Abstract
Naturalists as early as Darwin observed terrestrial basking in green turtles (Chelonia mydas), but the distribution and environmental influences of this behaviour are poorly understood. Here, we examined 6 years of daily basking surveys in Hawaii and compared them with the phenology of local sea surface temperatures (SST). Data and models indicated basking peaks when SST is coolest, and we found this timeline consistent with bone stress markings. Next, we assessed the decadal SST profiles for the 11 global green turtle populations. Basking generally occurs when winter SST falls below 23°C. From 1990 to 2014, the SST for these populations warmed an average 0.04°C yr−1 (range 0.01–0.09°C yr−1); roughly three times the observed global average over this period. Owing to projected future warming at basking sites, we estimated terrestrial basking in green turtles may cease globally by 2100. To predict and manage for future climate change, we encourage a more detailed understanding for how climate influences organismal biology.
Keywords: climate forcing, environmental variability, phenology, Fourier series
1. Introduction
Climate regulates marine biodiversity by directing ecosystem state, biogeography, community dynamics and reproductive fecundity [1,2]. Sea turtles, like many marine species, range over large geographical distances which exposes them to different ecological phenomena throughout development [3]. This spatial and demographic structure, once understood, is key for understanding climatic influences to population dynamics and can be applied both to interpret historical abundance and to forecast future trajectories [4]. It is therefore strategically important to link climate to specific aspects of organismal biology over the empirical record in order to manage for climate change [5].
Basking behaviour in reptiles has been linked to thermoregulation [6,7], and sea turtles are known to bask at ocean surfaces [8]. Over the past several centuries, terrestrial basking has been uniquely observed in green turtles of the Galapagos, Hawaiian (figure 1a) and Wellesley archipelagos [9–11]. In addition to warming internal temperatures, researchers have speculated such terrestrial basking may aid immune function, predator avoidance, digestion, egg development and even prevent unwanted courtship [11,12]. Although these ideas are largely untested, one study showed terrestrial basking raised the internal body temperatures of captive turtles by 3°C [12]. The relationship between terrestrial basking and climate, however, remains unexplored.
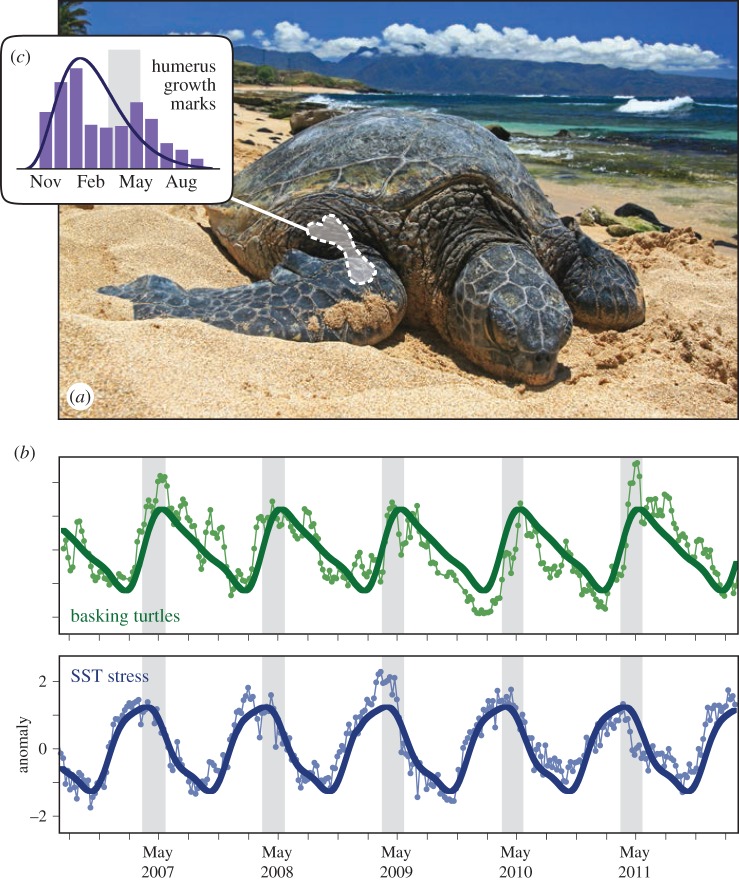
Figure 1.
Behavioural and physiological responses to SST stress in green turtles. (a) Green turtle basking at Paia, Maui (photo: Chris Stankis). (b) Basking varies seasonally in concert with cool SST. Green circles are standardized anomalies of the number of turtles observed basking weekly at Laniakea, Oahu. Blue circles are weekly AVHRR SST data for this location. Thick dark lines are the Fourier series for each timeseries. (c) Growth marks in turtle humeri (superimposed silhouette) peak in January, at the beginning of the coldest SST. Purple columns report the duration of growth marks from recaptured turtles. Line is fitted gamma probability distribution. Grey-shaded area spans the peak Fourier model values for SST and basking, retained for all plots.
Here, we assessed how climate influences terrestrial basking in green turtles across spatio-temporal scales. We analysed 6 years of daily turtle counts at one emergence site in Hawaii, fitting Fourier series to the counts and local SST series. We used the Fourier models to interpret the phenology of growth markings within humerus bones—apparent indicators of physiological stress [13]—for green turtles in Hawaii. Finally, we described the decadal SST profiles across green turtle populations, examining how SST might influence basking across the species' global range, and considering future warming. Besides improving our knowledge of climate impacts, these analyses also contribute to understanding growth rates and maturity [14] which are critical for conservation assessments [3].
2. Material and methods
NOAA's Pacific Islands Regional Office contracted the organization Mālama na Honu to census basking turtles at Laniakea Beach Park, Oahu (21.6° N, 158.1° W). Unique individuals, determined from carapace markings by NOAA staff (USFWS permit no. TE-72088A-0), were counted daily. This provided raw counts from July 2006 to March 2012, to which we applied a 21-day low pass filter similar to a LOESS model [15]. We compiled SST data at Laniakea and for the 11 major green turtle populations [3] worldwide from 1990 to 2014. Eight-day SST averages are the AVHRR Pathfinder v4.1 data from 1990 to 2002, and GAC series from 2003 to 2014 (see http://oceanwatch.pifsc.noaa.gov/las). Global surface temperature averages from 1900 to 2014 are from NASA GISS [16]. Bone cross-section images that identify growth marks are from a previous study [13].
We fit Fourier series to the Oahu basking and SST data using standard techniques [17]. We considered each dataset as an annual series with average weekly values, y(t), and fit the model:
Here, ω0 = 2π/T is the fundamental angular frequency, and T is the cycle time (1 year). A0 is the average of y(t). The parameters Ak and Bk are obtained from the Fourier inversion formulae (electronic supplementary material). Goodness-of-fit increased with the model order, K, and an AICc selected the highest-ranked model order [4]. As we are examining thermoregulation, we inverted the SST series (cold peaks, warm valleys). We represented SST and basking series as standardized anomalies [4].
We compared these results with bone stress marks from Hawaiian green turtles. A previous study [13] captured wild green turtles, injected them with fluorescent oxytetracycline (OTC), released them and later recovered a subset as dead strandings. Histological preparations of humeri cross sections from these turtles revealed OTC marks and dark lines of arrested growth (LAG) [13]. These LAGs are thought to reflect a low point in an annual cycle of bone growth, yet their phenology is unresolved. Using graphics software [18], we measured the radial tissue accretion in bone cross-section images for three turtles, noting where six LAGs begin and end. As bone growth varies seasonally (and figure 1b shows a correlation between basking and SST), we applied our SST Fourier model to growth chronology. We used δx = δx − 1 + f(t)Χ, where δx is the radial distance from the OTC mark, f(t) is the Fourier series value and X is a constant such that the final bone position, δn, equals the measured growth (electronic supplementary material). The Fourier process here is recalibrated from s(t) above so f(t) = −s(t) − s(t)min, and interpolated daily between weekly s(t) values. Having LAG positions and a chronology of bone growth, we recorded the Julian dates for LAGs, and fit Gamma distributions to the data using maximum likelihood estimation (MLE).
We calculated the decadal SST profiles for the 11 global green turtle populations, focusing on the primary nesting rookery locations [3]. In two of the three populations with basking, the major rookeries are also the primary basking sites. For the Eastern Pacific, however, we analysed SST at the primary basking location (Galapagos: 0° N, 90.5° W). For each population, we calculated the 95% confidence interval for annual and winter SST (12 coldest weeks annually). We estimated the observed rate of SST change over 1990–2014 as the slope of the linear model of annual SST averages (electronic supplementary material).
3. Results and discussion
Figure 1b shows that basking and SST stress have a similar phenology at the Laniakea site, measured over 6 consecutive years. The Fourier models demonstrate that, while there is some interannual variability, the series cycle annually with peak cold SST in late March and peak basking in early May. These phenologies are intuitive, and suggest terrestrial basking functions in thermoregulation as a response to seasonally cool ocean temperatures.
Figure 1c shows bone stress marks occur in winter, broadly consistent with the phenology of SST stress. The model fits suggest LAG occurrence peaks in January, but the raw data show a smaller peak later in May. Published studies disagree [13,14] about whether green turtle LAGs are strictly annual. We had too few samples to resolve this question. However, our analysis does indicate green turtle physiology is sensitive to SST phenologies, as reflected in basking (figure 1b) and bone LAGs (figure 1c). Environmental as well as demographic drivers may influence the bone LAG chronology, and as a result, this may vary between individuals. We encourage a more thorough treatment on this topic.
Figure 2 describes the annual SST profiles for green turtle populations globally. Hawaii has the coolest peak SST of all populations, expressed in both the extreme peak (28.5°C) and upper 95% interval (27.8°C). Moreover, as a set, the three basking populations have three of the four coolest winter SST profiles. The exception to the rule is the Mediterranean, which has the largest annual SST range and the coldest winter SST, yet basking does not occur there. Mediterranean sea turtles are anomalous in many respects [3,19]. For green turtles, this region is the most geographically restricted and least abundant of all such populations globally and it is currently under extreme conservation threat [3]. It is possible that terrestrial basking occurred in this population historically, but that it was selected against owing to harvest pressures in this densely populated area. By contrast, the three populations with contemporary basking are all archipelagos somewhat distant from human population centres.
Figure 2.
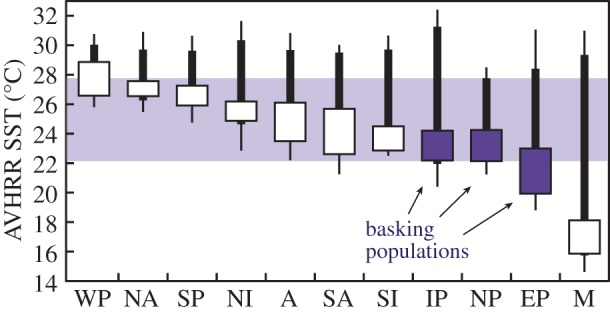
Terrestrial basking occurs in green turtle populations with colder winter SST. Data are weekly AVHRR SST (1990–2014) for each major population. Boxes are the winter 95% interval, thick lines are the annual 95% interval and thin lines are the extreme values. Light-shaded area is the annual SST interval for Hawaii (NP, North Pacific). The three basking populations (NP; IP, Indo-Pacific and EP, East Pacific) have some of the coldest documented winter SSTs. The Mediterranean (M), with the coldest winter and coldest extreme SST, is the exception. Hawaii has the coolest annual and extreme SST peak for all green turtle populations. WP, West Pacific; NA, North Atlantic; SP, South Pacific; NI, North Indian; A, Southwest Pacific; SA, South Atlantic and SI, South Indian.
How might climate change influence terrestrial basking? From 1990 to 2014, SST profiles at the primary green turtle nesting sites increased on average 1.0°C (range 0.3–2.2°C), which is 4.1°C century−1, and as high as 9.0°C century−1. This increase is three times (range 0.8–6.3 times) the average global surface temperature change (0.01°C yr−1) during this period [16]. These trends raise obvious concerns for conservation management, but they may also influence terrestrial basking. Except the Mediterranean, figure 2 shows populations with a minimum winter SST above 23°C do not bask on land. If warming continues at observed rates, we can calculate when winter SST minimums in basking populations will surpass 23°C, and basking may significantly decline or cease altogether. Based on observed SST changes, we estimate terrestrial basking may cease in Hawaii by 2039, in Australia by 2086, and in the Galapagos by 2102—all by century's end. Over time, these populations might shift toward in-water thermoregulation strategies, as are observed in other sea turtle species [19].
Terrestrial emergence is a risky behaviour that exposes turtles to human poaching and depredation from terrestrial carnivores. However, this habit must have evolved to serve a significant ecological function. Here, we described the spatio-temporal variability of basking and its correlation to ocean temperatures. Six years of daily monitoring in Hawaii shows basking peaks when ocean temperatures are coolest, and bone markings suggest this period is physiologically suboptimal. These correlations also play out at the global scale. The three populations with basking have some of the coolest temperature profiles. While this presents compelling evidence that terrestrial basking is climate-driven, warming SST trajectories may cause behavioural shifts to where terrestrial basking may decline or even disappear this century. We recommend future studies document how climate influences the biology of marine organisms and the impact of observed and future climate changes.
Supplementary Material
Supplementary Material
Acknowledgement
J. Pettigrew and P. Doyle supervised field counts of basking turtles. C. Stankis provided turtle images, M. Snover provided bone images and S. Murakawa digitized datasheets. F. Ascani, T. Jones, Y. Swimmer, J. Seminoff, S. Martin and two anonymous reviewers improved manuscript drafts.
Ethics statement
Our study followed the US Endangered Species Act guidelines and was approved by NOAA.
Data accessibility
Data used in this paper are presented in the electronic supplementary material.
Authors' Contributions
K.V. and J.H. analysed the data and wrote the manuscript. K.V. and W.M. digitized and quality-checked data. K.V. designed the study and prepared the figures. All authors reviewed the manuscript.
Funding statement
This work was supported by NOAA and by a Presidential Early Career Award in Science and Engineering (PECASE) to K.V.
Competing interests
The authors declare no competing interests.
References
- 1.Mantua N, Hare S. 2002. The Pacific decadal oscillation. J. Oceanogr. 58, 35–44. ( 10.1023/A:1015820616384) [DOI] [Google Scholar]
- 2.Nye JA, et al. 2013. Ecosystem effects of the atlantic multidecadal oscillation. J. Mar. Syst. 133, 103–116. ( 10.1016/j.jmarsys.2013.02.006) [DOI] [Google Scholar]
- 3.Seminoff JA, et al. 2014. Green turtle (Chelonia mydas) status review under the U.S. Endangered Species Act, 592 p Silver Spring, MD: NMFS. [Google Scholar]
- 4.Van Houtan KS, Halley JM. 2011. Long-term climate forcing in loggerhead sea turtle nesting. PLoS ONE 6, e19043 ( 10.1371/journal.pone.0019043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClure MM, et al. 2013. Incorporating climate science in applications of the US endangered species act for aquatic species. Conserv. Biol. 27, 1222–1233. ( 10.1111/cobi.12166) [DOI] [PubMed] [Google Scholar]
- 6.Bartholomew GA. 1966. A field study of temperature relations in the Galapagos marine iguana. Copeia 1966, 241–250. ( 10.2307/1441131) [DOI] [Google Scholar]
- 7.Boyer DR. 1965. Ecology of the basking habit in turtles. Ecology 46, 99–118. ( 10.2307/1935262) [DOI] [Google Scholar]
- 8.Sapsford CW, van der Riet M. 1979. Uptake of solar radiation by the sea turtle, Caretta caretta, during voluntary surface basking. Comp. Biochem. Physiol. A 63, 471–474. ( 10.1016/0300-9629(79)90174-9) [DOI] [Google Scholar]
- 9.Limpus C. 2008. A biological review of Australian marine turtle species, volume 2, 96 p. Brisbane, Australia: Queensland Environmental Protection Agency. [Google Scholar]
- 10.Snell HL, Fritts TH. 1983. The significance of diurnal terrestrial emergence of green turtles (Chelonia mydas) in the Galapagos archipelago. Biotropica 15, 285–291. ( 10.2307/2387653) [DOI] [Google Scholar]
- 11.Whittow G, Balazs G. 1982. Basking behavior of the Hawaiian green turtle (Chelonia mydas). Pac. Sci. 36, 129–140. [Google Scholar]
- 12.Swimmer JY. 2006. Relationship between basking and fibropapillomatosis in captive green turtles (Chelonia mydas). Chel. Conserv. Biol. 5, 305–309. ( 10.2744/1071-8443(2006)5[305:RBBAFI]2.0.CO;2) [DOI] [Google Scholar]
- 13.Snover ML, Hohn AA, Goshe LR, Balazs GH. 2011. Validation of annual skeletal marks in green sea turtles Chelonia mydas using tetracycline labeling. Aquat. Biol. 12, 197–204. ( 10.3354/ab00337) [DOI] [Google Scholar]
- 14.Van Houtan KS, Hargrove S, Balazs GH. 2014. Modeling sea turtle maturity age from partial life history records. Pac. Sci. 68, 465–477. ( 10.2984/68.4.2) [DOI] [Google Scholar]
- 15.Cleveland WS, Devlin SJ. 1988. Locally weighted regression: an approach to regression analysis by local fitting. J. Am. Stat. Assoc. 83, 596–610. ( 10.1080/01621459.1988.10478639) [DOI] [Google Scholar]
- 16.Hansen J, Ruedy R, Sato M, Lo K. 2010. Global surface temperature change. Rev. Geophys. 48, RG4004 ( 10.1029/2010RG000345) [DOI] [Google Scholar]
- 17.Percival DB, Walden AT. 1993. Spectral analysis for physical applications. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Adobe Systems Inc. 2011. Adobe Illustrator CS5. 15.1.0 edn Mountain View, CA: Adobe. [Google Scholar]
- 19.Schofield G, Bishop CM, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC. 2009. Microhabitat selection by sea turtles in a dynamic thermal marine environment. J. Anim. Ecol. 78, 14–21. ( 10.1111/j.1365-2656.2008.01454.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this paper are presented in the electronic supplementary material.