Short abstract
For patients suffering from severe coronary heart disease (CHD), the development of a cell-based tissue engineered blood vessel (TEBV) has great potential to overcome current issues with synthetic graft materials. While marrow stromal cells (MSCs) are a promising source of vascular smooth muscle cells (VSMCs) for TEBV construction, they have been shown to differentiate into both the VSMC and osteoblast lineages under different rates of dynamic strain. Determining the permanence of strain-induced MSC differentiation into VSMCs is therefore a significant step toward successful TEBV development. In this study, initial experiments where a cyclic 10% strain was imposed on MSCs for 24 h at 0.1 Hz, 0.5 Hz, and 1 Hz determined that cells stretched at 1 Hz expressed significantly higher levels of VSMC-specific genetic and protein markers compared to samples stretched at 0.1 Hz. Conversely, samples stretched at 0.1 Hz expressed higher levels of osteoblast-specific genetic and protein markers compared to the samples stretched at 1 Hz. More importantly, sequential application of 24–48 h periods of 0.1 Hz and 1 Hz strain-induced genetic and protein marker expression levels similar to the VSMC profile seen with 1 Hz alone. This effect was observed regardless of whether the cells were first strained at 0.1 Hz followed by strain at 1 Hz, or vice versa. Our results suggest that the strain-induced VSMC phenotype is a more terminally differentiated state than the strain-induced osteoblast phenotype, and as result, VSMC obtained from strain-induced differentiation would have potential uses in TEBV construction.
Keywords: mesenchymal stem cells, cyclic strain, tissue engineering, transdifferentiation
Introduction
CHD is the leading cause of morbidity and mortality in the U.S. [1]. For patients suffering from severe CHD, plaque build-up in the wall of the coronary arteries can cause inadequate oxygen supply to the heart, leading to cardiac ischemia and eventually death [2]. In order to restore adequate blood circulation, coronary artery bypass grafts (CABG) are performed. CABG procedures are highly invasive and involve the harvesting of a vessel at a secondary site [3,4]. Although synthetic materials such as Dacron and polytetrafluoroethylene are often used for vascular grafts, they can lead to thrombosis or neointimal hyperplasia [5]. More importantly, while synthetic grafts work well for arteries bigger than 12 mm, they are not suitable for the geometry of coronary arteries [6,7]. A cell-based TEBV has great potential to overcome the morbidity of autografts and the issues with synthetic materials.
In order to attain the proper organization and mechanical properties found in native vessels, VSMCs are used as a crucial component of TEBVs [8–11]. Physiological conditions expose native vessels to a constant cyclic mechanical strain of 9–12% strain at roughly 1 Hz [12]. Under pulsatile flow, VSMCs in culture have been shown to express a more organized and layered structure in addition to a higher level of extracellular matrix (ECM) reorganization, resulting in significantly higher Young's modulus and burst strength [13,14]. VSMCs play a crucial role in TEBVs and it is evident that the application of cyclic strain can help to duplicate their organization and mechanical properties found in native vessels. Nevertheless, isolation of patient VSMCs for graft construction is a highly invasive procedure and may not be the most efficient TEBV cell source.
With growing interest in stem cells, research has shown that MSCs, or mesenchymal stem cells, are a potential cell source for VSMCs. Compared to VSMCs, MSCs are more easily harvested and isolated, resulting in a process that is less invasive and leads to less morbidity than a vessel harvest for VSMCs [15]. In addition, MSCs have a much higher proliferative capacity and can potentially generate more tissue in less time than VSMCs [16,17]. The differentiation capacity of MSCs spans the broadest spectrum of terminally differentiated cell types among adult stem cells; the documented differentiation capacity of MSCs includes bone, cartilage, muscle, marrow stroma, tendon/ligaments, adipose tissue, connective tissues, and smooth muscle [17–22]. These combined factors make MSCs a potential cell source for vascular regeneration and a great candidate for the fabrication of tissue engineered vascular constructs.
Studies in recent years have shown bone marrow-derived MSCs to differentiate into VSMCs in response to transforming growth factor β-1 and ascorbic acid [23–26]. Despite success in static conditions, MSCs differentiated into VSMCs must retain their cell type under dynamic conditions to be useful in a functional TEBV. To this end, several studies have shown that mechanical stimulation involving cyclic strain was capable of inducing MSC differentiation into the VSMC lineage in as short as 24 h [27–30]. Higher strain frequencies were observed to yield more occurrences of VSMC commitment, while lower strain frequencies were often observed to induce osteogenesis [31,32]. The distinction between strain conditions suggests that there may be two distinct mechanical conditions capable of directing MSC differentiation, one leading to VSMCs and the other to osteoblasts. In order to implement MSCs in TEBV designs, it is essential to elucidate the stability of these lineage commitments.
In this study, we focused on the stability of MSC differentiation into VSMCs and osteoblasts when differentiation was induced solely by cyclic mechanical stimulation, thus mimicking the strain a TEBV would be exposed to in vivo. We apply conditions centering on the physiological conditions of 10% strain at 1 Hz [11] experienced by native VSMCs to study the effective frequency range of using mechanical conditioning for directed differentiation. By using single- and dual-stage conditioning, we investigated the stability of mechanically induced MSC differentiation into VSMCs, and in so doing determined whether any fluctuation in stretch frequency pre- or postimplantation could potentially compromise a constructed graft.
Materials and Methods
Cell Culture and Substrate Preparation.
Primary human MSCs (hMSCs; Lonza, Basel, Switzerland) were cultured in high glucose Dulbecco's modified eagle medium (DMEM; Gibco® Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 1% L-glutamine, and 1% penicillin-streptomycin (Invitrogen). Cells were maintained in a humidified incubator with 5% CO2 at 37 °C and passaged at 80% confluence. All experiments were conducted using cells between passages 4 and 10. Passages 4–10, while considered high for MSCs in most studies, were specifically chosen since differentiation capacity at higher passages is a crucial requirement for this study. This is because in order to construct a full vascular graft from marrow-isolated MSCs with efficiency, the isolated MSCs must undergo several population doublings prior to directed differentiation.
Cells were seeded onto nonreinforced, vulcanized, polydimethylsiloxane (PDMS) sheetings (Specialty Manufacturing, Inc., Pineville, NC) manufactured to a thickness of 254 μm. Sample substrates were cut into 1.5 cm × 4 cm rectangular films to fit the cell-stretching device. Prior to seeding, the PDMS films were treated with 3× layer-by-layer polyelectrolyte deposition of 1 mg/ml polyethyleneimine (Sigma Aldrich, St. Louis, MO) and 3 mg/ml polystyrene sulfonate (Alpha Aesar, Ward Hill, MA), followed by a coating of human fibronectin(Millipore, Billerica, MA) at 40 μg/ml. hMSCs were then seeded at a density of 1.0 × 104 cells/cm2 and allowed to attach for 24 h before loading onto the stretching device.
Uniaxial Mechanical Stimulation.
Samples were stretched using a modified version of a proprietary, uniaxial mechanical cell stretcher [33]. Conditions for single-stage experiments were set to 24 h durations at 10% strain under 1 Hz, 0.5 Hz, and 0.1 Hz frequencies as well as a static unstretched condition for control. Control experiments had determined that the strain in the center 10 mm × 10 mm section of the slide varied by only 0.3%. Conditions for dual-stage experiments were set to 24 h durations at 10% strain under 1 Hz and 0.1 Hz frequencies. The first and second conditions had alternating frequencies applied; if the first condition was 1 Hz, the second condition was set to 0.1 Hz and vice versa. Switching from one condition to the other was done immediately following the termination of the first condition (Fig. 1). Dual-stage conditions were repeated using 48 h durations for each condition to study the effects of more prolonged stimulation on transdifferentiation. Cell samples were analyzed immediately following the conclusion of each experiment.
Fig. 1.
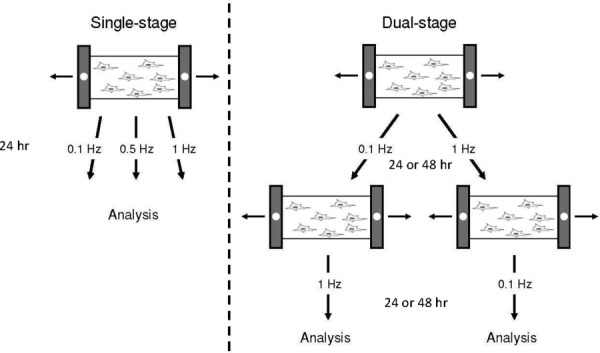
Uniaxial mechanical stimulation. Single- and dual-stage mechanical stimulation were delivered at 10% strain under the specified conditions. Dual-stage mechanical stimulation consists of two distinct conditions delivered sequentially.
Image Analysis of Cell Alignment.
Samples were fixed in cold 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) for 15 min and washed three times with phosphate buffered saline (PBS; Invitrogen). Bright field images were acquired using an Axiovert S-100 microscope system (Carl Zeiss, Inc., Thornwood, NY) equipped with metamorph imaging software under 10× magnification (Molecular Devices, Sunnyvale, CA).
Images of cells in the 10 mm × 10 mm region of consistent strain were analyzed using imagej software and Image-Pro Plus (Media Cybernetics, Silver Spring, MD). Cell outlines were manually traced, and the orientation of the cells were determined by measuring the major axis of each cell relative to the direction of applied strain. Measured angles varied from 0 deg to 90 deg, signifying a range from perfect alignment with the strain to alignment perpendicular to the strain, respectively (Fig. 2). Twenty images were acquired for each condition without overlap of field of view. Each image was then divided into a 5 × 5 grid, and one cell at the center of each grid was measured for polarity for a total of 500 measurements per condition. This promoted a measurement collection process that was unbiased from localized cell-to-cell interaction-influenced orientation.
Fig. 2.
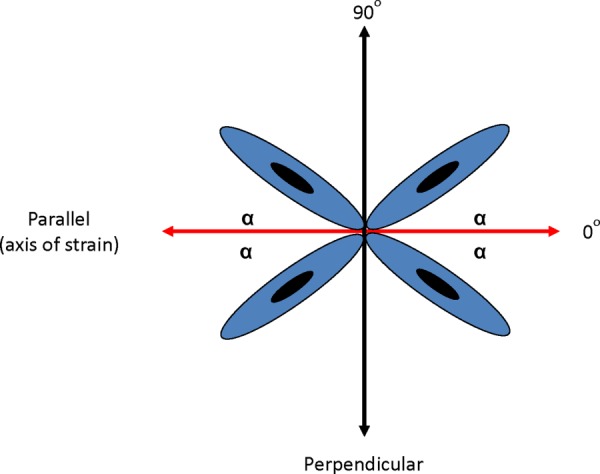
Measurement of cell orientation relative to the direction of mechanical strain. Cellular orientation relative to the direction of strain was measured from the angle α.
Quantitative Real-Time-Polymerase Chain Reaction (RT-PCR) Analysis of Lineage-Specific Markers.
To quantify the degree of lineage commitment of the MSCs, quantitative RT-PCR (qRT-PCR) was performed using primers that targeted lineage-specific genes. Total ribonucleic acid (RNA) was extracted with RNeasy Mini Kit (Qiagen, Germantown, MD) and reverse transcribed into complementary DNA (cDNA) deoxyribonucleic acid by high capacity RNA-to-cDNA Master Mix (Applied Biosystems, Carlsbad, CA). RT-PCR was then performed with an Applied Biosystems 7300 RT-PCR system using Taqman Gene Expression Assays) and Taqman Fast Universal PCR Master Mix (both Applied Biosystems). The Taqman PCR primers used are summarized in Table 1. Duplex PCR reactions were conducted for the simultaneous quantification of the housekeeping gene Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (VIC® dye) and a given target gene (FAMTM dye), starting with 1 μl of cDNA sample in a total volume of 20 μl. Three separate experiments were analyzed, each containing groups subjected to various strain conditions and a control group subjected to no strain (i.e., 0 Hz). For a given experiment, target gene expression in each group was first quantified relative to that of GAPDH. Using these values, the expression of target genes in experimental groups was then expressed relative to that in the control (0 Hz) group, giving the strain-induced change (increase or decrease). The average fold changes for three experiments are shown in Figs. 3 and 6.
Table 1.
RT-PCR primers for amplification of lineage-specific genes and the length of amplified products
| Lineage | Gene | Taqman Assay ID | Amplicon Length |
|---|---|---|---|
| GAPDH* | Hs999999905_m1 | 122 | |
| Osteoblast | BGLAP (osteocalcin, OC) | Hs00609452_g1 | 74 |
| Osteoblast | SPP1 (osteopontin, OPN) | Hs00959010_m1 | 84 |
| SMC | ACTA2 (SM-α actin) | Hs00426835_g1 | 105 |
| SMC | MYH11 (SM-MHC) | Hs00224610_m1 | 78 |
| SMC | CNN1 (calponin) | Hs00154543_m1 | 93 |
Housekeeping gene
Fig. 3.
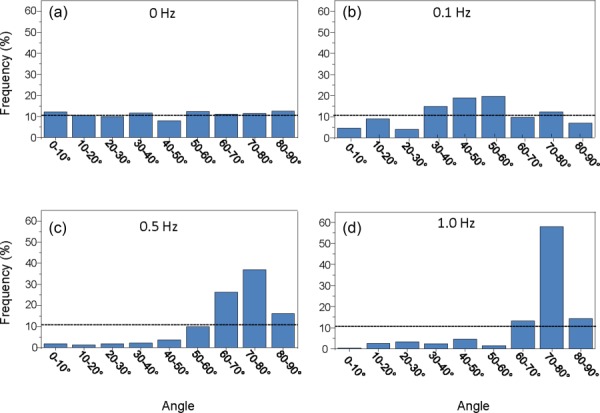
MSC alignment increased as a function of stretch frequency. MSC alignment distribution after 24 h of mechanical stimulation at (a) 0 Hz, (b) 0.1 Hz, (c) 0.5 Hz, and (d) 1 Hz frequencies shows an alignment shift toward the direction perpendicular to the axis of strain, as the strain frequency increases from 0 to 1 Hz. The dotted line depicts the frequency that would be expected at each angle grouping with random MSC alignment.
Fig. 6.
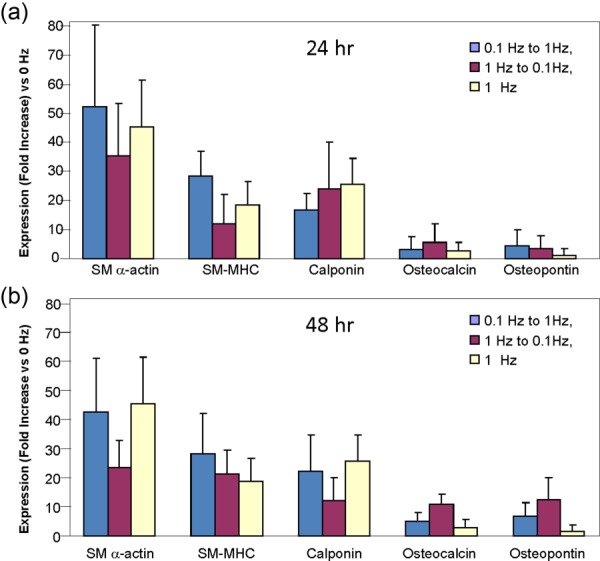
MSC lineage-specific gene expression after dual-stage mechanical stimulation indicates MSC transdifferentiation to a VSMC phenotype. (b) Expression of VSMC-specific genes was upregulated compared to osteoblast-specific genes when transitioned from either 0.1 Hz to 1 Hz condition or 1 Hz–0.1 Hz. The gene marker distribution was similar to that seen with single-stage 1 Hz condition. The same observation was made for both the 24 and 48 h duration conditions, suggesting the capacity of transdifferentiation in only one direction regardless of duration. Each bar represents the mean ± SD, n = 3.
Immunocytochemical Analysis of MSCs.
Samples were fixed in cold 4% PFA (electron microscopy sciences) for 15 min and washed three times with cold PBS (Invitrogen). To permeabilize the cells, 0.1% Triton X-100 (Sigma) in PBS solution was added for 10 min. The samples were then washed three times with cold PBS and incubated for 1 h at 37 °C in 0.1% bovine serum albumin (BSA; Sigma Aldrich) in PBS and goat serum (Millipore) at 15 μl/2 ml to block nonspecific binding. After blocking, the samples were incubated for 2 h at 37 °C in 0.1% BSA and the appropriate dilution of primary antibody. The primary antibodies used were monoclonal anti-actin, α-smooth muscle mouse IgG, clone 1A4 (Product No. A5228, Sigma Aldrich) at a 1:200 dilution and human osteocalcin antibody, monoclonal mouse IgG, clone 190125 (Catalog No. MAB1419; R&D Systems, Minneapolis, MN) at a 1:40 dilution. After three washes with 0.1% BSA, the samples were incubated for 1 h at room temperature in 0.1% BSA and a 1:200 dilution of goat anti-mouse IgG-FITC (Product No. F2012; Sigma Aldrich). The samples were washed three times with 0.1% BSA in PBS and then incubated for 10 min at room temperature in 0.1% BSA and a 1:400 dilution of Hoechst 33342 nuclear dye (Invitrogen). After three washes with PBS, the samples were imaged immediately using the Axiovert S-100 microscope (Carl Zeiss, Inc.) and assessed for protein expression.
Statistical Analysis.
Each experiment was performed at least in triplicate. Statistical significance between multiple groups was determined by analysis of variance, with intergroup significances identified by Tukey's post hoc test.
Results
MSC Alignment Increased as a Function of Stretch Frequency.
While cellular orientation for unstretched cells remained random and evenly distributed (Fig. 3(a)), a pattern for orientation was observed once mechanical stimulation was applied for 24 h. At 0.1 Hz, a gradual shift in alignment toward 40 deg–60 deg was observed in the orientation distribution (Fig. 3(b)). Fewer cells possessed orientations parallel or perpendicular to the axis of strain while over 38% of the cells lay within 40 deg–60 deg with respect to the axis of strain. At 0.5 Hz, the alignment of MSCs further shifted toward the direction perpendicular to the axis of strain (Fig. 3(c)). Over 78% of the cells possessed an orientation greater than 60 deg with respect to the axis of strain. This shift in orientation was significantly emphasized at 1 Hz strain frequency, with over 85% of the cells at an angle greater than 60 deg with respect to the axis of strain (Fig. 3(d)). These results show that MSC alignment shifts toward the direction perpendicular to the axis of strain as uniaxial cyclic strain frequency increases from 0 to 1 Hz. This observation confirms previous results [34,35] and is consistent with the physiological organization of VSMCs in the media layer of the artery.
MSC Differentiation Into VSMC and Osteoblast Lineages Was Induced by Distinct Stretch Frequencies.
Quantitative analysis of lineage-specific genetic markers showed that the mRNA expression of the VSMC markers smooth muscle actin (SM-α actin), smooth muscle myosin heavy chain (SM-MHC), and calponin significantly increased as the stretch frequency rose from 0.1 Hz to 1 Hz stretch. Conversely, the expression of the osteoblast marker osteocalcin significantly decreased under these conditions, and the marker osteopontin strongly trended downward (Fig. 4(a)). There were no significant differences in expression of any of the genes between the 0.5 Hz and either 0.1 or 1 Hz groups.
Fig. 4.
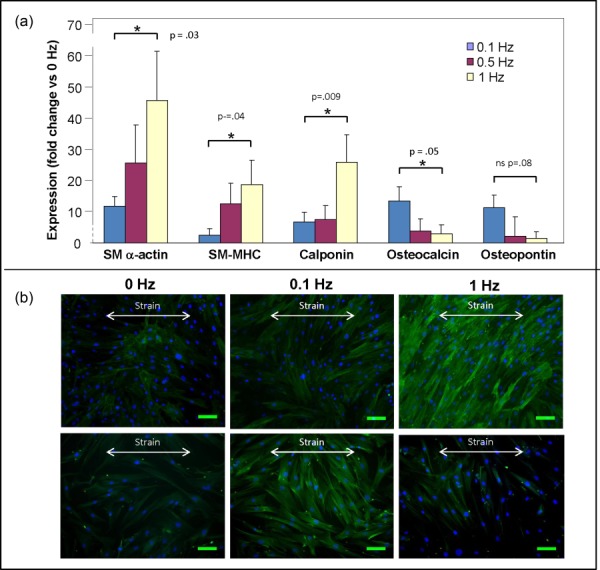
Expression of lineage-specific genes and protein shows MSC differentiation into VSMCs and osteoblasts under distinct frequencies. (a) At 1 Hz stretch frequency, VSMC-specific genes (SM-α actin, SM-MHC, and calponin) were highly upregulated compared to osteoblast-specific genes (osteocalcin and osteopontin). Conversely, osteoblast-specific genes were relatively upregulated at the 0.1 Hz frequency. Each bar represents the mean ± SD, n = 3, * p < 0.05. (b) Bright field and fluorescent images of MSCs show highly expressed SM-α actin under the 1 Hz condition and a positive expression of osteocalcin under 0.1 Hz condition. Scale bar is 100 μm.
To supplement the findings at the RNA level, the strained cells were stained for SM-α actin and osteocalcin, and the expression of these lineage-specific proteins was determined by immunocytofluorescence. Fluorescence microscopy confirmed the qRT-PCR findings. Expression of SM-α actin was significantly higher for the 1 Hz frequency condition than in the unstretched and 0.1 Hz conditions. Similarly, in the 0.1 Hz condition, expression of osteocalcin was higher than in the unstretched and 1 Hz conditions (Fig. 4(b)).
MSCs Showed Pronounced Alignment Regardless of Sequence and Duration of Dual-Stage Mechanical Stimulation.
While cellular reorientation is well documented as a response to mechanical stimulation, the stability of this response to further changes in mechanical stimulation remains to be elucidated. Accordingly, we delivered dual-stage mechanical stimulation using both 0.1 Hz and 1 Hz frequencies in both orders at 24-h and 48-h durations. Cells in the group first exposed to 0.1 Hz and then to 1 Hz (24 h each) showed a final alignment profile almost identical to that observed for cells receiving single-stage 1 Hz stimulation. The alignment profile for the 1 Hz–0.1 Hz group was also similar to that for the single-stage 1 Hz group, although there did appear to be a small, partial drift back toward the single-stage 0.1 Hz profile. Virtually identical alignment profiles were observed when stage durations were extended from 24 to 48 h (Figs. 4 and 5), indicating that 24 h of strain at a given frequency is sufficient to elicit any changes in cell orientation that may occur.
Fig. 5.
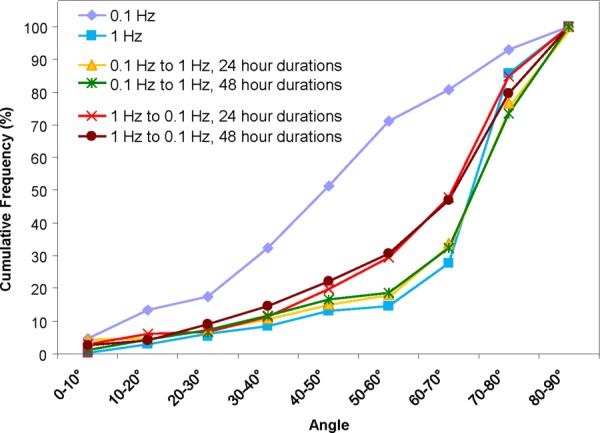
MSC alignment distributions after dual-stage mechanical stimulation indicate MSC transdifferentiation to a VSMC phenotype. (a) MSC alignment distribution for all dual-stage combinations shows a profile similar to that observed with single-stage 1 Hz stretching, expressing a pronounced shift toward the direction perpendicular to the axis of strain.
Mechanical Stimulation Was Capable of One-Directional Transdifferentiation of MSCs From the Osteoblast to the VSMC Lineage.
qRT-PCR analysis was next done after our dual-stage studies to examine the mRNA expression of SM-α actin, SM-MHC, calponin, osteocalcin, and osteopontin. The mRNA levels for all genes observed after dual-stage strain administered in either order were not statistically different than those observed with 1 Hz single-stage strain regardless of whether the stages were performed for 24 h (Fig. 6(a)) or 48 h (Fig. 6(b)).
Fluorescence microscopy showed confirmation to our qRT-PCR findings. For each of the four combinations of conditions, the samples stained highly positive for SM-α actin and positive but with low expression for osteocalcin (Fig. 7). These results coincide directly with the alignment results from dual-stage stretch conditions, indicating differentiation into the VSMC phenotype regardless of the direction of frequency transition and condition duration.
Fig. 7.
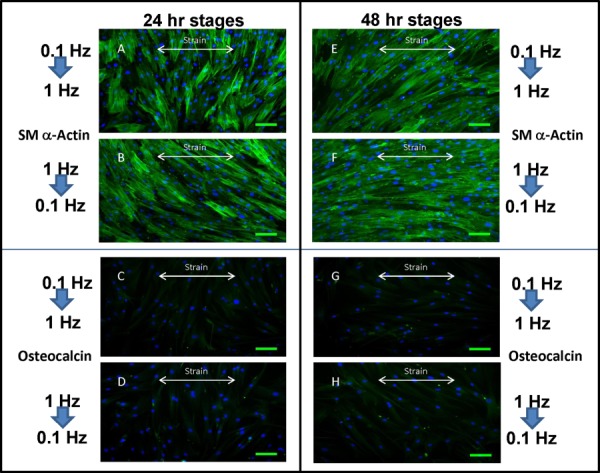
SM-α actin and osteocalcin staining of MSCs showed evidence of VSMC lineage commitment in all dual-stage mechanical stimulation conditions. A four two-stage protocols resulted in the same increased level of SM-α actin expression and low level of osteocalcin expression, which would be indicative of MSC commitment into the VSMC lineage. (a)–(d): 24 h stages, (e)–(h): 48 h stages. Scale bar is 100 μm.
Discussion
The initial investigations of this study confirm that the frequency of mechanical stimulation on MSCs directly influences cellular phenotype as determined by cell orientation, and gene and protein expression. Thus, the 1 Hz directional strain, which was modeled after the physiological pulsatile frequency, induced a cellular alignment perpendicular to the direction of strain, similar to that observed in VSMC in muscularized blood vessels. Furthermore, the expression of VSMC genetic markers was significantly increased at this frequency. In contrast, the 0.1 Hz directional strain induced only a partial shift in cellular orientation and induced a gene expression profile consistent with that of osteoblasts.
The VSMC markers were chosen for the specific indication of VSMCs of the contractile phenotype. SM-α actin alone is not sufficient as it can also be expressed in fibroblasts. However, the combination of SM-α actin, SMC-MHC, and calponin indicates the presence the contractile apparatus only present in mature VSMCs of the contractile phenotype [25,36,37]. The osteogenic markers chosen are common indicators of osteoblast formation. Osteocalcin and osteopontin play a major role in bone mineralization and thus indicate the presence of mature osteoblasts [38,39].
The most significant finding of this is the demonstration that a 1 Hz stretch frequency for at least 24 h is sufficient to induce a VSMC phenotype, regardless of any previous progression toward the osteoblast phenotype induced by 0.1 Hz strain. Furthermore, attaining the VSMC phenotype appears to largely preclude transdifferentiation back into the osteoblast phenotype. While there may have been a trend toward the acquisition of some osteoblast characteristics with second stage low frequency strain, this trend did not achieve statistical significance with respect to mRNA gene expression, and the protein expression data in Fig. 7 strongly support a VSMClike phenotype. Our data also indicate that extending the second stage 0.1 Hz strain to 48 h gave results almost identical to the 24-h second stage experiments. Overall, these results suggest that either the 1 Hz frequency was a threshold for permanent lineage commitment into the VSMC lineage or that the VSMCs were a dominant cell type for MSC differentiation.
Although the intracellular signaling mechanisms of mechanotransduction have not been well understood, the cytoskeleton is widely understood as an important signal transducer in stress-induced cell response (44–46). Liu et al. demonstrated the significance of actin stress fibers in the alignment response by showing the inability of smooth muscle cells to align under different stretch frequencies after using cytochalasin D to disrupt intact actin filaments [35]. Other studies have also implicated integrins and focal adhesions in alignment mechanotransduction [40–42]. It has also been suggested that stress fiber realignment occurs due to the inability of actin stress fibers to sufficiently relax at high frequencies [43,44]. Under these conditions, the cumulative time spent without tension leads to stress fiber degradation. Newly formed stress fibers oriented perpendicular to the axis of stretch are more stable because they experience less strain and would thus tend to accumulate over time. An alternate theory was proposed by Chen et al. [45] based on a minimal-model cellular mechanosensing study. It was demonstrated in this study that realignment of a stress fiber connecting to two focal adhesions at 1 Hz can be simply explained by mechanical focal adhesion destabilization and the intrinsic physical properties of a stress fiber. Regardless of the exact mechanism, realignment involves integrins and focal adhesions, which also have intracellular signaling functions through the c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) and focal adhesion kinase pathways. These pathways would thus have the potential to explain the changes in gene expression seen in the present study, although this remains to be demonstrated.
The two-dimensional strain system used in this study only allows for the formation of a single layer of SMCs, and as such would be best applied to the construction of small artificial arterioles. A next logical step would be to repeat this study with cells embedded in a 3D collagen-FN matrix, in order to establish conditions for the preparation of smooth muscle cell sheets containing multiple layers of cells. There are at least two distinct advantages to a 3D model. Recent studies have shown that 3D models promote better differentiation (reviewed in Ref. [46]), thus such a system would presumably produce a higher quality tissue sheet. More importantly, multilayer smooth muscle cell sheets would allow the engineering of larger arteries and arterioles.
Conclusions
The creation of a biological construct capable of mimicking the native structure and function of the medial layer of the artery would be a significant step toward a fully functional TEBV. Despite the benefits of MSCs as a cell source of VSMCs, the possibility of MSC transdifferentiation postimplantation is a potential complication that needs to be further understood. In working closer toward this goal, we discovered that the application of two distinct lineage-inductive mechanical conditions does indeed demonstrate the ability of MSC transdifferentiation. However, the observed transdifferentiation was only from the osteoblast lineage to the VSMC lineage under the condition of switching from 0.1 Hz stimulation frequency to 1 Hz. Reversing the sequence of stretch frequencies from 1 Hz to 0.1 Hz induced no transdifferentiation effects from the VSMC to the osteoblast lineage. Furthermore, the duration of each lineage-inductive condition also did not affect the observed one-directional transdifferentiation.
Acknowledgment
The authors acknowledge NIH HL072900 for financial support and the Center for Nanoscience and Nanobiotechnology at Boston University for partial financial support for R.Y. We thank Stanley Heydrick for careful reading and revision of the manuscript.
Contributor Information
Raphael Yao, Department of Biomedical Engineering, Boston University, 44 Cummington Mall, Boston, MA 02215 .
Joyce Y. Wong, Department of Biomedical Engineering, Boston University, 44 Cummington Mall, Boston, MA 02215, e-mail: jywong@bu.edu .
References
- [1]. Eagle, K. A. , Guyton, R. A. , Davidoff, R. , Edwards, F. H. , Ewy, G. A. , Gardner, T. J. , Hart, J. C. , Herrmann, H. C. , Hillis, L. D. , Hutter, A. M., Jr. , Lytle, B. W. , Marlow, R. A. , Nugent, W. C. , Orszulak, T. A. , Antman, E. M. , Smith, S. C., Jr. , Alpert, J. S. , Anderson, J. L. , Faxon, D. P. , Fuster, V. , Gibbons, R. J. , Gregoratos, G. , Halperin, J. L. , Hiratzka, L. F. , Hunt, S. A. , Jacobs, A. K. , and Ornato, J. P. , 2004, “ACC/AHA 2004 Guideline Update for Coronary Artery Bypass Graft Surgery: Summary Article. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery),” J. Am. Coll. Cardiol., 44(5), pp. e213–310. 10.1016/j.jacc.2004.07.021 [DOI] [PubMed] [Google Scholar]
- [2]. Vlodaver, Z. , 2012, Coronary Heart Disease: Clinical, Pathological, Imaging, and Molecular Profiles, Springer, New York. [Google Scholar]
- [3]. Serruys, P. W. , Morice, M. C. , Kappetein, A. P. , Colombo, A. , Holmes, D. R. , Mack, M. J. , Stahle, E. , Feldman, T. E. , Van Den Brand, M. , Bass, E. J. , Van Dyck, N. , Leadley, K. , Dawkins, K. D. , and Mohr, F. W. , 2009, “Percutaneous Coronary Intervention Versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease,” N. Engl. J. Med., 360(10), pp. 961–972. 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- [4]. Roger, V. L. , Go, A. S. , Lloyd-Jones, D. M. , Benjamin, E. J. , Berry, J. D. , Borden, W. B. , Bravata, D. M. , Dai, S. , Ford, E. S. , Fox, C. S. , Fullerton, H. J. , Gillespie, C. , Hailpern, S. M. , Heit, J. A. , Howard, V. J. , Kissela, B. M. , Kittner, S. J. , Lackland, D. T. , Lichtman, J. H. , Lisabeth, L. D. , Makuc, D. M. , Marcus, G. M. , Marelli, A. , Matchar, D. B. , Moy, C. S. , Mozaffarian, D. , Mussolino, M. E. , Nichol, G. , Paynter, N. P. , Soliman, E. Z. , Sorlie, P. D. , Sotoodehnia, N. , Turan, T. N. , Virani, S. S. , Wong, N. D. , Woo, D. , and Turner, M. B. , 2012, “Heart Disease and Stroke Statistics—2012 Update: A Report From the American Heart Association,” Circulation, 125(1), pp. e2–e220. 10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Weinberg, C. B. , and Bell, E. , 1986, “A Blood Vessel Model Constructed From Collagen and Cultured Vascular Cells,” Science, 231(4736), pp. 397–400. 10.1126/science.2934816 [DOI] [PubMed] [Google Scholar]
- [6]. Esquivel, C. O. , and Blaisdell, F. W. , 1986, “Why Small Caliber Vascular Grafts Fail: A Review of Clinical and Experimental Experience and the Significance of the Interaction of Blood at the Interface,” J. Surg. Res., 41(1), pp. 1–15. 10.1016/0022-4804(86)90002-8 [DOI] [PubMed] [Google Scholar]
- [7]. Tiwari, A. , Salacinski, H. J. , Hamilton, G. , and Seifalian, A. M. , 2001, “Tissue Engineering of Vascular Bypass Grafts: Role of Endothelial Cell Extraction,” Eur. J. Vasc. Endovasc. Surg., 21(3), pp. 193–201. 10.1053/ejvs.2001.1316 [DOI] [PubMed] [Google Scholar]
- [8]. Rhodin, J. A. G. , 1979, Architecture of the Vessel Wall. Handbook of Physiology, Section 2, Vol. 2 (RM Berne, ed). American Physiological Society, Baltimore, MD.
- [9]. Thakar, R. G. , Ho, F. , Huang, N. F. , Liepmann, D. , and Li, S. , 2003, “Regulation of Vascular Smooth Muscle Cells by Micropatterning,” Biochem. Biophys. Res. Commun., 307(4), pp. 883–890. 10.1016/S0006-291X(03)01285-3 [DOI] [PubMed] [Google Scholar]
- [10]. Krizmanich, W. J. , and Lee, R. M. , 1997, “Correlation of Vascular Smooth Muscle Cell Morphology Observed by Scanning Electron Microscopy With Transmission Electron Microscopy,” Exp. Mol. Pathol., 64(3), pp. 157–172. 10.1006/exmp.1997.2217 [DOI] [PubMed] [Google Scholar]
- [11]. Li, S. , Sims, S. , Jiao, Y. , Chow, L. H. , and Pickering, J. G. , 1999, “Evidence From a Novel Human Cell Clone That Adult Vascular Smooth Muscle Cells Can Convert Reversibly Between Noncontractile and Contractile Phenotypes,” Circ. Res., 85(4), pp. 338–348. 10.1161/01.RES.85.4.338 [DOI] [PubMed] [Google Scholar]
- [12]. Chapman, G. B. , Durante, W. , Hellums, J. D. , and Schafer, A. I. , 2000, “Physiological Cyclic Stretch Causes Cell Cycle Arrest in Cultured Vascular Smooth Muscle Cells,” Am. J. Physiol.: Heart Circ. Physiol., 278(3), pp. H748–H754.http://ajpheart.physiology.org/content/278/3/H748 [DOI] [PubMed] [Google Scholar]
- [13]. Niklason, L. E. , Abbott, W. , Gao, J. , Klagges, B. , Hirschi, K. K. , Ulubayram, K. , Conroy, N. , Jones, R. , Vasanawala, A. , Sanzgiri, S. , and Langer, R. , 2001, “Morphologic and Mechanical Characteristics of Engineered Bovine Arteries,” J. Vasc. Surg., 33(3), pp. 628–638. 10.1067/mva.2001.111747 [DOI] [PubMed] [Google Scholar]
- [14]. Kim, B. S. , Nikolovski, J. , Bonadio, J. , and Mooney, D. J. , 1999, “Cyclic Mechanical Strain Regulates the Development of Engineered Smooth Muscle Tissue,” Nat. Biotechnol., 17(10), pp. 979–983. 10.1038/13671 [DOI] [PubMed] [Google Scholar]
- [15]. Kirmani, B. , and Zacharias, J. , 2010, “Endoscopic Vein Harvesting: Does the Learning Curve Influence Outcomes?,” Ann. Thorac. Surg., 90(5), p. 1743. 10.1016/j.athoracsur.2010.04.094 [DOI] [PubMed] [Google Scholar]
- [16]. Ramalho-Santos, M. , Yoon, S. , Matsuzaki, Y. , Mulligan, R. C. , and Melton, D. A. , 2002, ““Stemness”: Transcriptional Profiling of Embryonic and Adult Stem Cells,” Science, 298(5593), pp. 597–600. 10.1126/science.1072530 [DOI] [PubMed] [Google Scholar]
- [17]. Pittenger, M. F. , Mackay, A. M. , Beck, S. C. , Jaiswal, R. K. , Douglas, R. , Mosca, J. D. , Moorman, M. A. , Simonetti, D. W. , Craig, S. , and Marshak, D. R. , 1999, “Multilineage Potential of Adult Human Mesenchymal Stem Cells,” Science, 284(5411), pp. 143–147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- [18]. Hwang, N. S. , Zhang, C. , Hwang, Y. S. , and Varghese, S. , 2009, “Mesenchymal Stem Cell Differentiation and Roles in Regenerative Medicine,” Wiley Interdiscip. Rev.: Syst. Biol. Med., 1(1), pp. 97–106. 10.1002/wsbm.26 [DOI] [PubMed] [Google Scholar]
- [19]. Minguell, J. J. , Erices, A. , and Conget, P. , 2001, “Mesenchymal Stem Cells,” Exp. Biol. Med., 226(6), pp. 507–520.http://ebm.rsmjournals.com/cgi/content/abstract/226/6/507 [DOI] [PubMed] [Google Scholar]
- [20]. Okita, K. , Ichisaka, T. , and Yamanaka, S. , 2007, “Generation of Germline-Competent Induced Pluripotent Stem Cells,” Nature, 448(7151), pp. 313–317. 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- [21]. Kern, S. , Eichler, H. , Stoeve, J. , Kluter, H. , and Bieback, K. , 2006, “Comparative Analysis of Mesenchymal Stem Cells From Bone Marrow, Umbilical Cord Blood, or Adipose Tissue,” Stem Cells, 24(5), pp. 1294–1301. 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- [22]. Caplan, A. I. , 1994, “The Mesengenic Process,” Clin. Plast. Surg., 21(3), pp. 429–435. [PubMed] [Google Scholar]
- [23]. Narita, Y. , Yamawaki, A. , Kagami, H. , Ueda, M. , and Ueda, Y. , 2008, “Effects of Transforming Growth Factor-Beta 1 and Ascorbic Acid on Differentiation of Human Bone-Marrow-Derived Mesenchymal Stem Cells into Smooth Muscle Cell Lineage,” Cell Tissue Res., 333(3), pp. 449–559. 10.1007/s00441-008-0654-0 [DOI] [PubMed] [Google Scholar]
- [24]. Gong, Z. , Calkins, G. , Cheng, E. C. , Krause, D. , and Niklason, L. E. , 2009, “Influence of Culture Medium on Smooth Muscle Cell Differentiation From Human Bone Marrow-Derived Mesenchymal Stem Cells,” Tissue Eng., Part A, 15(2), pp. 319–330. 10.1089/ten.tea.2008.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Galmiche, M. C. , Koteliansky, V. E. , Briere, J. , Herve, P. , and Charbord, P. , 1993, “Stromal Cells From Human Long-Term Marrow Cultures Are Mesenchymal Cells That Differentiate Following a Vascular Smooth Muscle Differentiation Pathway,” Blood, 82(1), pp. 66–76. [PubMed] [Google Scholar]
- [26]. Gong, Z. , and Niklason, L. E. , 2011, “Use of Human Mesenchymal Stem Cells as Alternative Source of Smooth Muscle Cells in Vessel Engineering,” Methods Mol. Biol., 698, pp. 279–294. 10.1007/978-1-60761-999-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Park, J. S. , Chu, J. S. , Cheng, C. , Chen, F. , Chen, D. , and Li, S. , 2004, “Differential Effects of Equiaxial and Uniaxial Strain on Mesenchymal Stem Cells,” Biotechnol. Bioeng., 88(3), pp. 359–368. 10.1002/bit.20250 [DOI] [PubMed] [Google Scholar]
- [28]. Kurpinski, K. , Park, J. , Thakar, R. G. , and Li, S. , 2006, “Regulation of Vascular Smooth Muscle Cells and Mesenchymal Stem Cells by Mechanical Strain,” Mol. Cell. Biomech., 3(1), pp. 21–34. [PubMed] [Google Scholar]
- [29]. Jang, J. Y. , Lee, S. W. , Park, S. H. , Shin, J. W. , Mun, C. , Kim, S. H. , and Kim, D. H. , 2011, “Combined Effects of Surface Morphology and Mechanical Straining Magnitudes on the Differentiation of Mesenchymal Stem Cells Without Using Biochemical Reagents,” J. Biomed. Biotechnol., 2011, p. 860652. 10.1155/2011/860652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Nieponice, A. , Maul, T. M. , Cumer, J. M. , Soletti, L. , and Vorp, D. A. , 2007, “Mechanical Stimulation Induces Morphological and Phenotypic Changes in Bone Marrow-Derived Progenitor Cells Within a Three-Dimensional Fibrin Matrix,” J. Biomed. Mater. Res., Part A, 81(3), pp. 523–530. 10.1002/jbm.a.31041 [DOI] [PubMed] [Google Scholar]
- [31]. Kearney, E. M. , Farrell, E. , Prendergast, P. J. , and Campbell, V. A. , 2010, “Tensile Strain as a Regulator of Mesenchymal Stem Cell Osteogenesis,” Ann. Biomed. Eng., 38(5), pp. 1767–1779. 10.1007/s10439-010-9979-4 [DOI] [PubMed] [Google Scholar]
- [32]. Jagodzinski, M. , Drescher, M. , Zeichen, J. , Hankemeier, S. , Krettek, C. , Bosch, U. , and Van Griensven, M. , 2004, “Effects of Cyclic Longitudinal Mechanical Strain and Dexamethasone on Osteogenic Differentiation of Human Bone Marrow Stromal Cells,” Eur. Cells Mater., 7, pp. 35–41.vol007a04 [pii] [DOI] [PubMed] [Google Scholar]
- [33]. Houtchens, G. R. , Foster, M. D. , Desai, T. A. , Morgan, E. F. , and Wong, J. Y. , 2008, “Combined Effects of Microtopography and Cyclic Strain on Vascular Smooth Muscle Cell Orientation,” J. Biomech., 41(4), pp. 762–769. 10.1016/j.jbiomech.2007.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Kanda, K. , Matsuda, T. , and Oka, T. , 1992, “Two-Dimensional Orientational Response of Smooth Muscle Cells to Cyclic Stretching,” ASAIO J., 38(3), pp. M382–M385. 10.1097/00002480-199207000-00060 [DOI] [PubMed] [Google Scholar]
- [35]. Liu, B. , Qu, M. J. , Qin, K. R. , Li, H. , Li, Z. K. , Shen, B. R. , and Jiang, Z. L. , 2008, “Role of Cyclic Strain Frequency in Regulating the Alignment of Vascular Smooth Muscle Cells in vitro,” Biophys. J., 94(4), pp. 1497–1507. 10.1529/biophysj.106.098574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Kinner, B. , Zaleskas, J. M. , and Spector, M. , 2002, “Regulation of Smooth Muscle Actin Expression and Contraction in Adult Human Mesenchymal Stem Cells,” Exp. Cell Res., 278(1), pp. 72–83. 10.1006/excr.2002.5561 [DOI] [PubMed] [Google Scholar]
- [37]. Worth, N. F. , Rolfe, B. E. , Song, J. , and Campbell, G. R. , 2001, “Vascular Smooth Muscle Cell Phenotypic Modulation in Culture Is Associated With Reorganisation of Contractile and Cytoskeletal Proteins,” Cell Motil. Cytoskeleton, 49(3), pp. 130–145. 10.1002/cm.1027 [DOI] [PubMed] [Google Scholar]
- [38]. Lian, J. B. , Stein, G. S. , Stein, J. L. , and Van Wijnen, A. J. , 1998, “Osteocalcin Gene Promoter: Unlocking the Secrets for Regulation of Osteoblast Growth and Differentiation,” J. Cell Biochem. Suppl., 30–31, pp. 62–72. [DOI] [PubMed] [Google Scholar]
- [39]. Jaiswal, N. , Haynesworth, S. E. , Caplan, A. I. , and Bruder, S. P. , 1997, “Osteogenic Differentiation of Purified, Culture-Expanded Human Mesenchymal Stem Cells in vitro,” J. Cell Biochem., 64(2), pp. 295–312. [DOI] [PubMed] [Google Scholar]
- [40]. Hsu, H. J. , Lee, C. F. , Locke, A. , Vanderzyl, S. Q. , and Kaunas, R. , 2010, “Stretch-Induced Stress Fiber Remodeling and the Activations of Jnk and Erk Depend on Mechanical Strain Rate, but Not Fak,” PLoS One, 5(8), p. e12470. 10.1371/journal.pone.0012470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Naruse, K. , Yamada, T. , Sai, X. R. , Hamaguchi, M. , and Sokabe, M. , 1998, “Pp125fak Is Required for Stretch Dependent Morphological Response of Endothelial Cells,” Oncogene, 17(4), pp. 455–463. 10.1038/sj.onc.1201950 [DOI] [PubMed] [Google Scholar]
- [42]. Sai, X. , Naruse, K. , and Sokabe, M. , 1999, “Activation of Pp60(Src) Is Critical for Stretch-Induced Orienting Response in Fibroblasts,” J. Cell Sci., 112(Pt. 9), pp. 1365–1373. [DOI] [PubMed] [Google Scholar]
- [43]. De, R. , Zemel, A. , and Safran, S. A. , 2007, “Dynamics of Cell Orientation,” Nat. Phys., 3(9), pp. 655–659. 10.1038/nphys680 [DOI] [Google Scholar]
- [44]. Hsu, H. J. , Lee, C. F. , and Kaunas, R. , 2009, “A Dynamic Stochastic Model of Frequency-Dependent Stress Fiber Alignment Induced by Cyclic Stretch,” PLoS One, 4(3), p. e4853. 10.1371/journal.pone.0004853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Chen, B. , Kemkemer, R. , Deibler, M. , Spatz, J. , and Gao, H. , 2012, “Cyclic Stretch Induces Cell Reorientation on Substrates by Destabilizing Catch Bonds in Focal Adhesions,” PLoS One, 7(11), p. e48346. 10.1371/journal.pone.0048346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Riehl, B. D. , Park, J. H. , Kwon, I. K. , and Lim, J. Y. , 2012, “Mechanical Stretching for Tissue Engineering: Two-Dimensional and Three-Dimensional Constructs,” Tissue Eng., Part B, 18(4), pp. 288–300. 10.1089/ten.teb.2011.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]


