Abstract
Social learning offers an efficient route through which humans and other animals learn about potential dangers in the environment. Such learning inherently relies on the transmission of social information and should imply selectivity in what to learn from whom. Here, we conducted two observational learning experiments to assess how humans learn about danger and safety from members (‘demonstrators') of an other social group than their own. We show that both fear and safety learning from a racial in-group demonstrator was more potent than learning from a racial out-group demonstrator.
Keywords: social learning, fear, safety
1. Introduction
Learning to predict potential dangers in the environment is critical for survival. Of equal importance is the ability to flexibly adapt to changing environmental contingencies, such as learning that a past threat is now safe. Whereas previous research has focused on how such predictions are formed and changed through own, direct experiences, much of what humans and other animals learn about the environment comes from social forms of learning, such as observing others [1]. Here, we assessed whether learning about danger and safety was determined by whether the demonstrator belonged to one's own (in-group) or another (out-group) racial group.
A substantial body of research on social learning supports that animals are selective with regards to whom they learn from. For example, although both primates [2–4] and rodents [5] can observationally acquire fears from unrelated conspecifics, the strength of such learning is enhanced by relatedness [5,6], familiarity and social status [6]. Such social learning biases might have conferred an adaptive advantage to animals, including humans, by facilitating the acquisition of locally relevant knowledge [1]. However, in increasingly diverse modern human societies, such social learning biases can be maladaptive, both because much important information is of global (as opposed to local) significance, and because traits, such as racial group membership, are less reliable indicators of whether or not individuals have locally relevant knowledge. Recent studies have shown that humans will more readily learn to fear racial out-group members [7], but it is unknown whether group membership affects the extent to which humans learn from others. We hypothesized that both fear and safety learning would be more potent when learned from an in-group demonstrator than from an out-group demonstrator. This was based on the presence of similarity-based learning biases in other animals [5,6], and a general tendency to display greater empathic and otherwise prosocial responses to in-group, when compared with out-group, members [8,9]. To test these hypotheses, we conducted two separate observational learning experiments based on previously established procedures for social fear and safety learning [4,10]. The first experiment was designed to measure observational fear learning, i.e. the acquisition of fear, and the second was designed to measure observational safety learning, i.e. the extinction of acquired fear. To assess learning, we used a standard index of conditioned response (CR) in humans: the skin conductance response (SCR), reflecting the phasic increase in skin conductance that occurs in response to physiologically arousing stimuli.
2. Material and methods
In both experiments 1 and 2, participants were White Swedish residents of European origin and were recruited on, or nearby, Karolinska Institutet campus. Participants were excluded if they either failed to report the correct contingency between the conditioned stimulus (CS) and the unconditioned stimulus (US) during acquisition [10] or their differential SCR was above or below 2.5 standard deviations from the group mean in the acquisition, extinction or test stage (see the electronic supplementary material). For both experiments, one image of a snake and one image of a spider served as CSs, and four different male individuals (two White and two Black counterbalanced across participants) served as demonstrators. Throughout all experimental stages, each CS was presented six times for 6 s in a pseudo-randomized order with an inter-trial interval ranging between 12 and 18 s. After both experiments, participants completed a computerized implicit association task [11]; assessing implicit racial bias and a questionnaire assessing explicit racial bias (modern racism scale questionnaire) [12].
In experiment 1, 46 White European participants (seven of whom were excluded; see the electronic supplementary material) first underwent an observational acquisition stage. During this stage, participants viewed a video depicting either the in- (White) or the out-group (Black) demonstrator displaying discomfort when receiving an electrical shock (the US) paired with the presentation of an image of a snake (the CS+), but never when paired with a spider image (the CS−). During a subsequent direct test, participants were re-exposed to the CSs, without being shocked, and in the absence of the demonstrator. Accordingly, any fear response to the CS+ was due to social learning taking place during the observational acquisition stage.
In experiment 2, 55 White European participants (10 of whom were excluded; see the electronic supplementary material) underwent an observational extinction paradigm developed by our group [10]. Briefly, participants first underwent standard fear conditioning during which they received a mild electrical shock (the US) when presented with an image of a snake (CS+), but never when presented with an image of a spider (CS−). Then, during observational extinction, participants watched a video depicting either the in- (White) or the out-group (Black) demonstrator acting calmly when exposed to presentations of both CSs. Finally, to assess fear recovery, participants underwent a standard reinstatement test during which they received three reminder shocks before they were re-exposed to the CSs during the direct test. This procedure has been shown to reinstate the expression of the original fear memory in both animals [13] and humans [14].
In both experiments, physiological fear responses were based on the mean SCR to the CS+ and CS− separately for each stage and analysed using separate mixed analysis of variance (ANOVA) with CS (CS+, CS−) as a within-subject factor, and group (in-group demonstrator, out-group demonstrator) as a between-subject factor. As CRs are extinguished rapidly in the absence of shocks [15], the direct test data in both experiments 1 and 2 were divided into an early and late stage, defined by the mean first three responses versus the mean subsequent three responses and analysed in a CS (CS+, CS−) × time (early, late) × group (in-group demonstrator, out-group demonstrator) mixed ANOVA.
3. Results
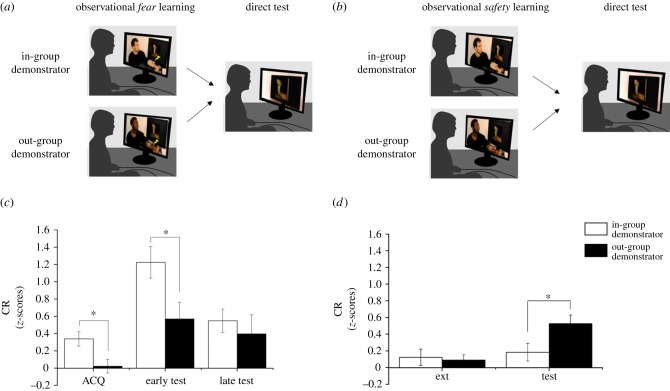
The CR was indexed as the differential SCR to the snake (CS+) and spider (CS−) image. The mean CR from both experiments is presented in figure 1.
Figure 1.
Experimental design and results from experiment 1 (left panels) and experiment 2 (right panels). (a,b) Both experiments were divided into an observational stage and a direct test stage. Note that in experiment 2, the observational extinction stage was preceded by a direct acquisition stage (see the electronic supplementary material, figure S1). (c,d) Mean CR (expressed as CS+/CS− SCR difference) as a function of in- and out-group demonstrator displayed for the observational and direct test stages separately for experiment 1 (left) and experiment 2 (right). Given the significant CS × time × group interaction during the test stage in experiment 1 (but not in experiment 2), data are plotted separately for the early and late stage of the direct test in experiment 1. Error bars indicate standard error of the mean. Asterisks indicate statistically significant differences (p < 0.05).
In experiment 1, we observed a significant group difference during observational acquisition, (CS × group: F1,37 = 7.57, p = 0.009, η2 = 0.17). Participants who were exposed to the in-group demonstrator acquired a CR (t(18) = 4.04, p = 0.001), whereas this effect was not significant in the group of participants exposed to the out-group demonstrator (t(19) = 0.28, p = 0.79). During the direct test, participants still differed during the early stage (CS × time × group: F1,37 = 4.21, p = 0.04, η2 = 0.10), but both groups of participants nevertheless expressed a significant CR (in-group demonstrator: t(18) = 6.68, p < 0.001; out-group demonstrator: t(19) = 2.95, p = 0.008). Interestingly, correlation analysis demonstrated that explicit, but not implicit, negative racial attitudes predicted CRs during the early test stage (r = −0.5, p = 0.026), so that participants with more negative racial attitudes expressed less learning from a racial out-group demonstrator (see the electronic supplementary material).
In experiment 2, participants showed equivalent levels of CR during direct acquisition (main effect of CS: F1,43 = 13.10, p = 0.001, η2 = 0.23), and there was a small remaining effect of CS during observational extinction (F1,43 = 4.11, p = 0.049, η2 = 0.09). Importantly, participants differed in CR during the subsequent direct test (CS × group: F1,43 = 4.85, p = 0.033, η2 = 0.10; CS × time × group: F1,43 = 0.15, p = 0.70). As predicted, the recovery of fear was stronger in the group of participants exposed to an out-group demonstrator (in-group: t(22) = 1.74, p = 0.095; out-group: t(21) = 5.07, p < 0.001). Finally, neither implicit nor explicit racial attitudes moderated the out-group learning bias in experiment 2 (see the electronic supplementary material).
4. Discussion
Our results show that both fear and safety learning are more potently learned from an in-group than from an out-group demonstrator. These findings concur with research in non-human animals showing that fears are readily learned from both social in- and out-group demonstrators [2,5], but that fear learning can be enhanced depending on the social relationship between the observer and demonstrator [5,6]. Experiment 2 demonstrated that the efficacy of social safety learning was restricted to conditions in which safety information was transmitted from an in-group demonstrator.
Taken together with previous research on social learning in non-human animals, the evolutionary interpretation of our findings suggests that humans are predisposed to learn about potential danger and safety from individuals belonging to their own social group. Because social categorization based on racial difference among humans occurred relatively recently in human evolutionary history [16], it is unlikely that responses to racial differences are under strong genetic influence [17], and therefore race per se is unlikely to explain our findings. Even if a genetic predisposition to preferentially learn from in-group individuals more generally exists, this is likely to be influenced by socially acquired attitudes concerning racial groups [18]. In support of this, we found a significant relation between negative racial attitudes and less expressed fear learning from a racial out-group member, stressing the importance of assessing how stereotypic beliefs about members of out-groups can bias both whom we learn from and what we learn from whom. Indeed, children show superior learning from similar others [19] and develop negative beliefs about racial out-group members early in life [20,21], and similar racial-biases have been demonstrated in related social situations that include interindividual encounters, such as cooperation and trust [22], empathy [9,23] and altruism [24].
Theoretical and empirical work highlights the tendency in animals to be selective with regards to when and from whom to learn, and that natural selection will favour the use of adaptive social strategies to guide the individual's reliance on social information [1]. Here, we demonstrate that human social learning is determined by the demonstrator's racial group. Given the possibility that using participants and demonstrators of other racial groups could produce different results, future work should address whether these effects generalize to other racial and social groups.
Supplementary Material
Acknowledgements
We thank Elizabeth A. Phelps and Carlos D. Navarrete for helpful comments on an earlier draft.
Ethics statement
The study was approved by the local ethics committee at Karolinska Institutet, and all participants gave written consent prior to participation.
Data accessibility
Data associated with the manuscript are accessible through Dryad: doi:10.5061/dryad.n9v18.
Author contributions
A. Golkar and A. Olsson developed the study concept and designed the experiments. Testing and data collection were performed by V. Castro. A. Golkar performed the data analysis and drafted the manuscript, and A. Olsson provided critical revisions. All authors approved the final version of the manuscript for submission.
Funding statement
This research was supported by an independent starting grant (284366; ELSI) from the European Research Council to A. Olsson.
Competing interests
The authors declare that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- 1.Laland KN. 2004. Social learning strategies. Learn. Behav. 32, 4–14. ( 10.3758/BF03196002) [DOI] [PubMed] [Google Scholar]
- 2.Cook M, Mineka S, Wolkenstein B, Laitsch K. 1985. Observational conditioning of snake fear in unrelated rhesus-monkeys. J. Abnorm. Psychol. 94, 591–610. ( 10.1037/0021-843X.94.4.591) [DOI] [PubMed] [Google Scholar]
- 3.Hygge S, Ohman A. 1978. Modeling processes in acquisition of fears: vicarious electrodermal conditioning to fear-relevant stimuli. J. Pers. Soc. Psychol. 36, 271–279. ( 10.1037/0022-3514.36.3.271) [DOI] [PubMed] [Google Scholar]
- 4.Olsson A, Phelps EA. 2004. Learned fear of ‘unseen’ faces after Pavlovian, observational, and instructed fear. Psychol. Sci. 15, 822–828. ( 10.1111/j.0956-7976.2004.00762.x) [DOI] [PubMed] [Google Scholar]
- 5.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. 2010. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488. ( 10.1038/nn.2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavaliers M, Colwell DD, Choleris E. 2005. Kinship, familiarity and social status modulate social learning about ‘micropredators’ (biting flies) in deer mice. Behav. Ecol. Sociobiol. 58, 60–71. ( 10.1007/s00265-004-0896-0) [DOI] [Google Scholar]
- 7.Olsson A, Ebert JP, Banaji MR, Phelps EA. 2005. The role of social groups in the persistence of learned fear. Science 309, 785–787. ( 10.1126/science.1113551) [DOI] [PubMed] [Google Scholar]
- 8.Hein G, Silani G, Preuschoff K, Batson CD, Singer T. 2010. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron 68, 149–160. ( 10.1016/j.neuron.2010.09.003) [DOI] [PubMed] [Google Scholar]
- 9.Xu XJ, Zuo XY, Wang XY, Han SH. 2009. Do you feel my pain? Racial group membership modulates empathic neural responses. J. Neurosci. 29, 8525–8529. ( 10.1523/jneurosci.2418-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golkar A, Selbing I, Flygare O, Ohman A, Olsson A. 2013. Other people as means to a safe end: vicarious extinction blocks the return of learned fear. Psychol. Sci. 24, 2182–2190. ( 10.1177/0956797613489890) [DOI] [PubMed] [Google Scholar]
- 11.Greenwald AG, mcghee DE, Schwartz JLK. 1998. Measuring individual differences in implicit cognition: the implicit association test. J. Pers. Soc. Psychol. 74, 1464–1480. ( 10.1037/0022-3514.74.6.1464) [DOI] [PubMed] [Google Scholar]
- 12.Akrami N, Ekehammar B, Araya T. 2000. Classical and modern racial prejudice: a study of attitudes toward immigrants in Sweden. Eur. J. Soc. Psychol. 30, 521–532. () [DOI] [Google Scholar]
- 13.Bouton ME. 2002. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry 52, 976–986. ( 10.1016/s0006-3223(02)01546-9) [DOI] [PubMed] [Google Scholar]
- 14.Haaker J, Golkar A, Hermans D, Lonsdorf TB. 2014. A review on human reinstatement studies: an overview and methodological challenges. Learn. Mem. 15, 424–440. ( 10.1101/lm.036053.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, U49–U51. ( 10.1038/nature08637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar S. 1998. Human variation: races, types, and ethnic groups, 4th edn Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 17.Cosmides L, Tooby J, Kurzban R. 2003. Perceptions of race. Trends Cogn. Sci. 7, 173–179. ( 10.1016/s1364-6613(03)00057-3) [DOI] [PubMed] [Google Scholar]
- 18.Fiske S, Taylor S. 2008. Social cognition: from brains to culture. New York, NY: McGraw-Hill. [Google Scholar]
- 19.Kinzler KD, Corriveau KH, Harris PL. 2011. Children's selective trust in native-accented speakers. Dev. Sci. 14, 106–111. ( 10.1111/j.1467-7687.2010.00965.x) [DOI] [PubMed] [Google Scholar]
- 20.Aboud FE. 1988. Children and prejudice. New York, NY: Blackwell. [Google Scholar]
- 21.Stanley DA, Sokol-Hessner P, Banaji MR, Phelps EA. 2011. Implicit race attitudes predict trustworthiness judgments and economic trust decisions. Proc. Natl Acad. Sci. USA 108, 7710–7715. ( 10.1073/pnas.1014345108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knauft BM. 1991. Violence and sociality in human-evolution. Curr. Anthropol. 32, 391–428. ( 10.1086/203975) [DOI] [Google Scholar]
- 23.Tajfel H, Billig MG, Bundy RP, Flament C. 1971. Social categorization and intergroup behavior. Eur. J. Soc. Psychol. 1, 149–177. ( 10.1002/ejsp.2420010202) [DOI] [Google Scholar]
- 24.De Waal FBM. 2008. Putting the altruism back into altruism: the evolution of empathy. In A. Rev. Psychol. 59, 279–300. ( 10.1146/annurev.psych.59.103006.093625) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with the manuscript are accessible through Dryad: doi:10.5061/dryad.n9v18.