Abstract
Insulin acutely stimulates the degradation of apolipoprotein B (apo B) which decreases very low density lipoprotein (VLDL) secretion by liver. Insulin-dependent apo B degradation (IDAD) occurs following phosphatidylinositide 3-kinase (PI3K) activation and involves lysosomal degradation. Insulin suppression of apo B secretion is blocked by over-expression of phosphatase and tensin homologue (PTEN) in McArdle RH7777 (McA) cells suggesting the importance of Class I PI3K generated PI (3,4,5) triphosphate (PIP3) in IDAD. Classical autophagy inhibitors including 3-methyladenine, L-asparagine and bafilomycin A1 also blocked the ability of insulin to suppress apo B secretion by rat hepatocytes (RH) suggesting that IDAD occurs through an autophagy-related mechanism. IDAD is also blocked following over-expression in McA cells of a dominant negative kinase-defective Vps34, a class III PI3K that generates PI 3-monophosphate required for autophagy. Vps34 inhibition of IDAD occurs without altering insulin-dependent S473 phosphorylation Akt indicating PI3K/PIP3/Akt signaling is intact. Cellular p62/SQSTM1, an inverse indicator of autophagy, is increased with insulin treatment consistent with the known ability of insulin to inhibit autophagy, and therefore the role of insulin in utilizing components of autophagy for apo B degradation is unexpected. Thapsigargan, an inducer of endoplasmic reticulum (ER) stress, and a recently demonstrated autophagy inhibitor, blocked apo B secretion which contrasted with other autophagy inhibitors and mutant Vps34 results which were permissive with respect to apo B secretion. Pulse chase studies indicated that intact B100 and B48 proteins were retained in cells treated with thapsigargan consistent with their accumulation in autophagosomal vacuoles. Differences between IDAD and ER stress-coupled autophagy mediated by thapsgargin suggest that IDAD involves an unique form of autophagy. Insulin action resulting in hepatic apo B degradation is novel and important in understanding regulation of hepatic VLDL metabolism.
Keywords: apo B, autophagy, PI3K, Vps34, thapsigargin, PTEN, VLDL, liver
Introduction
Dissection of the regulatory pathways involved in hepatic VLDL secretion is fundamental to understanding mechanisms involved in the development of hypertriglyceridemia in insulin resistant states [1]. Assembly of VLDL is complex involving fusion of triglyceride and apo B, a required structural protein, that is synthesized full length as B100 or shorter form as B48 [2]. Insulin suppresses VLDL apo B assembly and secretion downstream of Class I phosphatidylinositide 3-kinase (PI3K) activation [3,4]. Loss of insulin-dependent reduction in hepatic VLDL secretion during the postprandial period occurs early during development of hepatic insulin resistance, and may precede disordered glucose metabolism [5]. In HepG2 cells where lipid supply is restricted, a large portion of nascent B100 becomes ubiquitinated and undergoes endoplasmic reticulum-associated degradation (ERAD) by proteasomes [6]. Post-ER presecretory proteolysis (PERPP) of apo B occurs through autophagic degradation, and can be stimulated by oxidation [7], fatty acid-induced ER stress [8], and expression of missense mutations within the βα1 domain of apo B [9]. Little is known about the mechanism(s) responsible for insulin-dependent apo B degradation (IDAD) which occurs in a post-ER cellular compartment presumptively lysosomes [3]. IDAD favors B100 degradation over B48 [10], and recent studies demonstrate that insulin increases B100 binding to sortilin which may initiate selective post-ER degradation of B100 in lysosomes [11].
The current study was undertaken to define the role of PI3K, and to further characterize the role autophagy plays in IDAD. Since insulin inhibits autophagy by activation of the PI3K/Akt/mammalian target of rapamycin pathway [12], IDAD is a novel action of insulin. In McArdle RH7777 (McA) cells, over-expression of phosphatase and tensin homologue (PTEN), which mainly targets the 3’ phosphate of PI (3,4,5) triphosphate (PIP3) [13], prevented IDAD supporting a role for PIP3-generated by Class I PI3K. Classical autophagy inhibitors eliminated IDAD and normalized apo B secretory rates in the presence of insulin further supporting autophagy as the mechanism for IDAD. To test this possibility, we examined the role of Vps34, a Class III PI3K whose kinase activity is essential in liver autophagy [14]. Expression of a kinase-defective Vps34 mutant abolished IDAD in McA cells. Thapsigargan which induces ER stress and inhibits autophagy [15] resulted in cellular retention of intact B100 and B48 which contrasted with other autophagy inhibitors which were permissive for apo B secretion. The physiological importance of IDAD is that resistance in this pathway results in hypersecretion of VLDL which is the main cause of hypertriglyceridemia in metabolic syndrome and type 2 diabetes [16].
2. Materials and Methods
2.1 Materials
Wild type McA cells were obtained from the ATCC (Manassas, VA). Waymouth's 752/1 medium, Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum, horse serum, 3-methyladenine (3MA), L-asparagine (ASN), bafilomycin A1 (BafA1), puromycin (PURO), thapsigargan and most other chemicals were from Sigma-Aldrich (St. Louis, MO). Purified bovine serum albumin (BSA) was purchased from Serologicals Proteins, Inc. (Bayer Corp., Kankakee, IL). PROTEAN®TGX™ SDS polyacrylamide gels (4-15% (w/v) acrylamide), nitrocellulose and PVDF membranes, and ECL reagents were obtained from Bio-Rad Laboratories (Hercules, CA). Rabbit anti-phosphoAkt (S473, #9271)) and anti-Akt (#9272) antibodies were from Cell Signaling Technology (Danvers, MA). Mouse anti-glyceraldehyde phosphate dehydrogenase (GAPDH) and rabbit anti-PTEN antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-p62/SQSTM1 was from Medical & Biological Laboratories, Nagoya, Japan). Lipofectamine 2000, Plus™ Reagent, and rabbit anti-Vps34 antibody were from Invitrogen (Carlsbad, CA). Anti-rabbit and anti-mouse horseradish peroxidase (HRP)-linked IgG and Hyperfilm™ were purchased from GE Healthcare (Buckinghamshire, UK).
2.2 Cell Culture
Rat hepatocytes (RH) were isolated from Sprague-Dawley rat livers, and were cultured on collagen-coated dishes in Waymouth's 751/1 medium containing 0.2% (w/v) BSA as described previously [17]. Wild-type McA cells were maintained in culture in complete DMEM (cDMEM) [11]. Inhibitors were used at reported concentrations and validated in RH where cell toxicity was minimal as determined by LDH release.
2.3 Radioimmunoassay
Cellular and media apo B concentrations were determined in triplicate by competitive RIA as described previously [3] using rat VLDL apo B as standard and 125I-labeled mouse monoclonal antibody equally reactive to rat B100 and B48 [18]. Apo B concentrations were normalized to cell protein on a per dish basis. Protein concentrations were determined using Thermo Scientific Pierce bicinchoninic acid (BCA) protein assay (Rockland, IL).
2.4 Adenoviral transduction in vitro
Adenoviral (Ad) vectors Ad-CMV-human PTEN (#1547) and Ad-CMV-green fluorescent protein (GFP) (#1060) were purchased from VectorBiolabs (Philadelphia, PA). McA cells were seeded and cultured in cDMEM until reaching 50-60% confluence and were infected at 10 multiplicities of infection (MOI) with Ad-CMV-PTEN or Ad-CMV-GFP for 24 h. Afterwards, plates were rinsed, fresh cDMEM medium was added for a 6 h recovery period, and cells were reincubated in DMEM containing 1% BSA (1% BSA/DMEM) overnight for 12-14 h. Cells were then incubated in 1% BSA (w/v)/DMEM ± insulin (final, 500 nM) for 6 h; medium was collected and apo B concentration was quantified by RIA (3-100 mm plates per condition) in each experiment).
2.5 McArdle RH7777 cell transfections
The pcDNA3 PURO plasmid encoding a rat kinase defective Vps34p (Vps34mt) was a kind gift from Dr. Howard W. Davidson [19]. The pcDNA3-Vps34mt and empty vector plasmids were transfected into McA cells using Lipofectamine 2000 with Plus™ Reagent (Invitrogen) according to manufacturer's instructions. Transfected McA cells were selected in cDMEM containing puromycin (2.5 μg/mL), and passaged 3 times in selection medium prior to study. After reaching 70-80% confluence, transfected McA cells were rinsed and reincubated for 12-14 h in DMEM containing 1% (w/v) BSA. After a medium change, cells were treated ± insulin (final, 500 nM) for 6 h; medium was collected and apo B concentration was quantified by RIA (3-100 mm plates per condition) for each experiment.
2.6 Pulse chase studies in RH
Pulse chase studies were carried out using EXPRE35S35S-protein labeling as described [20]. Briefly, hepatocytes were pre-incubated in depletion medium containing 1 μM thapsigargan or equivalent DMSO for 45 min followed by addition of 130-175 μCi (Spec. Act. 1175 Ci/mmol) EXPRE35S35S-protein labeling mix (NEG-072, Perkin-Elmer Life Sciences, Boston, MA). Incubation was continued for 30 min, and cells were reincubated in chase medium containing 10 mM L-methionine and 2.5 mM L-cysteine and 1 μM thapsigargan or DMSO. 35S-apo B was immunoprecipitated from cells and media using rabbit anti-rat apo B polyclonal antibody [20]. Immunoprecipitated 35S-labeled apo B was eluted into SDS-gel loading buffer, and B100 and B48 were separated by SDS-PAGE [20]. 35S-labeled B100 and B48 were quantified by PhosphorImager analysis using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
2.7 Western immunoblotting
Western immunoblotting analysis of cellular protein expression levels were carried as described previously following protein separation by SDS-PAGE on TGX SDS-gels (4-15%) and electrophoretic transfer to PVDF membranes [11]. After membrane blocking and incubations with primary antibodies, secondary HRP-linked anti-rabbit or anti-mouse antibody binding to membranes was evaluated by ECL chemiluminescence detection using Hyperfilm™ or imaged directly using the BioRad Laboratories ChemiDocXRS+ system (Hercules, CA).
2.8 Statistics
Unless otherwise stated, data are expressed as mean ± S.E.M. where n = the number of independent experiments and where 3-5 individual plates per condition were analyzed in each experiment. Significant differences were determined using Student's t-test with p-values < 0.05 being considered significant.
3. Results and Discussion
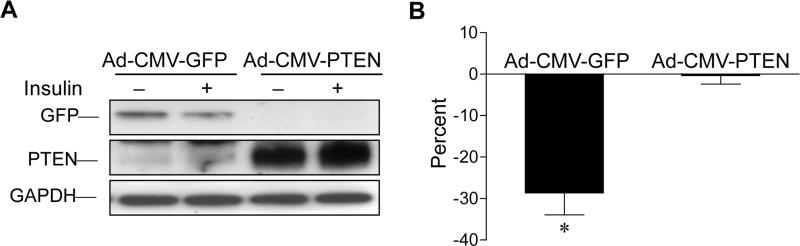
Previous studies using broad specificity inhibitors of PI3K, wortmannin and LY294002, indicated that insulin-stimulated PI3K activity is necessary for IDAD [3,4,20]. The role of Class I PI3K in initiating IDAD is supported by studies showing that expression of constitutively active p110α subunit of Class I PI3K suppresses apo B secretion in the absence of insulin [11]. PTEN is a lipid phosphatase whose main catalytic function is to dephosphorylate the 3’ phosphate group of PIP3 [21]. Over-expression of PTEN (2-4 fold) in McA cells by Ad-CMV PTEN infection (Fig. 1A) prevented the 29% ± 5% reduction in apo B secretion induced by insulin in McA cells expressing GFP (Fig. 1B). Insulin eliminated 89% ± 3.4 % of the insulin effect observed in McA cells expressing GFP (p < 0.05). Considering the relative specificity of PTEN for PIP3 [13], loss of insulin suppression with increased PTEN expression provides additional support for PIP3, the product of insulin-stimulated Class I PI3K, in IDAD.
Fig. 1.
Insulin suppression of apo B secretion by McA cells is prevented by PTEN over-expression. McA cells were infected with either Ad-CMV-GFP or Ad-CMV-PTEN, and following serum starvation, were incubated ± insulin for 6 h and media apo B quantified by RIA. (A) Cellular GFP and PTEN expression were determined by western immunoblotting. (B) Differences in media apo B ± insulin as a percentage of the no insulin condition are presented as mean percent ± S.E.M. from 3 independent studies. *Indicates that the percent reduction of apo B secretion with insulin in GFP expressing cells differs significantly from that of PTEN expressing cells at p < 0.01.
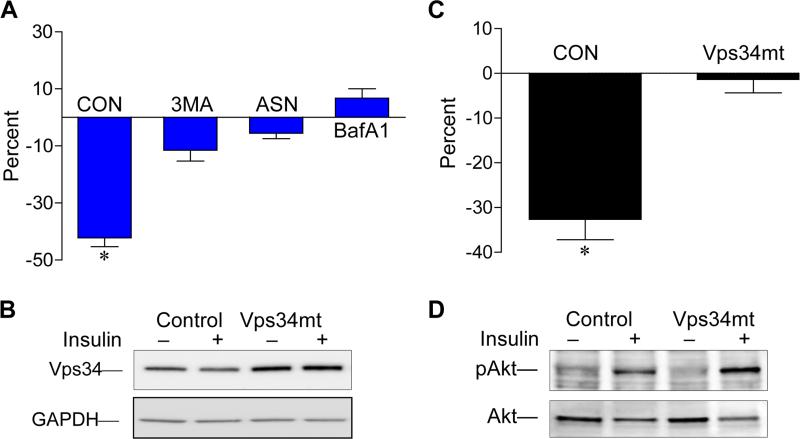
We first suggested autophagy as a mechanism for IDAD [22]. To examine this possibility, we tested classical autophagy inhibitors for their ability to block IDAD including 3MA, ASN and BafA1. 3MA blocks autophagic sequestration [23], while ASN interferes with amphisome-lysosome fusion [24], and BafA1 acts by inhibiting the vacuolar H+-ATPase which prevents vesicle acidification and limits lysosomal degradation [25]. Pre-incubation of RH with each of the three inhibitors was followed by insulin addition (final, 100 nM), and media apo B secreted in 5 h was measured. All three inhibitors blocked the ability of insulin to suppress the secretion of apo B (Fig. 2A) compared with insulin in the absence of inhibitors which suppressed apo B on average by 42% ± 9% (p < 0.001). Insulin suppression was reduced to 12% ± 3.8% in 3MA treated cells (n = 7); 6% ± 2.5% in ASN treated cells (n = 3) and was increased by 5% ± 2.1% (n = 3) in BafA1-treated cells, and these changes were significantly different than the reduction observed with insulin alone.
Fig. 2.
Autophagy inhibitors block insulin suppression of apo B secretion by RH. (A) RH were pre-treated with 10 mM 3MA, 20 mM ASN or 100 nM BafA1 or vehicle control (CON) for 45 min followed by ± insulin addition (final, 100 nM) and incubations were continued for 5 h. Media apo B concentrations were determined by RIA, and the reduction with insulin was calculated as a percentage change from the no insulin condition. Results are mean percent ± S.E.M. from 3-6 independent rat liver preparations using 3-5 dishes per condition. *Indicates that the percent reduction with insulin is significantly different only in control RH at p < 0.001. (B) Vps34mt or empty vector plasmids were expressed in McA cells followed by ± insulin treatment for 6 h and media apo B was quantified by RIA. (B) Cellular Vps34 and GAPDH (loading control) were evaluated by western immunoblotting. (C) Reduced media apo B content with insulin as a percentage of no insulin at 6 h for empty vector control (CON) and Vps34 mt expressing cells is shown as mean percent ± S.E.M. where 3-4 plates were analyzed per condition in 4 independent studies. *Indicates reduced apo B secretion with insulin is significant at p < 0.01. (D) Insulin-dependent phosphorylation of S463 of pAkt relative to Akt mass as determined by western immunoblotting.
Apo B is known to be degraded by the proteasome through ERAD which is inhibited by 10 μM lactacystin (LAC) [6]. To exclude a role for ERAD in insulin-dependent degradation, RH were preincubated with 10 μM LAC (45 min) followed by incubation ± insulin (final, 100 nM) for 5 h and media apo B concentrations analyzed. Insulin inhibited apo B secretion in the absence of LAC by 51% ± 5% (n = 5, p < 0.001), and in presence of LAC by 67% ± 14% (n = 5, p < 0.001). These results indicate that IDAD is not mediated by proteasomal degradation, and favor autophagy as the relevant mechanism involved.
We next examined the role of Class III PI3K Vps34 which plays an essential role in liver autophagy [26] and whose kinase activity produces PI3P, a phospholipid critical in membrane trafficking. We used a kinase-deficient mutant Vps34 (Vps34mt) [19] which we expressed in McA cells by transfection (Fig. 2B). Over-expression of Vps34mt significantly reduced the ability of insulin to suppress apo B secretion to only 2% ± 3% compared with an average reduction of 33% ± 5% in control McA cells transfected with empty vector (Fig. 2C). However, insulin maintained its ability to stimulate S473 phosphorylation of Akt indicating that insulin stimulated the PI3K/PIP3/Akt pathway (Fig. 2D). Cellular p62/SQSTM1 levels were significantly increased by an average of 22% by insulin (mean ± S.D.: 0.72 ± 14 vs 0.88 ± 0.16, n = 4 independent studies) indicative of autophagy inhibition [27], and consistent with the known effect of insulin to inhibit autophagy [28]. The ability of insulin to inhibit autophagy yet initiate degradation of apo B through Vps34 is unexpected. As Vps34 is not known to be activated by insulin [29], our results suggest an initiating event prior to entry of apo B into an autophagy pathway which we speculate involves Class I PI3K and PIP3 generation.
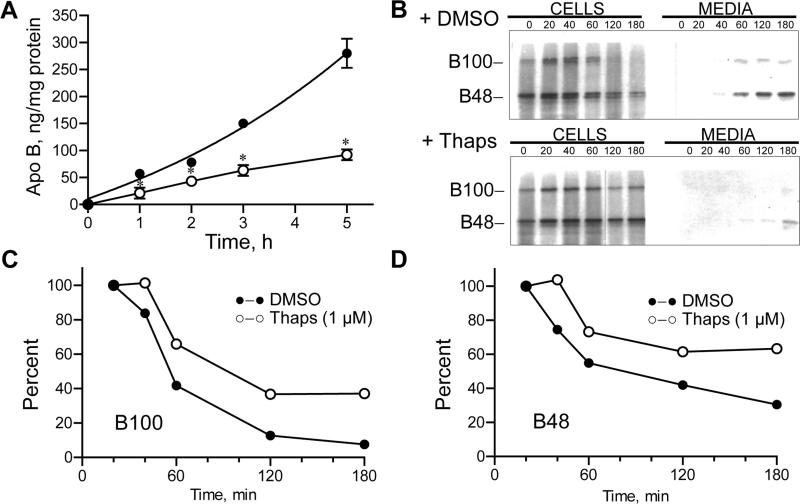
Thapsigargan inhibits the ER Ca2+ ATPase and induces ER stress by inhibiting Rab7 dependent ER stress induced autophagosomal vacuolar (AV) fusion with endosomal/lysosomal structures [15]. In RH thapsigargin rapidly suppressed apo B secretion (Fig. 3A), and by 5 h media apo B was reduced by 67% ± 10% (p < 0.01, mean ± S.D., n = 4). Similar results were obtained in McA cells where apo B secretion was reduced by 84% ± 13% (p < 0.01, n = 3). Pulse chase studies demonstrated an almost complete blockade of 35S-B100 and 35S-B48 secretion by thapsigargin with cellular retention of intact B100 and B48 (Fig 3B). Kinetic analysis confirmed that 40% of B100 and 64% of newly synthesized B100 and B48 were retained in cells by 3 h (Fig. 3C,D). These results are consistent with thapsigargan causing AV accumulation and inhibiting autophagic degradation of apo B not degraded by the proteasome [15]. Pulse chase studies in McA cells yielded similar results with 48% of B100 and 52% of B48 being retained in cells by 3 h of chase (data not shown). Retention of both B100 and B48 with thapsigargan contrasts with IDAD which shows selectivity for B100 and when IDAD is inhibited permits apo B to be secreted.
Fig. 3.
Thapsigargan treatment of RH blocks apo B secretion, and leads to cellular retention of intact B100 and B48. (A) RH were incubated with 1 μM thapsigargan (Thaps) (○−○) or DMSO (●−●), and media apo B was measured by RIA at various times thereafter. Average media apo B ± S.D. (n = 4 replicate plates) is plotted time. (B) RH were incubated with 1 μM Thaps for 45 min and subjected to pulse chase analysis. Cell and media 35S-labeled apo B were isolated and 35S-B100 and 35S-B48 were visualized by Phosphoimager. (C, D) Cellular 35S-apo B is plotted as percent remaining of pulse labeled 35S-apo B at 20 min against time of chase for B100 (C) and for B48 (D).
In RH insulin rapidly stimulates Class I PI3K which translocates to low density microsomes where apo B synthesis and VLDL assembly is initiated [4]. Disappearance of apo B following insulin requires movement to a post-ER compartment for degradation [3]. Reversal of insulin suppression of apo B secretion by PTEN over-expression provides new evidence supporting the role of insulin-activated Class I PI3K and PIP3 in initiating IDAD. Supporting this conclusion are the following findings. Constitutively active Class I PI3K is sufficient to mediate suppressed apo B secretion in the absence of insulin [11]. Insulin receptor-mediated tyrosine phosphorylation of insulin receptor substrates induces activation of Class I PI3K, and vanadate, which blocks dephosphorylation, mimics insulin suppression of apo B [30]. Furthermore, increased expression of protein tyrosine phosphatase 1B, an ER-localized phosphatase, leads to stabilization of cellular apo B [31]. We hypothesize that PIP3 generation from ER-localized Class I PI3K targets B100 in an unknown manner leading to increased interaction with sortilin [11], a Golgi localized sorting protein [32]. Considering that insulin inhibits the maturation phase of VLDL assembly by preventing bulk lipid transfer to pre-VLDL [33] which also takes place in the Golgi [34], it is possible that by blocking maturation, immature pre-VLDL-apo B particles are shunted to lysosomes employing elements of autophagy including Vps34.
Class II PI3Ks have also been shown to be activated by insulin mainly producing PI3P [35] which is central to autophagy and membrane trafficking [36]. Recently, Class II PI3K-C2γ and PI (3,4) biphosphate product have been shown to be involved in IDAD rather than class I PI3K and PIP3 [37]. Insulin maintained its suppressive effect on apo B secretion in the presence of PIK75, a Class I PI3K inhibitor [37]. However, PIK75 preferentially inhibits p110α and incompletely blocks insulin signaling to Akt leaving open the possibility that p110β, which is not inhibited by PIK75, plays a role in IDAD [38]. Recent studies indicate that p110β may function as a positive regulator of autophagy [39]. More research will be necessary to define roles for different classes of PI3K, and the required product phospholipids for each step in IDAD.
We propose a sequence of events in IDAD whereby activated Class I PI3K translocates to the ER generating PIP3 and initiating IDAD [4]. As apo B moves in the secretory pathway, B100 binding to sortilin is increased [11] followed by a complex sequence of events involving activated PI3K and elements of autophagy ultimately leading to apo B degradation in lysosomes. Increasing our knowledge of how IDAD is regulated is fundamental to our understanding of hepatic VLDL metabolism. In humans IDAD is reduced with insulin resistance, and is associated with hypersecretion of triglyceride and associated hypertriglyceridemia [1]. Identifying each step in the IDAD pathway will provide future therapeutic targets for modification of hepatic triglyceride metabolic pathways.
Abbreviations
- ERAD
endoplasmic reticulum-associated degradation
- PI3K
phosphatidylinositide 3-kinase
- PIP3
phosphatidylinositide (3,4,5) triphosphate
- PI3P
phosphatidylinositide 3 monophosphate
- PTEN
phosphatase and tensin homologue
- PERPP
post-ER presecretory proteolysis
- IDAD
insulin-dependent apolipoprotein B degradation
- VLDL
very low density lipoprotein
References
- 1.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 2.Davidson NO, Shelness GS. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr. 2000;20:169–193. doi: 10.1146/annurev.nutr.20.1.169. [DOI] [PubMed] [Google Scholar]
- 3.Sparks JD, Phung TL, Bolognino M, Sparks CE. Insulin-mediated inhibition of apolipoprotein B secretion requires an intracellular trafficking event and phosphatidylinositol 3-kinase activation: studies with brefeldin A and wortmannin in primary cultures of rat hepatocytes. Biochem J. 1996;313(Pt 2):567–574. doi: 10.1042/bj3130567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phung TL, Roncone A, Jensen KL, Sparks CE, Sparks JD. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J Biol Chem. 1997;272:30693–30702. doi: 10.1074/jbc.272.49.30693. [DOI] [PubMed] [Google Scholar]
- 5.den Boer MA, Voshol PJ, Kuipers F, Romijn JA, Havekes LM. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am J Physiol Endocrinol Metab. 2006;291:E1360–1364. doi: 10.1152/ajpendo.00188.2006. [DOI] [PubMed] [Google Scholar]
- 6.Fisher EA, Zhou M, Mitchell DM, Wu X, Omura S, Wang H, Goldberg AL, Ginsberg HN. The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J Biol Chem. 1997;272:20427–20434. doi: 10.1074/jbc.272.33.20427. [DOI] [PubMed] [Google Scholar]
- 7.Pan M, Maitin V, Parathath S, Andreo U, Lin SX, St Germain C, Yao Z, Maxfield FR, Williams KJ, Fisher EA. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci U S A. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong S, Magnolo AL, Sundaram M, Zhou H, Yao EF, Di Leo E, Loria P, Wang S, Bamji-Mirza M, Wang L, McKnight CJ, Figeys D, Wang Y, Tarugi P, Yao Z. Nonsynonymous mutations within APOB in human familial hypobetalipoproteinemia: evidence for feedback inhibition of lipogenesis and postendoplasmic reticulum degradation of apolipoprotein B. J Biol Chem. 2010;285:6453–6464. doi: 10.1074/jbc.M109.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparks JD, Sparks CE. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem. 1990;265:8854–8862. [PubMed] [Google Scholar]
- 11.Chamberlain JM, O'Dell C, Sparks CE, Sparks JD. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochem Biophys Res Commun. 2013;430:66–71. doi: 10.1016/j.bbrc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang YY, Juhasz G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufeld TP. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans. 2009;37:232–236. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- 13.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 14.Jaber N, Dou Z, Lin RZ, Zhang J, Zong WX. Mammalian PIK3C3/VPS34: the key to autophagic processing in liver and heart. Autophagy. 2012;8:707–708. doi: 10.4161/auto.19627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganley IG, Wong PM, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 2011;42:731–743. doi: 10.1016/j.molcel.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 17.Sparks JD, Chamberlain JM, O'Dell C, Khatun I, Hussain MM, Sparks CE. Acute suppression of apo B secretion by insulin occurs independently of MTP. Biochem Biophys Res Commun. 2011;406:252–256. doi: 10.1016/j.bbrc.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparks JD, Bolognino M, Trax PA, Sparks CE. The production and utility of monoclonal antibodies to rat apolipoprotein B lipoproteins. Atherosclerosis. 1986;61:205–211. doi: 10.1016/0021-9150(86)90139-5. [DOI] [PubMed] [Google Scholar]
- 19.Row PE, Reaves BJ, Domin J, Luzio JP, Davidson HW. Overexpression of a rat kinase-deficient phosphoinositide 3-kinase, Vps34p, inhibits cathepsin D maturation. Biochem J. 2001;353:655–661. doi: 10.1042/0264-6021:3530655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirieac DV, Davidson NO, Sparks CE, Sparks JD. PI3-kinase activity modulates apo B available for hepatic VLDL production in apobec-1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G382–388. doi: 10.1152/ajpgi.00472.2005. [DOI] [PubMed] [Google Scholar]
- 21.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 22.Sparks JD, Sparks CE. Overindulgence and metabolic syndrome: is FoxO1 a missing link? J Clin Invest. 2008;118:2012–2015. doi: 10.1172/JCI35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarze PE, Seglen PO. Reduced autophagic activity, improved protein balance and enhanced in vitro survival of hepatocytes isolated from carcinogen-treated rats. Exp Cell Res. 1985;157:15–28. doi: 10.1016/0014-4827(85)90148-x. [DOI] [PubMed] [Google Scholar]
- 24.Hoyvik H, Gordon PB, Berg TO, Stromhaug PE, Seglen PO. Inhibition of autophagic lysosomal delivery and autophagic lactolysis by asparagine. J Cell Biol. 1991;113:1305–1312. doi: 10.1083/jcb.113.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–950. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- 26.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 28.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 29.Juhasz G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, Neufeld TP. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson TK, Salhanick AI, Sparks JD, Sparks CE, Bolognino M, Amatruda JM. Insulin-mimetic effects of vanadate in primary cultures of rat hepatocytes. Diabetes. 1988;37:1234–1240. doi: 10.2337/diab.37.9.1234. [DOI] [PubMed] [Google Scholar]
- 31.Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, Adeli K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem. 2002;277:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown AM, Gibbons GF. Insulin inhibits the maturation phase of VLDL assembly via a phosphoinositide 3-kinase-mediated event. Arterioscler Thromb Vasc Biol. 2001;21:1656–1661. doi: 10.1161/hq1001.096640. [DOI] [PubMed] [Google Scholar]
- 34.Gusarova V, Seo J, Sullivan ML, Watkins SC, Brodsky JL, Fisher EA. Golgi- associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J Biol Chem. 2007;282:19453–19462. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- 35.Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, Cazzolli R, Shepherd PR, James DE, Maffucci T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem. 2007;282:28226–28236. doi: 10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- 36.Burman C, Ktistakis NT. Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett. 2010;584:1302–1312. doi: 10.1016/j.febslet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Andreo U, Guo L, Chirieac DV, Tuyama AC, Montenont E, Brodsky JL, Fisher EA. Insulin-Stimulated Degradation of Apolipoprotein B100: Roles of Class II Phosphatidylinositol-3-Kinase and Autophagy. PLoS One. 2013;8:e57590. doi: 10.1371/journal.pone.0057590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaussade C, Rewcastle GW, Kendall JD, Denny WA, Cho K, Gronning LM, Chong ML, Anagnostou SH, Jackson SP, Daniele N, Shepherd PR. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. Biochem J. 2007;404:449–458. doi: 10.1042/BJ20070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dou Z, Chattopadhyay M, Pan JA, Guerriero JL, Jiang YP, Ballou LM, Yue Z, Lin RZ, Zong WX. The class IA phosphatidylinositol 3-kinase p110-beta subunit is a positive regulator of autophagy. J Cell Biol. 2010;191:827–843. doi: 10.1083/jcb.201006056. [DOI] [PMC free article] [PubMed] [Google Scholar]