Abstract
One of the oldest known fossil scorpions, a new species from the mid-Silurian Eramosa Formation (430 myr) of Ontario, Canada, exhibits several surprising features. The depositional environment and associated biota indicate a marine habitat; however, the leg morphology of this scorpion, which has a short tarsus in common with all Recent scorpions, suggests that a key adaptation for terrestrial locomotion, the ability to support its weight on a subterminal ‘foot’, appeared remarkably early in the scorpion fossil record. Specimens are preserved intact and undisturbed in a splayed posture typical of moults rather than carcasses. We postulate that these animals were aquatic, but occasionally ventured into extremely shallow water, or onto a transient subaerially exposed surface while moulting, before returning to deeper water. Shed exuviae were preserved in situ by rapid overgrowth of bacterial biofilm.
Keywords: scorpion, Silurian, locomotion, behaviour, exuviae, new species
1. Introduction
Scorpions are the oldest known arachnids, dating back to the Silurian Period [1,2]. The oldest known, recognizable as a scorpion only in outline, comes from the late Llandovery series (433–438 myr) of Scotland [1,3]. Here, we document a well-preserved assemblage of slightly younger scorpions from the early Wenlock (430–433 myr) Eramosa Formation of the Bruce Peninsula, Ontario, Canada. Eramoscorpius brucensis gen. et sp. nov. is the oldest known occurrence of a fossil scorpion bearing anatomically modern walking legs, in which the next-to-terminal element, the tarsus, is shorter than the preceding basitarsus (figures 1 and 2). This provides the potential for a so-called plantigrade stance, with the short tarsus (foot) placed flat on the substrate, interpreted here as evidence that a key adaptation for life on land appeared remarkably early in the scorpion fossil record. By contrast, other Silurian scorpions have either stubby, pointed, rather ‘crab-like’ legs [4,5], or legs in which the tarsus is distinctly longer than the basitarsus [6,7], in either of which cases the animal must have walked on its ‘toes’ (digitigrade stance). The original habitat of the earliest scorpions remains controversial, and previous suppositions that most mid-Palaeozoic taxa were marine have been questioned [8]. Its coxosternal morphology as well as the depositional environment and associated fauna imply that E. brucensis was primarily an aquatic animal, yet we document limbs consistent with the potential for terrestrial locomotion.
Figure 1.
Eramoscorpius brucensis gen. et sp. nov. All scale bars, 10 mm. (a,c,d,f,g,h) Complete to near complete specimens demonstrating the range of sizes; (a,c,d and f) appear to represent a single size class; (f,h) well-preserved telson with aculeus; (g,h) the largest and the smallest complete individuals respectively. (b,e) Detail of coxosternal area demonstrating the lack of coxapophyses (I and II). Modern (terrestrial) scorpions all have projections of the anterior coxae. Short pectines (arrows) are clearly evident on (b) and faintly visible on (e). (i). Detail of walking legs III and IV demonstrating claws and short tarsus with respect to basitarsus. (a,b), Ventral aspect, holotype ROM53247; (c) dorsal, ROM49276; (d,e) ventral, ROM60063, the only specimen with no original exoskeleton preserved; (f) ROM56751; (g) ROM58778; (h) dorsal ROM58777; (f,g) inferred to be ventral because of the lack of obvious carinae on the last mesosomal somite; (i) ROM50048. (j) Sketch of scorpion leg IV comparing three Silurian fossils with a Recent scorpion. ta, tarsus; ba, basitarsus; ti, tibia; pa, patella. From left to right: Palaeophonus with stubby ‘crab-like’ leg, with all elements about the same length; Proscorpius with tarsus distinctly longer than basitarsus (both slightly younger than Eramoscorpius); Eramoscorpius with tarsus distinctly shorter than basitarsus; Buthus, a modern scorpion with tarsus distinctly shorter than basitarsus. The Eramoscorpius leg is well on its way to being modern with its short tarsus, but is not as gracile as a typical modern scorpion leg, which shows a notable lengthening of the patella. (Online version in colour.)
Figure 2.
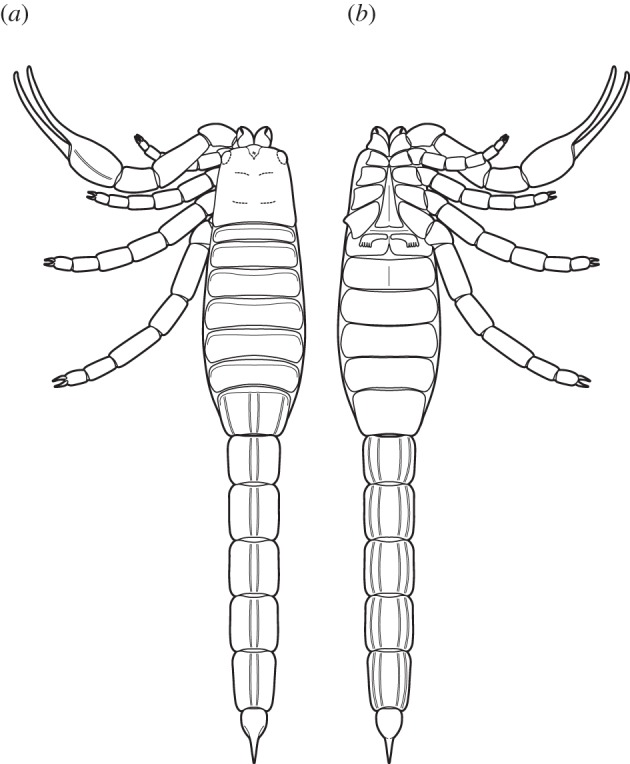
Idealized reconstruction of Eramoscorpius brucensis gen. et sp. nov. in dorsal (a) and ventral (b) view based on a composite of available material.
2. Systematic palaeontology
Order Scorpiones Koch, 1837.
Family undetermined.
Eramoscorpius brucensis.
gen. et sp. nov.
N. Gen. A and N. Gen. B Jeram 1998 [2].
(a). Etymology
The genus is named for the Eramosa Formation, the species after the Bruce Peninsula, Ontario.
(b). Material
With one exception, specimens were collected by quarry workers who did not divulge precise location information, or were discovered in quarried stone delivered to landscaping projects. All specimens are in the invertebrate fossils collection at the Royal Ontario Museum (ROM). ROM 53247 (holotype), 49275, 53248, 58777, 58931, 50048, 58778, 60063, 61159 and 59322 from the Eramosa Formation at Wiarton; ROM 56751 from the northern Bruce Peninsula, Ontario.
(c). Diagnosis of genus and species
Fossil scorpions in which the coxae of walking legs II–IV are fully separated by a narrow triangular sternum with a distinct medial furrow; coxae of leg I abut the sternum and meet in front of it; coxapophyses are absent. Chelicerae small; pedipalps large and robust, with long fingers. Legs long, laterally compressed, with two claws; walking leg IV reaches beyond end of mesosoma; tarsus of all legs shorter than basitarsus.
(d). Description
Because total length ranges from an inferred minimum of 29 mm up to ca 165 mm, dimensions are expressed as ratios rather than as absolute measurements. Carapace smooth, about one-seventh of total length, subquadrate with a slightly curved anterior margin and a faint pattern of transverse sulci. Chelicerae small with only the distal articles visible, free finger quite short in relation to the total length. Pedipalps large and robust; femur longer than patella, chela making up just under half total pedipalp length, and more than one and a half times carapace length. Maximum width of manus about one-third of total chela length; free finger long, making up about two-thirds of length of chela. Patella distinctly shorter than manus; manus bears a dorsal keel; no apparent ventral ornament.
Ventrally, the sternum is long, a little over two-thirds length of prosoma, narrow and triangular, more than twice as long as its maximum width, with distinct median furrow. Coxae of legs II–IV abut sternum, but do not extend in front of it, coxae I also abut sternum, meet in front of it; significantly, there are no coxapophyses—projections from the anterior limb coxae which contribute to a pre-oral chamber in living scorpions permitting extra-oral digestion. Walking legs long, laterally compressed, lengthening progressively from I to IV with leg IV longest and extending beyond the end of the mesosoma. Marked differentiation in length of articles. In common with all Recent scorpions, tarsus is distinctly shorter than basitarsus. Walking legs end in two short claws.
Mesosoma length about one-third of total, maximum width less than half its total length. Tergite I short, tergites II–VI about equal in length, apparently with a low transverse ridge at the anterior border; tergite VII longer, bearing two pairs of carinae, one pair mesodorsal, the other laterodorsal. Six somites visible ventrally; faint but distinct traces of short pectines are present in two specimens, with the posterior margin normal to the midline and each rachis bearing less than ten rounded teeth. The first visible sternite (second somite) has a triangular break at the posterior edge; first and second sternites appear to have a median slit. Metasoma makes up about half the total length and is composed of five subrectangular segments, all about the same size, longer than wide. Carinae difficult to distinguish, but there appear to be a single pair of dorsal carinae and possibly two pairs of ventral carinae on the metasoma. Telson well exposed in four specimens and bears a distinct aculeus making up about half its length. Total telson length slightly less than that of a metasomal tergite.
3. Discussion
The slender sternum with distinct median sulcus is reminiscent of the giant (approx. 1 m long) Devonian scorpion Praearcturus gigas, which does exhibit coxapophyses but for which distal appendage elements are unknown [9]; but the combination of characters in Eramoscorpius, in particular, the lack of coxapophyses, together with its ‘modern’ limb morphology, do not fit any previously diagnosed genera in the literature. Fossil scorpion systematics suffers from the highly typological system of Kjellesvig-Waering [9], and in the absence of a robust phylogenetic framework, we prefer to leave the familial position of our new specimens open.
Specimens all derive from the mid-Silurian (Wenlock, Sheinwoodian) Eramosa Lagerstätte of the Bruce Peninsula, Ontario. All occur on bedding plane surfaces of finely crystalline, laminated, organic-rich dolostones typical of the so-called Lithofacies 1 of the lower Interbedded Unit [10]. These are interpreted as shallow marginal marine deposits, representing restricted lagoonal to inter-reefal environments. Laminations are highly organic, indicating repeating microbial mat surfaces [11]. Several specimens are associated with bedding plane structures interpreted as adhesion ripples [10] or wrinkle surfaces [11] suggestive of brief subaerial exposure. The fossils are remarkably complete and well preserved, occurring in undisturbed dorsoventral aspect, indicating little to no transport before burial. The scorpions occur on slabs with no associated macrofossils, but the rich Eramosa Lagerstätte biota, in general, contains no terrestrial elements, and many of its component taxa are unequivocally marine (echinoderms, trilobites, brachiopods, cephalopods) [12]. Specimens were apparently preserved by microbial mat biostabilization in a depositional environment lacking significant terriginous input.
The oldest scorpions have been assumed by many authors to have been aquatic [9] although this assertion has been challenged [8,13]. Preservation of respiratory organs in early fossil scorpions is extremely rare. Two Devonian scorpions show hints of lungs [13,14]. The oldest unequivocally lung-bearing scorpions come from the Early Carboniferous of Scotland [15]. Where respiratory organs are absent, evidence for original habitat must be inferred either from sedimentology and/or any associated biota (see above) or from other anatomical features.
The contemporary Silurian scorpions Allopalaeophonus caledonicus and Palaeophonus nuncius with short, stubby, pointed and rather ‘crab-like’ limbs [4,7,9], and the slightly younger Late Silurian Proscorpius osborni, with the tarsus demonstrably longer than the basitarsus [7], all suggest a digitigrade stance in which the scorpion effectively walked on the tips of its toes, suggesting an aquatic habitat. In contrast, the short tarsus-to-basitarsus length ratio of E. brucensis is regarded as a prerequisite to a plantigrade stance allowing locomotion unsupported by water, as the body weight is spread over the larger surface area of the whole tarsus [7]. Together with the long legs, laterally compressed podomeres, and sturdy coxosternal structure this suggests an animal capable of locomotion on land, whereas the absence of coxapophyses, consistent with aquatic feeding, draws into question how such an animal would feed in a terrestrial setting.
Recent scorpion moults and carcasses are about equally durable [16] and a moult resembles a complete, but empty animal. Thus, an intact exoskeleton is not by itself a reliable indicator of a carcass. Exuviae of living scorpions, however, show a highly consistent arrangement of limbs, and the pose of all E. brucensis specimens, with extended pedipalps, chelicerae and walking legs, coupled with the occurrence of telescoped limb elements, suggests that these specimens are in situ moults that have not been appreciably disturbed or transported [17,18]. An apparent lack of obvious flexure between the tarsus and basitarsus (to form an ‘ankle’) may be real, or may be an artefact of the moult process causing straightening and minor telescoping of the joint. As aquatic scorpions would have been extremely vulnerable while moulting, selection for a leg structure that permitted short-term locomotion into very shallow water or onto a temporarily emergent surface to moult, safe from, for example eurypterid or cephalopod predators, would confer a decided advantage during ecdysis. In this scenario, the exuviae were quickly overgrown by a bacterial film before physical disturbance or degradation. All known specimens of E. brucensis are interpreted as exuviae, and the preferred habitat of the scorpions between moults cannot be determined. While perhaps not all animals actually ventured onto temporarily emergent surfaces to moult, the exuviae of those that did would have had an improved chance of being coated by biofilm and thus preserved.
Supplementary Material
Acknowledgements
Kirby Bransfield and the Saxe family, Louise and Chris Johnstone, Jane and Gary Kiyonaga, Paul Perry, Denis Tetreault and the late Harold Stobbe donated specimens to the ROM. Additional specimens were purchased with generous support of the Royal Ontario Museum Louise Hawley Stone Charitable Trust and M.A. Fritz endowment. Andrew Jeram first recognized the ‘modern’ nature of the limb structure. Henry Choong assisted with figure composition. Danielle Dufault produced the reconstruction drawing. Greg Edgecombe and an anonymous reviewer provided helpful suggestions for improvement.
Authors' contributions
All authors contributed equally to the main text. D.R. took the photographs and composed figure 1; J.D. produced the leg drawing in figure 1j; J.W. measured the specimens.
Funding statement
There is no funding to report for this paper.
Competing interests
The authors have no competing interests.
References
- 1.Dunlop JA, Selden PA. 2013. Scorpion fragments from the Silurian of Powys, Wales. Arachnology 16, 27–32. ( 10.13156/arac.2013.16.1.27) [DOI] [Google Scholar]
- 2.Jeram AJ. 1998. Phylogeny, classification and evolution of Silurian and Devonian scorpions. In Proc. the 17th European Colloquium of Arachnology, Edinburgh, 1997 (ed. Selden PA.), pp. 17–31. See http://www.european-arachnology.org/proceedings/17th/3Jeram.pdf. [Google Scholar]
- 3.Laurie M. 1899. On a Silurian scorpion and some additional eurypterid remains from the Pentland Hills. Trans. R. Soc. Edinb. 39, 575–590. ( 10.1017/S0080456800035109) [DOI] [Google Scholar]
- 4.Pocock RI. 1901. The Scottish Silurian scorpion. Q. J. Microscop. Sci. 44, 291–311. See http://jcs.biologists.org/content/s2-44/174/291.full.pdf+html. [Google Scholar]
- 5.Thorell T, Lindström G. 1885. On a Silurian scorpion from Gotland. Kongl. Sv. Vet.-Akad. Handl. 21, 1–33. [Google Scholar]
- 6.Whitfield RP. 1885. An American Silurian scorpion. Science 6, 87–88. ( 10.1126/science.ns-6.130.87) [DOI] [PubMed] [Google Scholar]
- 7.Dunlop JA, Tetlie OE, Prendini L. 2008. Reinterpretation of the Silurian scorpion Proscorpius osborni (Whitfield): integrating data from Palaeozoic and Recent scorpions. Palaeontology 51, 303–320. ( 10.1111/j.1475-4983.2007.00749.x) [DOI] [Google Scholar]
- 8.Scholtz G, Kamenz C. 2006. The book-lungs of Scorpiones and Tetrapulmonata (Chelicerata: Arachnida): evidence for homology and a single terrestrialisation event of a common arachnid ancestor. Zoology 109, 2–13. ( 10.1016/j.zool.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 9.Kjellesvig-Waering EN. 1986. A restudy of the fossil Scorpionida of the world. Palaeontogr. Am. 55, 1–287. See http://www.biodiversitylibrary.org/item/91625#page/6/mode/1up. [Google Scholar]
- 10.Armstrong DK, Meadows JR. 1988. Stratigraphy and resource potential of the Eramosa Member (Amabel Formation), Bruce Peninsula, Ontario. Ont. Geol. Surv. Open File Report 5662, 1–90. See http://classify.oclc.org/classify2/ClassifyDemo?swid=150463218. [Google Scholar]
- 11.Noffke N, Gerdes G, Klenke T, Krumbein WE. 2001. Microbially induced sedimentary structures: a new category within the classification of primary sedimentary structures. J. Sediment. Res. 71, 649–656. ( 10.1306/2DC4095D-0E47-11D7-8643000102C1865D) [DOI] [Google Scholar]
- 12.Collette JH, Rudkin DM. 2010. Phyllocarid crustaceans from the Silurian Eramosa Lagerstätte (Ontario, Canada): taxonomy and functional morphology. J. Paleont. 84, 118–127. ( 10.1666/08-174.1) [DOI] [Google Scholar]
- 13.Kühl G, Bergmann A, Dunlop J, Garwood RJ, Rust J. 2012. Redescription and palaeobiology of Palaeoscorpius devonicus Lehmann, 1944 from the Lower Devonian Hunsrück Slate of Germany. Palaeontology 55, 775–787. ( 10.1111/j.1475-4983.2012.01152.x) [DOI] [Google Scholar]
- 14.Shear WA, Gensel PG, Jeram A. 1996. Fossils of large terrestrial arthropods from the Lower Devonian of Canada. Nature 384, 555–557. ( 10.1038/384555a0) [DOI] [Google Scholar]
- 15.Selden PA, Jeram AJ. 1989. Palaeophysiology of terrestrialisation in the Chelicerata. Trans. R. Soc. of Edinb., Earth Sci. 80, 303–310. ( 10.1017/S0263593300028741) [DOI] [Google Scholar]
- 16.McCoy VE, Brandt DS. 2009. Scorpion taphonomy: criteria for distinguishing fossil scorpion molts and carcasses. J. Arachnol. 37, 312–320. http://www.americanarachnology.org/JoA_free/JoA_v37_n3/arac-37-03-312.pdf ( 10.1636/SH09-07.1) [DOI] [Google Scholar]
- 17.Gaban RD, Farley RD. 2002. Ecdysis in scorpions: supine behaviour and exuvial ultrastructure. Invert. Biol. 121, 136–147. ( 10.1111/j.1744-7410.2002.tb00054.x) [DOI] [Google Scholar]
- 18.Tetlie OE, Brandt DS, Briggs DEG. 2008. Ecdysis in sea scorpions (Chelicerata: Eurypterida). Palaeogeogr. Palaeoclimatol. Palaeoecol. 265, 182–194. ( 10.1016/j.palaeo.2008.05.008) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



