOACs, novel oral anticoagulants; AF, atrial fibrillation; VKA, vitamin K antagonist; INR, internationalized therapeutic ratio.
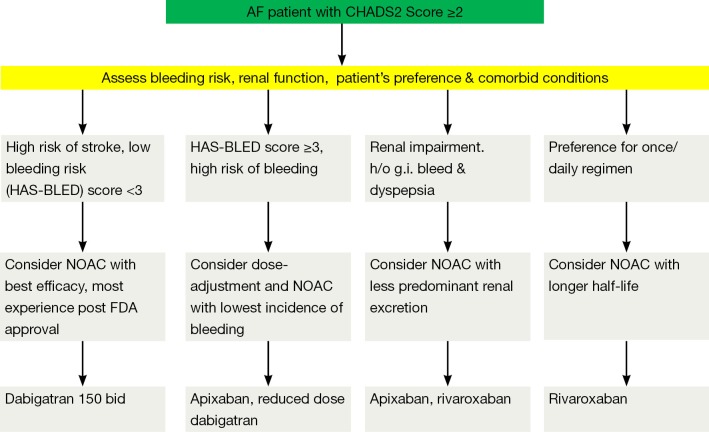
Several practical considerations can be helpful in the selection of an appropriate agent for an individual patient (Figure 1). The use of dabigatran might be less suitable in the patients with propensity to develop dyspepsia/gastrointestinal bleeding and those with a substantial risk of coronary events. There is also a consensus for reduction of dose of dabigatran in the patients with impaired renal function and low body weight. In routine clinical practice, prior to the initiation of dabigatran it is recommended that renal function [by measuring creatinine clearance calculated using the Cockcroft-Gault formula (7)] should be assessed in all patients. The assessment of renal function at baseline and during follow-up is also relevant in the treatment with other NOACs considering the renal elimination of the active bioavailable drug of dabigatran, rivaroxaban and apixaban is estimated to be 80%, 33% and 27% respectively (8-10). The European Society of Hematology 2012 guidelines recommend the assessment of renal function (CrCl) prior to the initiation of all NOACs. In the patients with normal (CrCl ≥80 mL/min) or mild (CrCl 50-79 mL/min) renal impairment, renal function should be assessed annually and in the patients with moderate (CrCl 30-49 mL/min) a more frequent assessment (2-3 times/year) should be performed (11).
Figure 1.
A proposed algorithm to aid in the selection of NOACs in the patients with non-valvular AF for prevention of stroke and systemic embolism.
It is also pertinent to acknowledge that the patients with severe impairment of renal function were excluded from the landmark clinical trials [patients with a CrCl of <30 mL/min were excluded from the RE-LY trial (12) and CrCl <25 mL/min in the ROCKET-AF (13) and ARISTOTLE (14) trials]. In the ROCKET-AF trial, the dose of rivaroxaban was reduced to 15 mg once daily in the patients with moderate renal impairment (defined by CrCl of 30-49 mL/min), similarly in the ARISTOTLE trial, moderate renal impairment (defined by serum creatinine ≥1.5 mg/dL) was one of the factors considered for administration of a reduced dose of apixaban (2.5 mg bid instead of 5 mg bid). In the post-hoc analysis of the ROCKET-AF trial, impaired renal function was also identified to be an independent risk factor for an increased risk of bleeding (14). Analyses from the three major landmark trials observed a greater number of thromboembolic and major bleeding events in the patients with renal dysfunction as compared to patients with normal renal function; this effect was observed in both the NOAC arm as well as warfarin arm (12-16).
Patient’s age should also be an important consideration while recommending the use of NOACs. In the RE-LY trial the risk of bleeding in the dabigatran arm was significantly influenced by the patient’s age. In younger patients (age <75 years) both the doses of dabigatran were observed to reduce the risk of bleeding as compared to warfarin [RR for bleeding in dabigatran 110 and 150 mg arm were 0.62 (95% CI: 0.50-0.77) and 0.70 (95% CI: 0.57-0.86)], however in the patients older than 75 years, the bleeding risk was similar for dabigatran 110 mg vs. warfarin (RR: 1.01; 95% CI: 0.83-1.23) and showed a higher trend in the dabigatran 150 mg arm (RR: 1.18; 95% CI: 0.98-1.42) (17).
Cost-effectiveness of NOACs over warfarin is another important factor which can affect patient preference and compliance in routine clinical practice. Although there is promising data which favor the cost-effectiveness of NOACs as compared to warfarin (18-20), but patients might be hesitant to switch to NOACs considering the increased “out-of-pocket” expense. Results from a single-center based study showed that if the patients with non-valvular AF were switched from warfarin to dabigatran then the hospital’s overall expense increased by $3 million attributed to the drug-cost alone (21). It is also important to acknowledge that the current data on cost-efficacy of various NOACs is predominantly derived from the three landmark clinical trials. In future as the use of NOACs is expected to increase in the routine clinical practice and data from such real-world based cohorts will yield further insights regarding the cost-efficacy of each of the individual NOACs.
Another important factor which is particularly relevant in the AF patients is the effect of interaction with other drugs. Considering the epidemiological observation that AF coexists with other common cardiovascular conditions, it is relatively common for these patients to be taking antiarrhythmics, antiplatelet and rate control medications. These drug-drug interactions between these agents and NOACs should be kept in mind. Although dabigatran carries an overall low potential for interaction with other drugs, caution is recommended if the patients are concomitantly taking the drugs which are likely to inhibit the activity of the permeability glycoprotein (P-gp). Dabigatran should not be administered with any P-gp inhibitor if the patient also has coexisting moderate renal impairment. Special attention should be also paid if the patient is also taking verapamil (increased bleeding risk of dabigatran) and proton pump inhibitor (decreased efficacy of dabigatran) (22). The drug-metabolism of rivaroxaban is at least partly mediated by several cytochrome P450 enzymes (CYP3A4/5 and CYP2J2) (23). Intuitively the co-administration of strong inducers of these cytochrome enzyme systems (such as ketoconazole, ritonavir) can lead to a significant increase in the anticoagulant effect of rivaroxaban. Based on the current data, it is recommended that the co-administration of rivaroxaban with strong inhibitors of both CYP3A4 and P-gp should be avoided (24). The CYP3A4 system also plays a significant role in the metabolism of apixaban; also apixaban is a substrate of P-gp. Therefore the co-administration of apixaban with drugs which strongly inhibit both CYP3A4 and P-gp is not recommended (25). No dose-adjustment for apixaban is required if it is co-administered with relatively less potent inhibitors of CYP3A4 and/or P-gp (e.g., amiodarone, naproxen, diltiazem, and verapamil) (Table 3).
Table 3. Summary of clinically relevant drug-drug interactions with the use of NOACs in clinical practice.
| NOACs | Co-administered drugs | Comments/recommendations |
|---|---|---|
| Dabigatran | Strong inhibitors of P-gp | Dabigatran dose should be reduced to 75 mg bid if co-administration is planned in presence of moderate renal impairment (Crcl 30-50 mL/min) |
| Less potent inhibitors of P-gp | No dose-adjustment is recommended | |
| P-gp inducers | Co-administration is not recommended | |
| Rivaroxaban | Strong inhibitors of P-gp and CYP3A4 | Co-administration not recommended, risk of increased bleeding risk |
| Less potent inhibitors of P-gp and CYP3A4 | No dose-adjustment is recommended | |
| Strong inducers of CYP3A4 and/or P-gp | Co-administration is recommended with caution, leads to decreased effect of rivaroxaban | |
| Apixaban | Strong dual inhibitors of P-gp and/or CYP3A4 | Recommended to reduce the dose to 2.5 mg bid If the patient is already taking apixaban 2.5 mg bid, avoid co-administration of the strong dual P-gp and CYP3A4 inhibitors |
| Less potent inhibitors of CYP3A4 and/or P-gp | No dose-adjustment is recommended | |
| Strong inducers of P-gp and/or CYP3A4 | Avoid the concomitant use of these agents to prevent decrease in the efficacy of apixaban |
(I) Strong inhibitors of P-gp and/or CYP3A4 (e.g., ketoconazole, itraconazole, ritonavir, clarithromycin); (II) less potent inhibitors of CYP3A4 and/or P-gp (e.g., amiodarone, diltiazem, verapamil); (III) strong inducers of P-gp and/or CYP3A4 (e.g., rifampin, carbamazepine, phenytoin, St. John Wort). AF, atrial fibrillation; NOACs, novel oral anticoagulants; P-gp, permeability glycoprotein.
Temporary interruption of NOACs
For the patients anticipated to undergo catheter ablation, it is important to evaluate the aspect of temporary interruption of NOACs prior to the procedure. Currently available literature of investigations of clinical outcomes after temporary interruption of NOACs in clinical setting remains limited. The duration of interruption of anticoagulation is also particularly relevant especially to balance the risk of bleeding and stroke in the peri-procedural period. A post-hoc analysis of the RE-LY trial by Healey et al. reported key data regarding the pre-procedural discontinuation of dabigatran in the patients anticipated to undergo an invasive procedure. In the dabigatran arm, the last dose of the drug was given 49 [35-85] hours prior to an elective invasive procedure as compared to the 114 [87-144] hours in the warfarin arm. There were no significant differences in the rate of major bleeding between the patients receiving dabigatran 110 mg (3.8%) or dabigatran 150 mg (5.1%) or warfarin (4.6%). Dabigatran 110 mg vs. warfarin RR: 0.83; 95% CI: 0.59-1.17, P=0.28, dabigatran 150 mg vs. warfarin RR: 1.09, 95% CI: 0.80-1.49, P=0.58. These results suggest that the use of dabigatran could have facilitated a shorter interruption of anticoagulation (26). The guidance in the package insert approved by the U.S. Food and Drug Administration (FDA) also provides useful recommendations for the interruption of dabigatran based on the renal function (27): “If possible, discontinue pradaxa 1 to 2 days (CrCl ≥50 mL/min) or 3-5 days (CrCl ≤50 mL/min) before invasive or surgical procedures because of an increased risk of bleeding”.
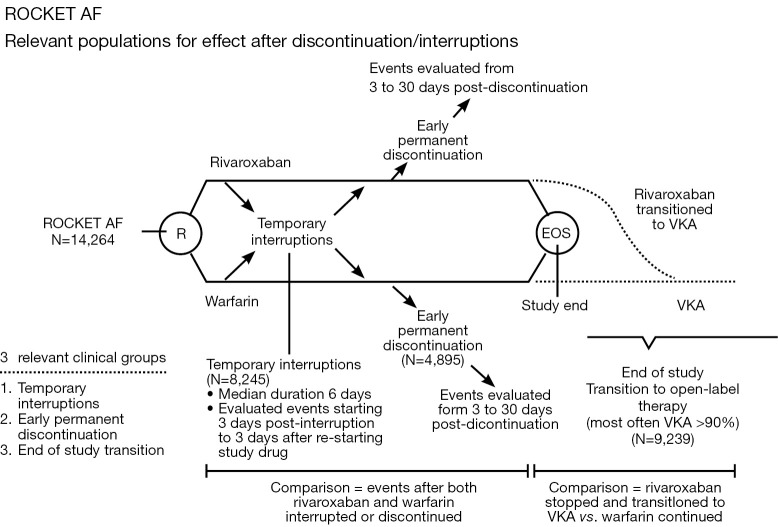
Patel et al. report data from a post-hoc analysis of the ROCKET AF trial which evaluated the incidence of stroke, systemic embolism and thrombotic events in patients who had either a temporary interruption (defined as any interruption of more than 3 days) or an early permanent study drug discontinuation and all patients who completed the clinical trial and transitioned to an open-label therapy (study design explained in Figure 2). The most common reasons for temporary discontinuation of the study drugs were either surgical or invasive procedures (38.2%) or adverse effects (40.2%). The rate of stroke or non-CNS embolism was observed to be similar after temporary interruption of rivaroxaban and warfarin (rivaroxaban, n=9, warfarin: n=8, 6.20 vs. 5.05 per 100 patient-years, HR: 1.28, 95% CI: 0.49-3.31, P=0.62). Similar findings were observed after an early permanent discontinuation in both arms (rivaroxaban: n=42, warfarin: n=36, 25.60 vs. 23.28 per 100 patient-years, HR: 1.10, 95% CI: 0.71-1.72, P=0.66). Contrary to these observations, the incidence of stroke was significantly higher in the rivaroxaban treated patients who were transitioned to an open-label VKA therapy at the completion of the study as compared to the patients in the warfarin arm (n=22 in rivaroxaban vs. warfarin treated patients: n=6, HR: 3.72, 95% CI: 1.51-9.16, P=0.0044) (28). The investigators also observed that more than 60% of the warfarin-treated patients completing the study had a therapeutic INR (2.0-3.0) at first protocol-mandated check at 3 days. Comparatively, less than 50% of the rivaroxaban-treated patients who were transitioned to an open-label VKA (predominantly warfarin) achieved the therapeutic range INR. This observation of subtherapeutic INR in the rivaroxaban arm could partly contribute to the increased incidence of stroke during the transition to warfarin at the completion of the study. Although the mechanism for this delay in the achievement of a therapeutic INR during the period of transition from rivaroxaban to warfarin is difficult to explain; but suggests that if feasible, interruptions in the anticoagulation therapy for such patients should be kept at a minimum in routine clinical practice. Considering the lack of robust data specifically addressing this aspect, these findings also warrant a careful consideration during the period of transition from rivaroxaban to warfarin.
Figure 2.
Figure demonstrating the ROCKET AF study flow with relevant populations for effect after discontinuation/interruptions. EOS, end of study; VKA, vitamin K antagonists [Reproduced with permission from (28)].
Current data on studies specifically addressing the duration and safety of pre-procedural interruption of apixaban remain limited. In the absence of robust data, an individualized approach (based on the risk of thromboembolism, bleeding and presence of comorbidities) seems to be acceptable. The guidelines listed in the FDA approved package insert recommend that “Eliquis should be discontinued at least 48 hours prior to the elective surgical or invasive procedures with a moderate to high risk of clinically significant bleeding”. For the procedures which entail a relatively lower risk of bleeding, discontinuation of apixaban 24 hours prior to the procedure seems to be acceptable. For a discontinuation period of 24-48 hours, bridging therapy with heparin is not recommended (25), whereas in patients with a longer interruption (>48 hours to 5 days: such as in the setting of a procedure with a high risk of bleeding in the presence of renal and/or hepatic impairment), it might be preferable to use the bridging therapy with heparin (29).
Monitoring of anticoagulant effect of NOACs
Accurate assessment of the anticoagulant effect of NOACs could be especially relevant in the immediate pre-procedural phase to determine the timing of the procedure. Monitoring is also particularly relevant in the assessment of pre-procedural bleeding risk and the management of procedure-related bleeding complications. Until robust clinical data becomes available regarding the use of accurate coagulation assays to assess the anticoagulant effect of NOACs, the conventional coagulation tests can be used as a screening tool. A recent study by Hawes et al. investigated the role of various coagulation assays for the measurement of dabigatran’s anticoagulant effect. The investigators reported that prothrombin time (PT) was not sensitive to detect subtherapeutic and therapeutic levels of dabigatran. However, a prolongation in PT was observed at plasma dabigatran concentration above the 75th percentile. Based on these results, PT can be considered for ruling out supratherapeutic levels of dabigatran, but its utility in measurement of therapeutic levels of dabigatran cannot be recommended (30).
Activated partial thromboplastin time (aPTT) appears to be more sensitive than PT to the action of dabigatran. aPTT follows a curvilinear dose-response relationship with dabigatran, with a steep increase at low concentrations and linearity above dabigatran concentration of 200 ng/mL (31,32). A normal aPTT is likely to exclude the therapeutic anticoagulation effect of dabigatran. Activated clotting time (ACT) which is routinely utilized for measurement of anticoagulation effect during catheter ablation exhibits a flatter dose-response curve with dabigatran and therefore is limited in its utility in this setting (33). Thrombin time (TT) is sensitive to the lower plasma concentration of dabigatran and also displays a linear dose-response to dabigatran. Based on these two properties, it seems to be a promising option as an accurate screening assay for the anticoagulant effect of dabigatran (32,33).
The anticoagulant effect of rivaroxaban seems to be best estimated by the use of PT. PT follows a linear concentration-response relationship over a broad range of drug concentration. PT also seems to have a higher sensitivity to the concentration of rivaroxaban as compared to the aPTT (34). It is important to emphasize that the use of PT for assessment of anticoagulation effect of rivaroxaban is dependent on the type of PT reagent used. For a laboratory which predominantly intends to use PT for the assessment of anticoagulation effect of rivaroxaban, the sensitivity of a known reagent should be standardized. Chromogenic substrate based anti FXa assays can also be utilized to quantify the effect of rivaroxaban (34-36). An approach based on the combination of PT and the chromogenic substrate based anti FXa assay can be utilized in clinical practice to estimate the effect of rivaroxaban.
Current data is limited regarding the role of various coagulation assays for the assessment of anticoagulation effect of apixaban. As a result of the inhibition of Factor Xa, apixaban leads to prolongation of PT and aPTT but this effect seems to be variable based on the reagent used (37). The poor sensitivity of PT for estimation of anticoagulant effect of apixaban limits its use in clinical setting to exclude the drug-effect of apixaban. A dilute prothrombin time (dPT) achieved by diluting the thromboplastin reagent in 100 mmol/L CaCl2 has also been observed to be more sensitive to the anticoagulant effect of apixaban, however there is still a lack of robust data on its utility for the measurement of anticoagulant effect of apixaban (38). Similar to the use of chromogenic FXa assays for rivaroxaban, these tests also have some utility for the measurement of anticoagulant effect of apixaban. Different chromogenic assays either followed a linear or an exponential drug concentration-response relationship depending on the reagent and the methodology used. Until further data become available the use of these assays remains limited to experimental settings (Table 4).
Table 4. Role of various coagulation assays in detection of the anticoagulant effect of NOACs.
| Coagulation assay | Dabigatran | Rivaroxaban | Apixaban | Comments |
|---|---|---|---|---|
| PT | Lacks sensitivity to detect therapeutic levels, prolonged with supratherapeutic levels | Linear concentration-response curve over a broad range but marked variability between PT reagents | Prolonged, but poor correlation with apixaban concentration Dilute prothrombin time (dPT) may be used to increase sensitivity of standard PT§ |
Can be considered to exclude the supratherapeutic levels of dabigatran Normal PT can be used to exclude the anticoagulant effect of rivaroxaban Not recommended to assess the effects of apixaban |
| aPTT | More sensitive than PT to anticoagulant effect, however the dose-response relationship is lost at concentrations >200 ng/mL | Curvilinear dose-response; poor correlation particularly with higher drug concentration | Concentration-response plateaus after 200 ng/mL, limits utility as drug levels increase | Might be useful to detect supratherapeutic levels of dabigatran Inferior than PT to assess the effect of rivaroxaban Not recommended to assess the effects of apixaban |
| TT | Correlates well with the dabigatran concentration* Normal levels suggest either absence or minimal plasma levels of dabigatran |
Not affected | Not affected | Might be used a screening tool to exclude the anticoagulant effect of dabigatran |
| ACT | Follows a flatter dose-response curve, limited utility | Limited data | Limited data | Might offer quantitative benefit over the use of aPTT for dabigatran, but clinical data remain limited |
| Chromogenic FXa assay | Not applicable | Offers benefit of quantitative estimation of rivaroxaban | Linear to exponential concentration-response | Could be used in conjunction with PT to estimate the effect of FXa inhibitors |
§, dilution of prothrombin time achieved by diluting the thromboplastin reagent in 100 mmol of CaCl2; *, the use of TT assay depends on the coagulometer and also on the reagent (thrombin) used for measurement. NOACs, novel oral anticoagulants; PT, prothrombin time; aPTT, activated partial thromboplastin time; TT, thrombin time; ACT, activated clotting time.
Cardioversion in patients on NOACs
Cardioversion (both electric and pharmacological) has been associated with an increased risk of thromboembolism, particularly if the patients have not been adequately anticoagulated (39-41). Current guidelines recommend anticoagulation for at least 3 weeks prior to and 4 weeks after cardioversion for patients with AF of unknown duration or duration >48 hours (42,43). Considering the advent of NOACs in clinical practice is still relatively new, there is a current paucity of data regarding the use of these agents in the setting of cardioversion.
Dabigatran for cardioversion
Nagrakanti et al. reported the largest cardioversion experience based on a post hoc analysis of the RE-LY trial. A total of 1,983 cardioversions were performed on 1,270 patients in the RE-LY trial (647, 672 and 664 in the dabigatran 110, 150 mg and warfarin arm respectively). No statistically significant differences were reported in the incidence of stroke and systemic embolism in between these three groups (0.77%, 0.30% and 0.60% in the dabigatran 110, 150 mg and warfarin arm respectively, dabigatran 110 mg vs. warfarin, P=0.71 and dabigatran 150 mg vs. warfarin, P=0.45). It is also worth pointing out that the transesophageal echocardiography (TEE) was performed in a higher proportion of patients in the dabigatran arm as compared to the warfarin arm (24-26% vs. 13%). However, the rate of stroke and systemic embolism were also similar in both conventional and TEE-guided cardioversions. These findings suggest that it might be reasonably safe to perform cardioversion in patients on dabigatran without a pre-cardioversion TEE (44).
Rivaroxaban for cardioversion
A recently published post-hoc analysis of the ROCKET AF trial by Piccini et al. compared the 30-day outcomes of systemic embolism and bleeding in the patients randomized to the rivaroxaban and warfarin study arms. A total of 147 and 138 cardioversions were performed in the respective drug groups. The investigators reported a relatively similar incidence of stroke and systemic embolism (1.88% in rivaroxaban arm vs. 1.86% in warfarin arm, P=NS) and overall bleeding events (18.75% in rivaroxaban arm vs. 13.04% in warfarin arm, P=NS*, *: final analysis also included a minor percentage of patients who underwent catheter ablation) (45). These results indicate that rivaroxaban can be a safer alternative to warfarin in the patients in the setting of cardioversion. The use of rivaroxaban may also offer the potential benefit of a steady anticoagulation effect which also can also reduce the requirement of anticoagulation bridging in the post-cardioversion period.
Apixaban for cardioversion
A recently published study by Flaker et al. described the post-hoc analysis from the Aristotle trial. A total of 743 electric cardioversions were performed in 540 patients, the minimum duration of therapy prior to cardioversion was 4 days in the warfarin arm vs. 1 day in the apixaban arm. After a follow up period of 30 days, a similar incidence of major bleeding was reported in both the study arms (0.3% in apixaban arm vs. 0.2% in the warfarin arm, P=NS). No stroke and systemic embolism events occurred in either of the study arms (46). This analysis also reported a similar 30-day mortality in both arms (2 in apixaban arm and 2 in warfarin arm, P=NS) (Table 5).
Table 5. Comparison of bleeding and thromboembolism rates from the post-hoc analysis of the major clinical trial comparing NOACs and warfarin in the setting of cardioversion.
| Drug trial | Total number of cardioversions | Bleeding incidence | Thromboembolism incidence |
|---|---|---|---|
| RE-LY (44) | 647 in D 110 mg arm 672 in D 150 mg arm 664 in warfarin arm |
Major bleeding: 1.7% in D 110 mg arm, 0.6% in D 150 mg arm, 0.6% in warfarin arm D 110 mg vs. warfarin, P=0.06 D 150 mg vs. warfarin, P=0.99 |
0.8% in D 110 mg arm, 0.3% in D 150 mg arm and 0.6% in warfarin arm D 110 mg vs. warfarin, P=0.71 D 150 mg vs. warfarin, P=0.40 |
| ROCKET-AF (45) | 147 in rivaroxaban arm 138 in warfarin arm |
18.75% in rivaroxaban arm vs. 13.04% in warfarin arm, P=NSɅ | 1.88% in rivaroxaban arm vs.1.86% in warfarin arm, P=NS |
| ARISTOTLE (46) | 743 in 540 patients | Major bleeding: 0.3% in apixaban arm vs. 0.2% in warfarin arm, P=NS | None in each drug arm |
Ʌ, the investigators reported the composite of major and non-major clinically relevant bleeding. NOACs, novel oral anticoagulants.
Based on these data, the use of NOACs appears to be a reasonable alternative to warfarin in the patients planning to undergo cardioversion. The rapid onset of action of NOACs also offers the potential benefit of their initiation in the outpatient setting and can potentially reduce the rate of hospitalization and associated costs.
Catheter ablation in the patients with NOACs
Catheter ablation is associated with a transiently increased thrombogenic state, at least in the immediate peri- and post-procedural period. This effect has been attributed to an increased atrial inflammatory state, endothelial denudation, local trauma and char formation secondary to the introduction of ablation catheters and creation of ablation lines (47-50). All these factors underscore the importance of adequate anticoagulation during the peri-procedural period. With the increased use of NOACs in clinical practice, the questions regarding their use in the setting of catheter ablation have also emerged.
Dabigatran for catheter ablation
Various studies have investigated the safety and efficacy of NOACs in the setting of catheter ablation. Most of the currently published studies did not use NOACs in a truly “uninterrupted” fashion. A multicentric study by Lakkireddy et al. investigated the incidence of thromboembolic and bleeding complications in patients undergoing catheter ablation on dabigatran and compared them with age, gender and AF type matched controls who underwent ablation on uninterrupted warfarin therapy (dose adjusted to maintain an INR between 2.0 and 3.5). The dabigatran arm consisted of patients who had received dabigatran 150 mg bid regimen starting at least 30 days prior to the ablation and withheld the dose of dabigatran on the day of the procedure. After catheter ablation, dabigatran was resumed within 3 hours after hemostasis and patients in the warfarin arm received their warfarin dose on the evening of the procedure. The analysis based on 30-day follow up events observed that the patients taking dabigatran had a significantly higher major bleeding rate (6% vs. 1% respectively, P=0.019), overall bleeding rate (14% vs. 6%, P =0.031), and composite of bleeding and thromboembolic complications (16% vs. 6%, P=0.009) as compared to patients on uninterrupted warfarin therapy prior to and after catheter ablation (51).
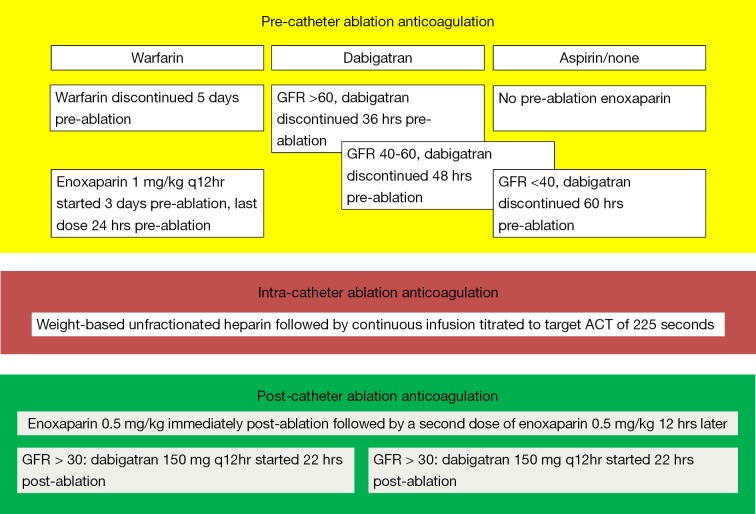
Another study by Winkle et al. reported a single-center based experience on 123 AF patients undergoing catheter ablation. This was a prospective observational study in which 56 (45.5%) patients were on warfarin and 34 (27.6%) patients were taking dabigatran prior to catheter ablation. Patients receiving pre-procedural warfarin were continued on warfarin until 5 days before catheter ablation and were bridged using low-molecular weight heparin (LMWH) 3 days prior to ablation. Patients receiving dabigatran had the drug discontinued 36-60 hours prior to ablation using a GFR based algorithm (Figure 3), no bridging was performed in the dabigatran patients. A GFR-adjusted dabigatran dose was started in all patients (120 patients receiving 150 mg bid regimen and remaining 3 patients receiving 75 mg bid regimen) 22 hours after catheter ablation. At a 30-day follow-up time interval, no patients receiving dabigatran experienced stroke, systemic embolism, transient ischemic attack (TIA) or any bleeding complications (52). Another recently published study by Kim et al. also observed findings similar to those reported by Winkle et al. In this study based on a total of 763 patients (191 in dabigatran arm and 572 in the warfarin arm) the rate of overall bleeding complications was similar in the two groups (4.7% in dabigatran arm vs. 5.4% in warfarin arm, P=0.85). The investigators in this study withheld the dose of dabigatran on the morning of the procedure and resumed it 4 hours after vascular hemostasis was achieved (53). Further studies with similar “interrupted” NOAC-administration regimen in the dabigatran arm were also conducted at their respective centers by Imamura et al. (54) and Kaiser et al. (55). The investigators in the study by Imamura et al. withheld the dose of dabigatran 12-24 hours prior to the ablation and restarted it 3 hours after the procedure (54). Similarly in their study, Kaiser et al. withheld the dose of dabigatran 24-30 hours prior to the procedure and restarted it 4-6 hours after post-ablation hemostasis was achieved. Both of these studies also observed comparatively similar bleeding and thromboembolic complication rate with the use of dabigatran and warfarin.
Figure 3.
Algorithm for pre-procedural, intraprocedural, and postprocedural anticoagulation in patients taking warfarin and dabigatran prior to undergoing catheter ablation for atrial fibrillation [Modified and reproduced with permission from (52)].
Similarly, another single-center based study by Snipelisky et al. also investigated the efficacy and safety of an “interrupted” dabigatran regimen vs. an INR-adjusted (to maintain a therapeutic range of 2.0-3.0) regimen of warfarin. The patients in the dabigatran arm had their dose withheld on the morning of the ablation procedure, whereas in the warfarin arm the medication dose was omitted on the evening prior to the day of ablation. The patients in these two study arms were compared for major, minor bleeding and the incidence of rebleeding at 48 hours and 1 week follow-up after ablation. There were no major bleeding events observed in either of the two drug-regimens. Furthermore, there was no significant difference in the incidence of minor bleeding in the two arms (19.4% in dabigatran arm vs. 16.8% in the warfarin arm, P=0.738). A trend towards an increased incidence of rebleeding was observed in the dabigatran arm (16.1% vs. 8.0% in the warfarin arm, P=0.172) (56).
An “uninterrupted” regimen of dabigatran in the peri-procedural period was first investigated in a single-center study by Maddox et al. Patients taking dabigatran (≥4 weeks prior to the procedure, n=212) were instructed to take the morning dose on the day of the ablation and were compared with continuous warfarin regimen (dose adjusted to maintain an INR of 2.0-3.0). After the ablation procedure, the patients were given their respective anticoagulant agents on the evening of the procedure. After the catheter ablation, a composite of thromboembolic and bleeding outcome was observed to be similar in both groups of patients (1.4% in dabigatran arm vs. 2.3% in warfarin arm, P=0.45) (57). Another study by Haines et al. also compared an “uninterrupted” pre-procedural dabigatran regimen with a dose-adjusted warfarin regimen. However, in the post-ablation phase of this study the average time taken to resume the first dose of dabigatran was 12.2±10.3 hours after the completion of ablation. The investigators did not observe any significant difference in the incidence of systemic embolism in the two study arms (1% in dabigatran arm vs. 0% in warfarin arm, P=NS). Similarly no significant difference was observed in the incidence of bleeding complications (2.48% in dabigatran arm vs. 1.49% in the warfarin arm, P=NS) (58) (Table 6).
Table 6. Current studies investigating dabigatran vs. warfarin in patients undergoing catheter ablation for atrial fibrillation.
| Study and pre-procedural anticoagulation | Dabigatran regimen | Overall thromboembolism (dabigatran vs. warfarin) | Bleeding complications |
|---|---|---|---|
| Interrupted NOAC regimen | |||
| Lakkireddy D, et al. (51) (dabigatran arm, n=145; warfarin arm, n=45) | Duration of dabigatran: ≥30 days pre-ablation TEE performed in dabigatran arm Post-ablation dose resumed 3 hours after hemostasis and when the patients were ready to take oral intake Discontinuation of dabigatran: morning dose held on the day of ablation |
2% in dabigatran arm vs. 0% in warfarin arm, P=0.25 | Total bleeding: 14% in dabigatran arm vs. 6% in warfarin arm, P=0.031 Major bleeding: 6% in dabigatran arm vs. 1% in the warfarin arm, P=0.019 Minor bleeding: 8% in dabigatran arm vs. 6% in warfarin arm, P=0.35 |
| Winkle RA, et al. (52) (dabiatran arm, n=34; warfarin arm, n=56) | Duration of dabigatran: NR Post-ablation dose resumed 22 hours after ablation Discontinuation of dabigatran: 36-60 hours prior to ablation* |
0 in dabigatran arm vs. 0 in warfarin arm, P=NS | Total bleeding: 0 in dabigatran arm vs. 0 in warfarin arm, P=NS§ |
| Kim JS, et al. (53) (dabigatran arm, n=191; warfarin arm, n=572) | Duration of dabigatran ≥4 weeks pre-ablation TEE performed in dabigatran arm Post-ablation dose was given 4 hours after vascular hemostasis following sheath removal Discontinuation of dabigatran: 24-30 hours prior to ablation |
0 in dabigatran arm vs. 0 in warfarin arm, P=1.0 | Total bleeding: 4.7% in dabigatran arm vs. 5.4% in warfarin arm, P=0.85 Major bleeding: 2.1% on dabigatran arm vs. 2.1% in warfarin arm Minor bleeding: 2.6% in dabigatran arm vs. 3.3% in warfarin arm, P=0.81 |
| Imamura K, et al. (54) (dabigatran arm, n=101; warfarin arm, n=126) | Duration of dabigatran: ≥1 month TEE performed in both study groups Post-ablation dose given 3 hours after the procedure Discontinuation of dabigatran: 12-24 hours prior to ablationψ |
1% in dabigatran arm vs. 0% in warfarin arm, P=0.45 | Major bleeding: 3.0% in dabigatran arm vs. 3.2% in warfarin arm, P=0.93 Minor bleeding: 5.0% in dabigatran arm vs. 4.0% in warfarin arm, P=0.54 |
| Kaiser DW, et al. (55) (dabigatran arm, n=122; warfarin arm, n=135) | Duration of dabigatran: NR Post-ablation dose resumed 4-6 hours after hemostasis Discontinuation of dabigatran: 24 hours to 5 days¶ |
2.5% in dabigatran arm vs. 0.7% in warfarin arm, P=0.28 | Major bleeding: 1.6% in dabigatran arm vs. 0.7% in warfarin arm, P=0.51 Minor bleeding: 2.5% in dabigatran arm vs. 7.4% in warfarin arm, P=0.09 |
| Snipelisky D, et al. (56) (dabigatran arm, n=31; warfarin arm, n=125) | Duration of dabigatran: ≥4 weeks Post-ablation dose resumed on evening of the procedure Discontinuation of dabigatran: morning dose held on the day of ablation |
0 in dabigatran arm vs. 0 in warfarin arm, P=NS | No major bleeding in either arms Minor bleeding: 19.4% in dabigatran arm vs. 16.8% in warfarin arm, P=0.74 |
| Uninterrupted dabigatran regimen | |||
| Maddox W, et al. (57) (dabigatran arm, n=212; warfarin arm, n=251) | Duration of dabigatran: ≥4 weeks No discontinuation of dabigatran dose TEE performed in both study groups Post-ablation dose resumed on the evening of procedure |
0.4% in dabigatran arm vs. 0% in warfarin arm, P=0.28 | Total bleeding: 0.9% in dabigatran arm vs. 2.3% in warfarin arm, P=0.23 |
| Haines DE, et al. (58) (dabigatran arm, n=202; warfarin arm, n=202) | Duration of dabigatran: NR No discontinuation of dabigatran dose TEE performed only in patients not taking OACs Post-ablation dose given 12.2±10.3 hoursɸ |
1% in dabigatran arm vs. 0% in warfarin arm, P=NS | Total bleeding: 2.5% in dabigatran arm vs. 1.5% in warfarin arm, P=NS, P value for combined endpoint of thrombotic complications and bleeding (P=0.12) |
*, duration of discontinuation of dabigatran was based on GFR (for patients with GFR >60 mL/min, dabigatran was discontinued 36 hrs pre-ablation, in patients with GFR 40-60 mL/min dabigatran was discontinued 48 hrs pre-ablation and in patients with GFR <40 mL/min dabigatran was discontinued 60 hrs pre-ablation; ¶, duration of discontinuation of dabigatran was based on CrCl (for patients with normal renal function, CrCl >50 mL/min, dabigatran was withheld 24-30 hrs prior to ablation, in patients with CrCl 15-30 mL/min dabigatran was withheld 3-5 days prior to ablation); §, the authors did not categorize bleeding into major or minor, total bleeding complications were adjudicated to include bruising and hematoma; Ψ, time of discontinuation of dabigatran was decided based on the chronology of AF and CHADS2 score (for paroxysmal AF and patients with a score of 0 or 1, dabigatran was discontinued 24 hrs prior to ablation, patients with persistent AF and CHADS2 score of ≥2, dabigatran was discontinued 12 hrs prior to ablation). NS, non-significant; NR, not reported.
It is worth discussing the contrary nature of results observed in the multicentre study by Lakkireddy et al. and various aforementioned single-center studies, we speculate that these difference in outcomes (bleeding and thromboembolic complications) could be potentially attributed to several factors: (I) Lakkireddy et al. withheld the dose of dabigatran for only approximately 12 hours prior to the ablation while various single-center studies favored continuation of dabigatran regimen closer to the time of ablation; (II) because of a shorter half-life of dabigatran (11-14 hours) (59), the anticoagulant effect of dabigatran could potentially decline in the 12 hour interruption period prior to ablation; (III) patients who had bleeding and thromboembolic complications in the study by Lakkireddy et al. were relatively older in age and had a higher incidence of chronic AF which could reflect an intrinsically increased risk of these complications and may not be a direct effect of dabigatran per se. In the post-ablation period, the relatively quicker onset of action of dabigatran might offer a superior alternative by obviating the requirement of anticoagulation bridging. This effect could be at least partially responsible for the shorter post-ablation hospital stay observed with the use of dabigatran (7.2±2.0 vs. 10.3±3.9 days with the use of warfarin by Imamura et al.) (54).
The interaction between dabigatran and heparin is also another relevant aspect regarding the use of dabigatran in the setting of catheter ablation. Snipelisky et al. reported in their study that a standard intraprocedural heparin protocol resulted in relatively delayed and lower levels of ACT during the procedure (56) Furthermore, Konduru et al. also reported an increased requirement of heparin dose up to 42% in the dabigatran group as well as an increased time required to achieve the targeted ACT levels (45 minutes in dabigatran group vs. 21 minutes in the warfarin group) (60). Current data regarding the mechanistic basis of interaction between dabigatran and heparin is limited, so it is important to be cognizant of an increased anticoagulant effect of dabigatran and heparin in the post-ablation period.
Rivaroxaban for catheter ablation
Current literature regarding the use of rivaroxaban in the periprocedural period of catheter ablation of AF remains limited. Very recently, Lakkireddy et al. investigated the safety and efficacy of a periprocedural “uninterrupted” regimen of rivaroxaban (n=321) with age, gender and AF type matched patients who were on an uninterrupted warfarin regimen (n=321) during the peri-ablation period. The patients in the rivaroxaban arm were instructed to take the medication dose on the evening prior to the ablation procedure. No bridging with heparin was performed in the rivaroxaban arm. A pre-ablation TEE was performed in every patient in the rivaroxaban arm. After the catheter ablation, the evening dose of rivaroxaban was resumed after a minimum post-hemostasis period of 3 hours. The results of this multicentric, observational study did not detect any significant differences in the incidence of major bleeding (1.6% vs. 1.9%, P=0.77), minor bleeding (5.0% vs. 5.9%, P=0.60) and embolic complications (0.3% vs. 0.3%, P=1.0) in the 30-day post-ablation follow up period between the rivaroxaban and warfarin groups (61).
Apart from this study, overall data regarding the use of rivaroxaban in the setting of catheter ablation remain limited. A post-hoc analysis of the ROCKET-AF trial by Piccini et al. provided some evidence by comparison of patients in the rivaroxaban and warfarin in the patients who underwent cardioversion and catheter ablation. Although this analysis did not reveal any significant differences in bleeding and thromboembolic complications between the two drug-groups, but the comparison were not made exclusively in the setting of catheter ablation. Also noteworthy is the observation that only 49% of the patients in the rivaroxaban arm were on the drug on the day of the ablation. It is also worth pointing out that at the time of enrollment, patients who had plans for either cardioversion or catheter ablations were excluded from participation. This factor could potentially limit the applicability of these data in the setting of catheter ablation (62). Another study by Eitel et al. compared the bleeding and thromboembolic complications in 259 patients undergoing catheter ablation on NOACs. Although the total number of patients included in the rivaroxaban arm in this study were only 16 (13 in the pre-ablation and 3 in the post-ablation period). There was also some interruption in the dosing regimen of rivaroxaban during the peri-ablation period; from the morning prior to the day of ablation to the evening of ablation. The study investigators did not report any bleeding or thromboembolic complications in the patients taking rivaroxaban (63). Providência et al. also recently published the results regarding the efficacy and safety of rivaroxaban based on their large volume single-center. The dose of rivaroxaban was interrupted 24-48 hours prior to the ablation procedure. In these patients, anticoagulation bridging was performed with subcutaneous heparin which was started 24 hours after the interruption of rivaroxaban. Post-procedurally, the dose of rivaroxaban was resumed 4-6 hours after the procedure. The use of rivaroxaban in the setting of catheter ablation was found to be associated with similar rates of thromboembolism (1.1% vs. 2.1% in the VKA arm, P=0.41) and major bleeding (1.6% in the rivaroxaban arm vs. 4.2% in the VKA arm, P=0.112) (64).
In future, the completion of VENTURE-AF trial (A study exploring two treatment strategies in patients with AF who undergo catheter ablation therapy), which is designed to compare the outcomes of catheter ablation on uninterrupted warfarin with uninterrupted rivaroxaban will provide key data on the safety and efficacy of rivaroxaban in the setting of catheter ablation (65). Current literature regarding the use of apixaban in the patients undergoing catheter ablation is very limited. In future the completion of a randomized multicentre trial (anticoagulation using the direct factor Xa inhibitor apixaban during AF catheter ablation: comparison to VKA therapy) (66) will yield useful information regarding the use of apixaban in the setting of catheter ablation.
Based on the available data so far, there are several considerations which can be kept in mind regarding the use of NOACs in the setting of catheter ablation. One of the strategies which can be considered in the patients taking NOACs is transitioning the patients to warfarin prior to ablation and then performing the procedure on an uninterrupted warfarin therapy. However, such a strategy might entail the risk of inconvenience to the patients especially during the periods of coming “on” and “off” from warfarin. This strategy also carries a theoretically increased risk of thromboembolism and bleeding complications during the drug-transition period. It is also important to emphasize that using a strict “uninterrupted” anticoagulation regimen is very important because it obviates the need for a pre-ablation TEE and also allows the administration of Protamine after ablation. The use of Protamine after ablation is needed to allow the reversal of heparin at the time of sheaths pull. If this is not done, then the sheaths are left in place for hours until the ACT drifts down. The use of a strictly “uninterrupted” NOAC regimen might also obviate the need of bridging with heparin during the pre- and post-ablation period. Therefore, such a strategy may also prove to be cost-effective by decreasing the length of hospital stay for the ablation procedure. Currently published studies investigating NOACs during the ablation period did not use these medications in a truly “uninterrupted” fashion. In the absence of a reliable assay to monitor the anticoagulant effect of NOACs, the use of pre-ablation TEE can be worth considering in the patients with a questionable drug-compliance. The timing of discontinuation of a NOAC prior to ablation can be determined by the individual drug’s half-life, renal and hepatic function of the patient and the availability of a reversal agent.
Management of catheter ablation related bleeding complications
It is pertinent to acknowledge that the bleeding complications associated with the use of NOACs in the peri-ablation period might not be solely attributed to the anticoagulation effect of these agents. The complexity of various integral components of catheter ablation also plays a significant role in the bleeding complications secondary to the procedure. The interaction of heparin products and NOACs in the peri-ablation period is also relevant to consider. Current data on the studies investigating this interaction are limited to dabigatran only (60). Future investigations will help elucidate this interaction with other NOACs and its implication in the setting of catheter ablation. Lack of availability of a specific antidote to reverse the anticoagulant effect of NOACs also limits therapeutic options for bleeding complications of these agents in the peri-ablation period. Although activated factor VII has been proposed as an emergency treatment for severe bleeding with these agents, but robust data regarding its use still remain limited (66). Marlu et al. compared the relative efficacy of prothrombin complex concentrate (PCC), factor VII and factor eight inhibitor bypass activity (FEIBA) for reversal of anticoagulant effect of rivaroxaban and dabigatran. Both factor VII and FEIBA were observed to reverse the anticoagulant effect of rivaroxaban and PCC seemed effective to reverse the anticoagulant effect of dabigatran (67,68). Using a strictly “uninterrupted” anticoagulation regimen during the peri-ablation period might also offer the benefit of a relatively convenient and a more uniform anticoagulation effect. By obviating the need for bridging with heparin, it might also decrease the potential for drug-drug interactions and therefore may decrease the incidence of bleeding complications. Further robust data are needed to test the safety and efficacy of this drug-regimen.
Conclusions
With the increasing use of NOACs in clinical practice, pertinent questions regarding their use in the patients undergoing cardioversion and catheter ablation have emerged. Based on the available data thus far, the use of these agents appears relatively safe and efficacious in comparison with warfarin. An approach combining the considerations based on the pharmacological properties, presence of comorbid conditions and risk-assessment of bleeding and thromboembolic complications in an individual patient appears to be the most useful in the setting of cardioversion and catheter ablation. Results from future studies will also yield further data to aid in the use of these agents.
Acknowledgements
Funding: E. Kevin Heist: Biotronik (research grant, honoraria), Boston Scientific (research grant, consultant, honoraria), Medtronic (honoraria), Sanofi (consultant), Sorin (consultant, honoraria), St. Jude Medical (research grant, consultant, honoraria); Jeremy N. Ruskin: Astellas/Cardiome-Consultant (significant); Biosense Webster-Consultant (modest) & Fellowship Support (significant); Boston Scientific-Fellowship Support (significant); CardioFocus-Clinical Oversight Committee (no compensation); CardioInsight-Scientific Advisory Board (modest); CryoCath-Scientific Steering Committee (no compensation); Medtronic-Consultant (modest) & Fellowship Support (significant); Med-IQ-Honoraria (modest); Pfizer-Consultant and Scientific Steering Committee (modest); Portola-Consultant & equity (modest); Sanofi-Consultant (modest), St. Jude Medical-Fellowship Support (significant); Third Rock Ventures- consultant (significant); Moussa Mansour: Biosense-Webster (consultant, research grant), Boston Scientific (research grant), St. Jude (consultant), Cardiofocus (research grant), Endosense (research grant), MC10 (research grant), St. Jude Medical (consultant, research grant), Voyage Medical (research grant).
Disclosure: The authors declare no conflict of interest.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-88. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517-84. [DOI] [PubMed] [Google Scholar]
- 3.McGrath ER, Kapral MK, Fang J, et al. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology 2013;81:825-32. [DOI] [PubMed] [Google Scholar]
- 4.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142-7. [DOI] [PubMed] [Google Scholar]
- 5.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385-413. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:1935-44. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. [DOI] [PubMed] [Google Scholar]
- 8.Stangier J, Rathgen K, Stähle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010;49:259-68. [DOI] [PubMed] [Google Scholar]
- 9.Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol 2010;70:703-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Y, Song Y, Wang J, et al. Single- and multiple-dose pharmacokinetics, pharmacodynamics, and safety of apixaban in healthy Chinese subjects. Clin Pharmacol 2013;5:177-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [DOI] [PubMed] [Google Scholar]
- 14.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [DOI] [PubMed] [Google Scholar]
- 15.Goodman SG, Wojdyla DM, Piccini JP, et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2014;63:891-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387-94. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363-72. [DOI] [PubMed] [Google Scholar]
- 18.Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Rognoni C, Marchetti M, Quaglini S, et al. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014;34:9-17. [DOI] [PubMed] [Google Scholar]
- 20.Lip GY, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192-210.e20. [DOI] [PubMed]
- 21.Atay JK, Fiumara K, Piazza G, et al. Hospital budget implications of substituting dabigatran for warfarin in an anticoagulation service. Clin Appl Thromb Hemost 2012;18:181-4. [DOI] [PubMed] [Google Scholar]
- 22.Huisman MV, Lip GY, Diener HC, et al. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost 2012;107:838-47. [DOI] [PubMed] [Google Scholar]
- 23.Mueck W, Kubitza D, Becka M.Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013;76:455-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xarelto: EPAR-Product Information-European Medicines Agency. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000944/WC500057108.pdf
- 25.Highlights of prescribing information. Available online: http://packageinserts.bms.com/pi/pi_eliquis.pdf
- 26.Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343-8. [DOI] [PubMed] [Google Scholar]
- 27.Highlights of prescribing information. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022512s000lbl.pdf
- 28.Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013;61:651-8. [DOI] [PubMed] [Google Scholar]
- 29.Ferrandis R, Castillo J, de Andrés J, et al. The perioperative management of new direct oral anticoagulants: a question without answers. Thromb Haemost 2013;110:515-22. [DOI] [PubMed] [Google Scholar]
- 30.Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013;11:1493-502. [DOI] [PubMed] [Google Scholar]
- 31.Douxfils J, Mullier F, Robert S, et al. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost 2012;107:985-97. [DOI] [PubMed] [Google Scholar]
- 32.Lindahl TL, Baghaei F, Blixter IF, et al. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost 2011;105:371-8. [DOI] [PubMed] [Google Scholar]
- 33.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116-27. [DOI] [PubMed] [Google Scholar]
- 34.Douxfils J, Mullier F, Loosen C, et al. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res 2012;130:956-66. [DOI] [PubMed] [Google Scholar]
- 35.Samama MM, Contant G, Spiro TE, et al. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012;107:379-87. [DOI] [PubMed] [Google Scholar]
- 36.Asmis LM, Alberio L, Angelillo-Scherrer A, et al. Rivaroxaban: Quantification by anti-FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res 2012;129:492-8. [DOI] [PubMed] [Google Scholar]
- 37.Douxfils J, Chatelain C, Chatelain B, et al. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost 2013;110:283-94. [DOI] [PubMed] [Google Scholar]
- 38.Barrett YC, Wang Z, Knabb RM. A novel prothrombin time assay for assessing the anticoagulant activity of oral factor Xa inhibitors. Clin Appl Thromb Hemost 2013;19:522-8. [DOI] [PubMed] [Google Scholar]
- 39.Airaksinen KE, Grönberg T, Nuotio I, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol 2013;62:1187-92. [DOI] [PubMed] [Google Scholar]
- 40.Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411-20. [DOI] [PubMed] [Google Scholar]
- 41.Saeed M, Rahman A, Afzal A, et al. Role of transesophageal echocardiography guided cardioversion in patients with atrial fibrillation, previous left atrial thrombus and effective anticoagulation. Int J Cardiol 2006;113:401-5. [DOI] [PubMed] [Google Scholar]
- 42.European Heart Rhythm Association ; European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [DOI] [PubMed] [Google Scholar]
- 43.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e531S-75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011;123:131-6. [DOI] [PubMed] [Google Scholar]
- 45.Piccini JP, Stevens SR, Lokhnygina Y, et al. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol 2013;61:1998-2006. [DOI] [PubMed] [Google Scholar]
- 46.Flaker G, Lopes RD, Al-Khatib SM, et al. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol 2014;63:1082-7. [DOI] [PubMed] [Google Scholar]
- 47.Viles-Gonzalez JF, Mehta D. Thromboembolic risk and anticoagulation strategies in patients undergoing catheter ablation for atrial fibrillation. Curr Cardiol Rep 2011;13:38-42. [DOI] [PubMed] [Google Scholar]
- 48.Maan A, Shaikh AY, Mansour M, et al. Complications from catheter ablation of atrial fibrillation: a systematic review. Crit Pathw Cardiol 2011;10:76-83. [DOI] [PubMed] [Google Scholar]
- 49.Dorbala S, Cohen AJ, Hutchinson LA, et al. Does radiofrequency ablation induce a prethrombotic state? Analysis of coagulation system activation and comparison to electrophysiologic study. J Cardiovasc Electrophysiol 1998;9:1152-60. [DOI] [PubMed] [Google Scholar]
- 50.Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010;121:2550-6. [DOI] [PubMed] [Google Scholar]
- 51.Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol 2012;59:1168-74. [DOI] [PubMed] [Google Scholar]
- 52.Winkle RA, Mead RH, Engel G, et al. The use of dabigatran immediately after atrial fibrillation ablation. J Cardiovasc Electrophysiol 2012;23:264-8. [DOI] [PubMed] [Google Scholar]
- 53.Kim JS, She F, Jongnarangsin K, et al. Dabigatran vs warfarin for radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm 2013;10:483-9. [DOI] [PubMed] [Google Scholar]
- 54.Imamura K, Yoshida A, Takei A, et al. Dabigatran in the peri-procedural period for radiofrequency ablation of atrial fibrillation: efficacy, safety, and impact on duration of hospital stay. J Interv Card Electrophysiol 2013;37:223-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser DW, Streur MM, Nagarakanti R, et al. Continuous warfarin versus periprocedural dabigatran to reduce stroke and systemic embolism in patients undergoing catheter ablation for atrial fibrillation or left atrial flutter. J Interv Card Electrophysiol 2013;37:241-7. [DOI] [PubMed] [Google Scholar]
- 56.Snipelisky D, Kauffman C, Prussak K, et al. A comparison of bleeding complications post-ablation between warfarin and dabigatran. J Interv Card Electrophysiol 2012;35:29-33. [DOI] [PubMed] [Google Scholar]
- 57.Maddox W, Kay GN, Yamada T, et al. Dabigatran versus warfarin therapy for uninterrupted oral anticoagulation during atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013;24:861-5. [DOI] [PubMed] [Google Scholar]
- 58.Haines DE, Mead-Salley M, Salazar M, et al. Dabigatran versus warfarin anticoagulation before and after catheter ablation for the treatment of atrial fibrillation. J Interv Card Electrophysiol 2013;37:233-9. [DOI] [PubMed] [Google Scholar]
- 59.Stangier J.Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285-95. [DOI] [PubMed] [Google Scholar]
- 60.Konduru SV, Cheema AA, Jones P, et al. Differences in intraprocedural ACTs with standardized heparin dosing during catheter ablation for atrial fibrillation in patients treated with dabigatran vs. patients on uninterrupted warfarin. J Interv Card Electrophysiol 2012;35:277-84. [DOI] [PubMed] [Google Scholar]
- 61.Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol 2014;63:982-8. [DOI] [PubMed] [Google Scholar]
- 62.Piccini JP, Stevens SR, Lokhnygina Y, et al. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol 2013;61:1998-2006. [DOI] [PubMed] [Google Scholar]
- 63.Eitel C, Koch J, Sommer P, et al. Novel oral anticoagulants in a real-world cohort of patients undergoing catheter ablation of atrial fibrillation. Europace 2013;15:1587-93. [DOI] [PubMed] [Google Scholar]
- 64.Providência R, Marijon E, Albenque JP, et al. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014;16:1137-44. [DOI] [PubMed] [Google Scholar]
- 65.A Study Exploring Two Treatment Strategies in Patients With Atrial Fibrillation Who Undergo Catheter Ablation Therapy (VENTURE-AF). Available online: http://www.clinicaltrials.gov/ct2/show/study/NCT01729871?show_locs=Y#locn
- 66.Anticoagulation using the direct factor Xa inhibitor apixaban during catheter ablation: comparison to vitamin K antagonist therapy. Available online: http://www.isrctn.com/ISRCTN87711003
- 67.Sartori MT, Imbergamo S, Zanon E, et al. Effect of recombinant activated factor VII in critical bleeding: clinical experience of a single center. Clin Appl Thromb Hemost 2009;15:628-35. [DOI] [PubMed] [Google Scholar]
- 68.Marlu R, Hodaj E, Paris A, et al. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012;108:217-24. [DOI] [PubMed] [Google Scholar]