Abstract
Like most benthic marine organisms, coral reef fishes produce larvae that traverse open ocean waters before settling and metamorphosing into juveniles. Where larvae are transported and how they survive is a central question in marine and fisheries ecology. While there is increasing success in modelling potential larval trajectories, our knowledge of the physical and biological processes contributing to larval survivorship during dispersal remains relatively poor. Mesoscale eddies (MEs) are ubiquitous throughout the world's oceans and their propagation is often accompanied by upwelling and increased productivity. Enhanced production suggests that eddies may serve as important habitat for the larval stages of marine organisms, yet there is a lack of empirical data on the growth rates of larvae associated with these eddies. During three cruises in the Straits of Florida, we sampled larval fishes inside and outside five cyclonic MEs. Otolith microstructure analysis revealed that four of five species of reef fish examined had consistently faster growth inside these eddies. Because increased larval growth often leads to higher survivorship, larvae that encounter MEs during transit are more likely to contribute to reef populations. Successful dispersal in oligotrophic waters may rely on larval encounter with such oceanographic features.
Keywords: larval dispersal, larval fish growth, otolith microstructure, mesoscale eddies, reef fish, population replenishment
1. Introduction
The complex life history of most benthic marine organisms includes a larval stage which disperses in the open ocean for days to months before settling to the benthos. As direct tracking of larvae is challenging, modelling this transit is an important tool for predicting recruitment and population dynamics. Modelled larval trajectories through the ocean are typically a function of physical oceanography and active larval behaviour and new climatologically driven hydrodynamic models are providing increasing resolution for predicting larval dispersal [1]. Sophisticated biophysical and individual-based models also incorporate larval feeding and mortality [2]. As prey fields are patchy and largely driven by the physical environment, larval survival is tightly linked to oceanographic features encountered by larvae during the pelagic stage. However, owing to the relatively few empirical data on the effect of larval encounter with particular oceanographic features, there is generally a mismatch between the resolution of physical oceanography and our knowledge of larval fish response [3].
Mesoscale eddies (MEs) are ubiquitous features in the world's oceans and the upwelling dynamics of eddies can support increased primary [4] and secondary productivity [5]. In addition, both predators and spawners often target convergent zones of eddies [6,7], suggesting that both the nutrient enrichment and concentration of prey provided by MEs are important to a diversity of organisms. Cyclonic MEs frequently pass through the Straits of Florida (SOF) and are important in transporting reef fish larvae to the Florida Keys reef [8]. However, because of the increased production resulting from upwelling in the centre of cyclonic MEs, the ecological importance of these pelagic habitats may extend beyond transport and play a vital role in providing enhanced feeding opportunities for larval fishes during their journey to the reef.
To date, the hypothesis that residence in an eddy leads to higher larval growth rates is largely untested (but see [9]), yet there is much speculation as to the role of MEs as essential larval habitat [10,11]. The objective of our study was to directly sample MEs in the SOF to determine the influence of eddies on larval reef fish growth rates.
2. Material and methods
Sampling was conducted during three 15–16-day cruises in the summers of 2007 (May 29–June 13, July 30–August 13 and 2008 (June 17–July 1). During each cruise, ichthyoplankton samples were collected at seven stations along each of six cross-shelf transects spanning the western SOF where MEs form coherent structures and have relatively long residence times. At each station we collected ichthyoplankton using a modified Multiple Opening Closing Net and Environmental Sensing System (MOCNESS [12]). The MOCNESS sampled discrete 20 m depth bins down to 80 m using paired nets (4 and 1 m2) fitted with 1 mm and 150 μm mesh, respectively. All tows were conducted during daylight hours and a flowmeter attached to the MOCNESS measured sample volume. All ichthyoplankton samples collected with the large-mesh nets were processed by separating all fish larvae from other plankton and identifying each specimen to the lowest possible taxonomic level.
The MEs were located using a suite of physical data (see electronic supplementary material S1). A ship-mounted 76.8 kHz RD Instruments Acoustic Doppler Current Profiler (ADCP) sampled the water column. Sensors attached to the MOCNESS collected temperature, salinity and fluorescence data, and two Lagrangian drifters (Technocean) drogued at 15 m were deployed during each cruise. Larvae collected at stations inside an eddy were classified as eddy (ED) fish while those collected well outside an eddy were termed non-eddy (NE) fish.
Based on sample sizes and the ability to morphologically identify individuals to the species level, five species of reef fish were chosen for otolith analysis: Xyrichtys novacula (pearly razorfish), Thalassoma bifasciatum (bluehead wrasse), Cryptotomus roseus (bluelip parrotfish), Sphyraena barracuda (great barracuda) and Stegastes partitus (bicolour damselfish). We followed standard procedures for analysing otolith microstructure of a subset of fish (from all depth bins) from each species to obtain individual growth rates and ages ([13]; see also electronic supplementary material S2). Sagittal (X. novacula, T. bifasciatum, C. roseus) or lapillar (S. barracuda, S. partitus) otoliths were read at least twice along the longest axis at 400× magnification (or 1000× for S. barracuda).
We compared ‘recent larval growth’ between ED and NE groups by averaging growth of each individual over the last three full days of larval life as the exact timing of larval entrainment in each eddy could not be determined. This measure of recent growth was then compared between groups using analysis of covariance (ANCOVA) with age as a covariate. In instances where a significant interaction between age and group (i.e. ED and NE) precluded the interpretation of ANCOVA results, ED and NE fish were split into a young group (youngest half of the samples) and an old group (oldest half of the samples) and an ANCOVA conducted separately for each age group, also with age as a covariate.
3. Results
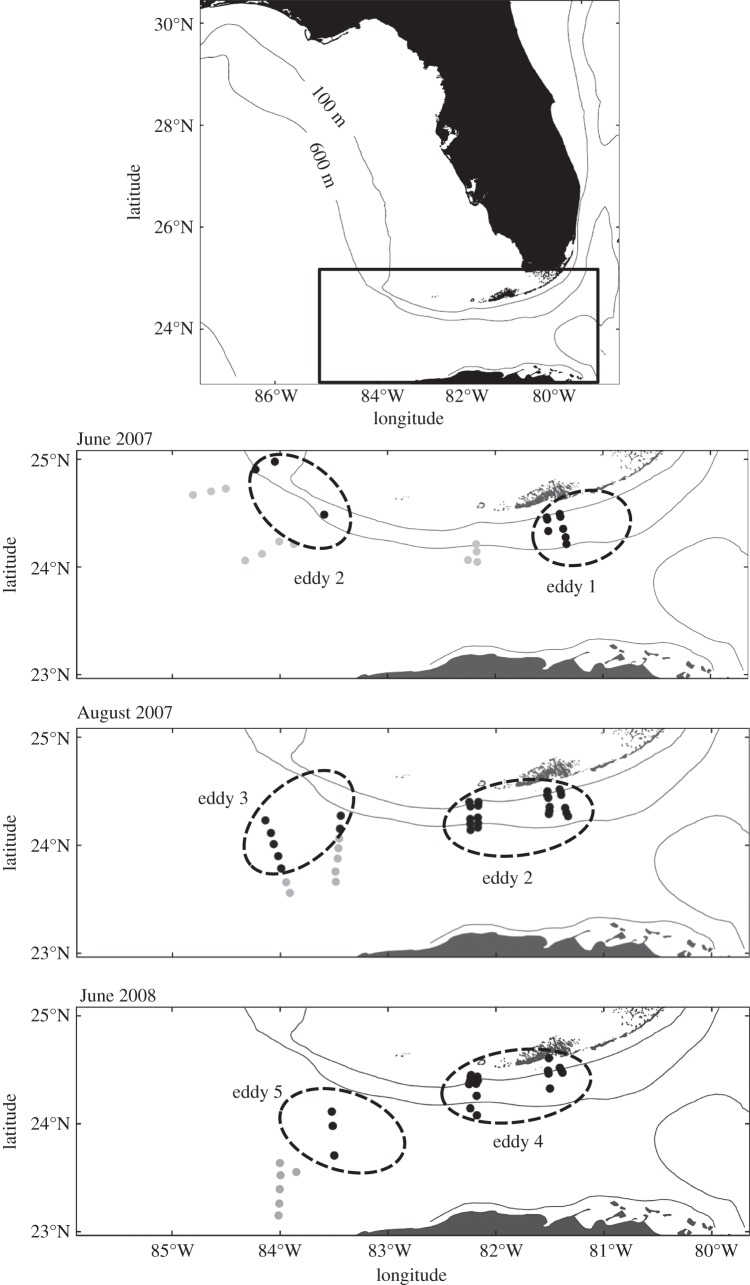
Two cyclonic MEs were present during each of the three cruises, with one (eddy 2) present during two sampling periods (figure 1; see also electronic supplementary material S1). Sample sizes permitted tests of growth differences between ED and NE larvae for X. novacula and T. bifasciatum in June and August 2007, for C. roseus in August 2007 and June 2008, and for S. barracuda and S. partitus in August 2007. Of these eight tests, three (i.e. T. bifasciatum, C. roseus and S. barracuda from August 2007) resulted in a significant interaction between age and group necessitating the division of samples into young and old groups for separate ANCOVA analysis. After dividing samples, only one significant interaction between age and group remained (i.e. T. bifasciatum young fish), so this group was removed from the analysis.
Figure 1.
Map of study area for each of the three sampling periods, indicating positions of mesoscale eddies (dashed lines) and locations of eddy (ED, black points) and non-eddy (NE, grey points) stations. Eddies are identified numerically in the order in which they propagated through the Straits of Florida.
For four of the five species of reef fish, ED larvae had significantly faster recent growth than NE larvae (figure 2). For X. novacula, T. bifasciatum and C. roseus, higher recent growth of ED fish was evident in each of two different time periods. For S. barracuda, young ED larvae grew significantly faster than young NE larvae in August 2007. Recent growth of S. partitus did not differ between ED and NE larvae.
Figure 2.
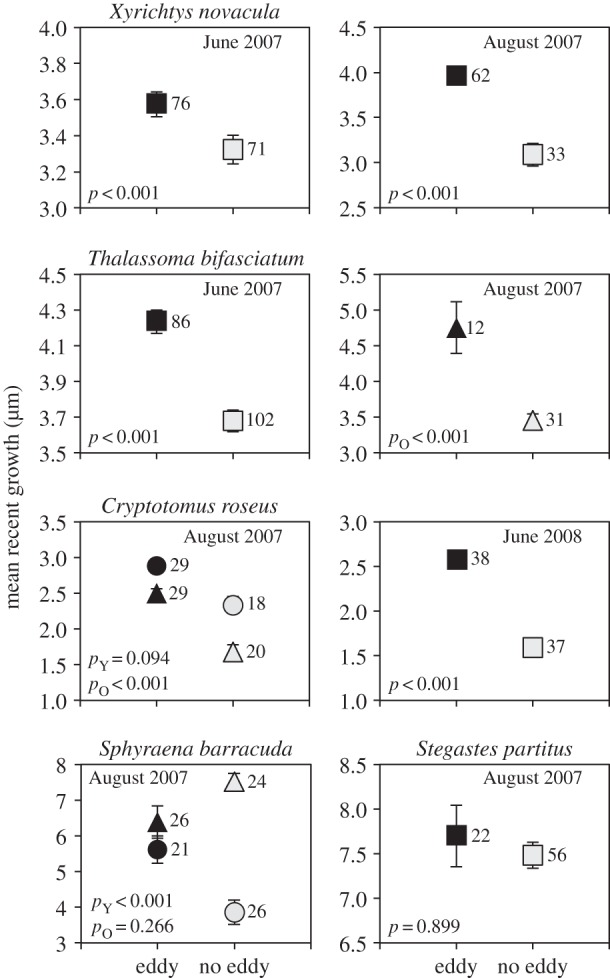
Comparisons of recent larval growth between larvae collected inside (ED; black) and outside (NE; grey) mesoscale eddies. Squares, comparisons with all ages included; circles, young age group only; and triangles, old age group only. pY, p-value for young age group comparisons; pO, p-value for old age group comparisons. Sample size indicated to the right of each data point.
4. Discussion
Larval reef fishes must obtain sufficient food to grow, survive and successfully disperse from source to settlement habitats. Here we demonstrate that larval encounter with distinct physical oceanographic features that promote productivity significantly enhances larval growth and probably contributes to successful population replenishment. Using otolith microstructure to examine the recent growth of larvae sampled inside and outside five cyclonic MEs, we show that in 7 of 10 comparisons, growth was significantly faster in larvae sampled from eddy stations. Fast growth of larvae in MEs was consistent across three sampling periods over two summers and across four species of reef fish from three different families. This finding echoes a study showing that 6–8 mm (but not 9–11 mm) anchovy larvae were of higher condition (RNA/DNA ratio) in a frontal eddy of the Kuroshio Current than they were in inshore and offshore stations [9]. Reef fish larvae in our study ranged in size from approximately 3 to 11 mm (standard length) and larvae of all sizes exhibited faster growth in MEs.
While there are few studies comparing growth and condition of fish larvae sampled inside and outside eddies, a large body of empirical data points to likely trophic mechanisms of fast growth for larval fishes in eddies. High levels of primary and secondary productivity have been identified in MEs across a range of geographical locations (e.g. [14,15]). Similarly, cyclonic MEs in the SOF are highly productive, with upwelling of cool water at the centre of these eddies leading to increased levels of nutrients, chlorophyll a and copepod abundances [16,17]. Copepods are a major component of the diet of a variety of larval reef fish [18], and fuller guts have been associated with higher growth in reef fishes [13]; thus the high productivity of MEs probably cascades up to enhance larval growth. Higher growth in eddies was not temperature-related as temperature was lower at ED than at NE stations (electronic supplementary material, table S2). Likewise, similar age/size distributions across stations indicate that growth differences were probably not due to differential predation (electronic supplementary material S2).
The fast growth we observed for larvae sampled in MEs has important implications beyond the larval stage. Higher growth is generally associated with higher survivorship [19], and thus higher recruitment to benthic populations [20]. Furthermore, faster growth during the larval stage has been shown to lead to increased survival in juveniles on the reef [21]. If fast growth of larvae from MEs leads to increased recruitment and these larvae preferentially survive as juveniles, then larvae retained in such eddies may contribute disproportionately to the replenishment of reef fish populations.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the scientific party and R/V Walton Smith crew for their contributions to the field sampling. T. Murphy, L. Parsons, J. Downing, K. Doering and K. Ternus helped process the samples, and J. Llopiz provided guidance with larval identification and data processing. Earlier drafts benefited from comments of A. Bakun, G. Hitchcock and S. Sogard.
Data accessibility
Biological data are available from the Biological and Chemical Oceanography Data Management Office (http://www.bco-dmo.org/dataset/529658) and physical data from the National Oceanographic Data Center (http://data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.nodc:0066847).
Funding statement
This study was supported by grant no. OCE 0550732 from the National Science Foundation. K.S. also was supported during this work by a ‘Science Made Sensible’ fellowship from NSF and, during manuscript preparation, S.S. received support from NOAA under award NA11NOS4780045.
References
- 1.Werner FE, Cowen RK, Paris CB. 2007. Coupled biological and physical models. Oceanography 20, 54–69. ( 10.5670/oceanog.2007.29) [DOI] [Google Scholar]
- 2.Kristiansen T, Vikebo F, Sundby S, Huse G, Fiksen O. 2009. Modeling growth of larval cod (Gadus morhua) in large-scale seasonal and latitudinal environmental gradients. Deep-Sea Res. II 56, 2001–2011. ( 10.1016/j.dsr2.2008.11.011) [DOI] [Google Scholar]
- 3.Sponaugle S, Pinkard DR. 2004. Impact of variable pelagic environments on natural larval growth and recruitment of the reef fish Thalassoma bifasciatum. J. Fish Bio. 64, 34–54. ( 10.1111/j.1095-8649.2004.00279.x) [DOI] [Google Scholar]
- 4.McGillicuddy DJ, Robinson AR, Siegel DA, Jannasch HW, Johnson R, Dickey TD, McNeil J, Michaels AF, Knap AH. 1998. Influence of mesoscale eddies on new production in the Sargasso Sea. Nature 394, 263–266. ( 10.1038/28367) [DOI] [Google Scholar]
- 5.Lane PVZ, Smith SL, Graber HC, Hitchcock GL. 2003. Mesoscale circulation and the surface distribution of copepods near the south Florida Keys. Bull. Mar. Sci. 72, 1–18. [Google Scholar]
- 6.Kai ET, Marsac F. 2010. Influence of mesoscale eddies on spatial structuring of top predators’ communities in the Mozambique Channel. Progr. Oceanogr. 86, 214–223. ( 10.1016/j.pocean.2010.04.010) [DOI] [Google Scholar]
- 7.Richardson DE, Llopiz JK, Leaman KD, Vertes PS, Muller-Karger FE, Cowen RK. 2009. Sailfish (Istiophorus platypterus) spawning and larval environment in a Florida Current frontal eddy. Progr. Oceanogr. 82, 252–264. ( 10.1016/j.pocean.2009.07.003) [DOI] [Google Scholar]
- 8.Sponaugle S, Lee T, Kourafalou V, Pinkard D. 2005. Florida Current frontal eddies and the settlement of coral reef fishes. Limnol. Oceanogr. 50, 1033–1048. ( 10.4319/lo.2005.50.4.1033) [DOI] [Google Scholar]
- 9.Nakata H, Kimura S, Okazaki Y, Kasai A. 2000. Implications of meso-scale eddies caused by frontal disturbances of the Kuroshio Current for anchovy recruitment. ICES J. Mar. Sci. 57, 143–151. ( 10.1006/jmsc.1999.0565) [DOI] [Google Scholar]
- 10.Logerwell EA, Smith PE. 2001. Mesoscale eddies and survival of late stage Pacific sardine (Sardinops sagax) larvae. Fish. Oceanogr. 10, 13–25. ( 10.1046/j.1365-2419.2001.00152.x) [DOI] [Google Scholar]
- 11.Bakun A. 2006. Fronts and eddies as key structures in the habitat of marine fish larvae: opportunity, adaptive response and competitive advantage. Sci. Mar. 70, 105–122. ( 10.3989/scimar.2006.70s2105) [DOI] [Google Scholar]
- 12.Guigand CM, Cowen RK, Llopiz JK, Richardson DE. 2005. A coupled asymmetrical multiple opening closing net with environmental sampling system. Mar. Tech. Soc. J 39, 22–24. ( 10.4031/002533205787444042) [DOI] [Google Scholar]
- 13.Sponaugle S, Llopiz JK, Havel LN, Rankin TL. 2009. Spatial variation in larval growth and gut fullness in a coral reef fish. Mar. Ecol. Prog. Ser. 383, 239–249. ( 10.3354/meps07988) [DOI] [Google Scholar]
- 14.Bibby TS, Gorbunov MY, Wyman KW, Falkowski PG. 2008. Photosynthetic community responses to upwelling in mesoscale eddies in the subtropical North Atlantic and Pacific Oceans. Deep-Sea Res. II 55, 1310–1320. ( 10.1016/j.dsr2.2008.01.014) [DOI] [Google Scholar]
- 15.Govoni JJ, Hare JA, Davenport ED, Chen MH, Marancik KE. 2010. Mesoscale, cyclonic eddies as larval fish habitat along the southeast United States shelf: a Lagrangian description of the zooplankton community. ICES J. Mar. Sci. 67, 403–411. ( 10.1093/icesjms/fsp269) [DOI] [Google Scholar]
- 16.Lee TN, Clarke ME, Williams E, Szmant AF, Berger T. 1994. Evolution of the Tortugas Gyre and its influence on recruitment in the Florida Keys. Bull. Mar. Sci. 54, 621–646. [Google Scholar]
- 17.Hitchcock GL, Lee TN, Ortner PB, Cummings S, Kelble C, Williams E. 2005. Property fields in a Tortugas Eddy in the southern Straits of Florida. Deep-Sea Res. I 52, 2195–2213. ( 10.1016/j.dsr.2005.08.006) [DOI] [Google Scholar]
- 18.Llopiz JK, Cowen RK. 2009. Variability in the trophic role of coral reef fish larvae in the oceanic plankton. Mar. Ecol. Prog. Ser. 381, 259–272. ( 10.3354/meps07957) [DOI] [Google Scholar]
- 19.Anderson JT. 1988. A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J. NW Atlantic Fish. Sci. 8, 55–66. [Google Scholar]
- 20.Bergenius MAJ, Meekan MG, Robertson DR, McCormick MI. 2002. Larval growth predicts the recruitment success of a coral reef fish. Oecologia 131, 521–525. ( 10.1007/s00442-002-0918-4) [DOI] [PubMed] [Google Scholar]
- 21.Vigliola L, Meekan MG. 2002. Size at hatching and planktonic growth determine post-settlement survivorship of a coral reef fish. Oecologia 131, 89–93. ( 10.1007/s00442-001-0866-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Biological data are available from the Biological and Chemical Oceanography Data Management Office (http://www.bco-dmo.org/dataset/529658) and physical data from the National Oceanographic Data Center (http://data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.nodc:0066847).