Abstract
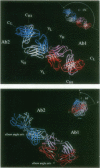
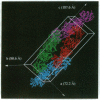
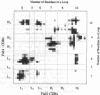
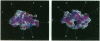
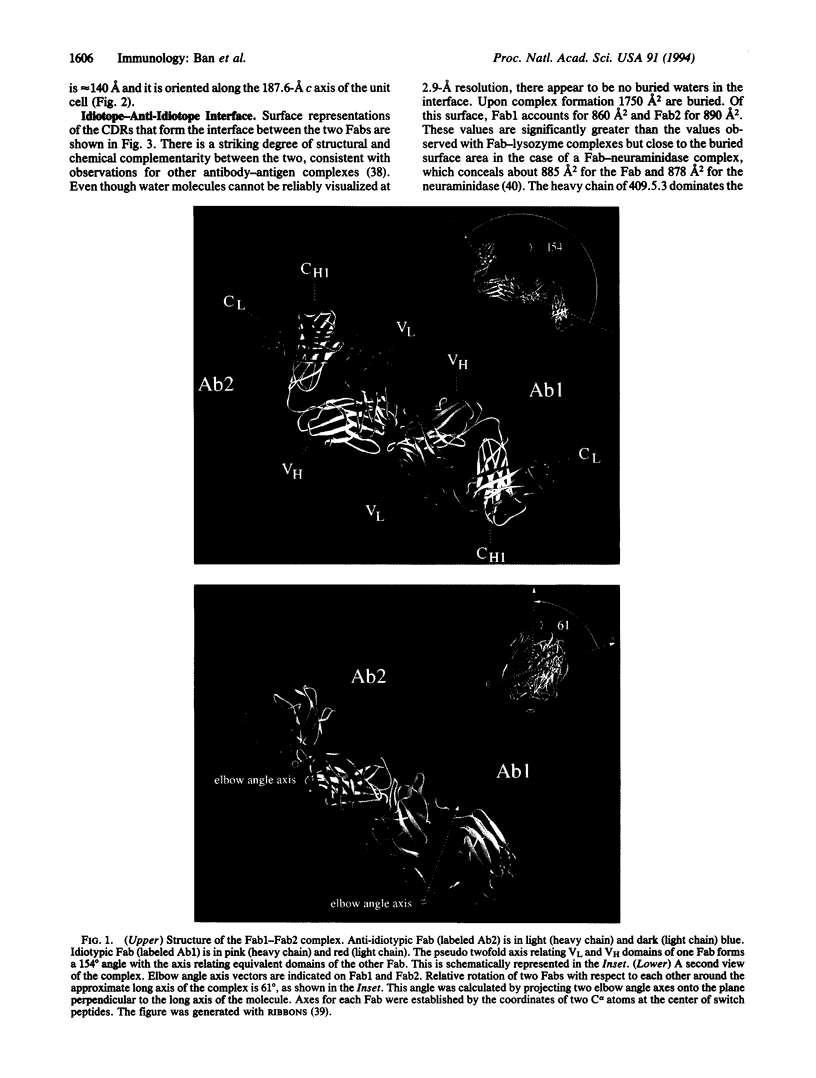
Anti-idiotypic monoclonal antibody 409.5.3 is raised against an antibody that neutralizes feline infectious peritonitis virus. This antibody, used as an immunogen, elicits the production of anti-anti-idiotypic antibodies that in turn neutralize the virus. The crystal structure of the complex between anti-idiotypic Fab 409.5.3 and idiotypic Fab fragment of virus-neutralizing antibody has been solved by molecular replacement using real-space Patterson search and filtering by Patterson correlation-coefficient refinement. The structure has been refined to an R value of 0.21 based on 21,310 unique reflections between 40.0 and 2.9 A. The three-dimensional structure reveals extensive, specific interactions that involve 118 van der Waals contacts and at least 9 probable hydrogen bonds. The two Fabs are rotated 61 degrees with respect to each other around the approximate long axis of the complex and are within 26 degrees being aligned along their major axes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ban N., Escobar C., Day J., Greenwood A., McPherson A. Preliminary crystallographic study of a complex between an Fab of a monoclonal feline peritonitis virus neutralizing antibody and its anti-idiotypic Fab. J Mol Biol. 1993 Dec 5;234(3):894–896. doi: 10.1006/jmbi.1993.1637. [DOI] [PubMed] [Google Scholar]
- Bentley G. A., Boulot G., Riottot M. M., Poljak R. J. Three-dimensional structure of an idiotope-anti-idiotope complex. Nature. 1990 Nov 15;348(6298):254–257. doi: 10.1038/348254a0. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Kennedy R. C. Anti-idiotypic antibodies as immunogens: idiotype-based vaccines. Vaccine. 1988 Jun;6(3):215–220. doi: 10.1016/0264-410x(88)90213-7. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Seiden M. V., Greenspan N. S., Lutz C. T., Bartholow T. L., Clevinger B. L. Structural correlates of idiotopes. Annu Rev Immunol. 1986;4:147–165. doi: 10.1146/annurev.iy.04.040186.001051. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Escobar J. C., Kochik S. A., Skaletsky E., Rosenberg J. S., Beardsley T. R. Immunization of cats against feline infectious peritonitis with anti-idiotypic antibodies. Viral Immunol. 1992 Spring;5(1):71–79. doi: 10.1089/vim.1992.5.71. [DOI] [PubMed] [Google Scholar]
- Garcia K. C., Ronco P. M., Verroust P. J., Brünger A. T., Amzel L. M. Three-dimensional structure of an angiotensin II-Fab complex at 3 A: hormone recognition by an anti-idiotypic antibody. Science. 1992 Jul 24;257(5069):502–507. doi: 10.1126/science.1636085. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Side-chain torsional potentials: effect of dipeptide, protein, and solvent environment. Biochemistry. 1979 Apr 3;18(7):1256–1268. doi: 10.1021/bi00574a022. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Lascombe M. B., Alzari P. M., Boulot G., Saludjian P., Tougard P., Berek C., Haba S., Rosen E. M., Nisonoff A., Poljak R. J. Three-dimensional structure of Fab R19.9, a monoclonal murine antibody specific for the p-azobenzenearsonate group. Proc Natl Acad Sci U S A. 1989 Jan;86(2):607–611. doi: 10.1073/pnas.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Silverton E. W., Sheriff S., Cohen G. H., Smith-Gill S. J., Davies D. R. Structure of an antibody-antigen complex: crystal structure of the HyHEL-10 Fab-lysozyme complex. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5938–5942. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt D. C., Jean-Francois M. J., Turnbull S., Macdonald L., Yasmeen D. Internal image (Ab2 beta) anti-idiotype vaccines. Theoretical and practical aspects. Vaccine. 1991 Nov;9(11):792–796. doi: 10.1016/0264-410x(91)90215-r. [DOI] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Smith J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987 Sep 20;197(2):273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8075–8079. doi: 10.1073/pnas.84.22.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S. W., Bhat T. N., Navia M. A., Cohen G. H., Rao D. N., Rudikoff S., Davies D. R. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-A resolution. Proteins. 1986 Sep;1(1):74–80. doi: 10.1002/prot.340010112. [DOI] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Webster R. G., Air G. M., Laver W. G., Colman P. M. Crystal structures of neuraminidase-antibody complexes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):257–263. doi: 10.1101/sqb.1989.054.01.032. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Weiner D. B., Kieber-Emmons T., Greene M. I. Antibody geometry and form: three-dimensional relationships between anti-idiotypic antibodies and external antigens. Trends Biotechnol. 1990 Sep;8(9):256–263. doi: 10.1016/0167-7799(90)90188-4. [DOI] [PubMed] [Google Scholar]