Abstract
Impulsivity, the widespread preference for a smaller and more immediate reward over a larger and more delayed reward, is known to vary across species, and the metabolic and social hypotheses present contrasting explanations for this variation. However, this presents a paradox for an animal such as the honeybee, which is highly social, yet has a high metabolic rate. We test between these two competing hypotheses by investigating the effect of hunger on impulsivity in bees isolated from their social environment. Using an olfactory conditioning assay, we trained individuals to associate a small and a large reward with or without a delay, and we tested their choice between the two rewards at different levels of starvation. We found an increase in impulsive behaviour and an associated increase in dopamine levels in the brain with increasing starvation. These results suggest that the energetic state of an individual, even in a eusocial group, is a critical driver of impulsivity, and that the social harmony of a group can be threatened when the energetic states of the group members are in conflict.
Keywords: impulsivity, self-control, energetic state, starvation, honeybee
1. Introduction
Impulsivity, defined as a preference for a smaller and more immediate reward, over a larger and more delayed one, is pervasive throughout the animal kingdom, and is considered to be adaptive as environments are uncertain and futures are doubtful [1]. The degree of impulsivity, however, varies across species, and two different functional hypotheses can explain this variation. The metabolic hypothesis argues that smaller animals with higher metabolic rates are at higher risk of starvation and are therefore more likely to exhibit higher impulsivity or less self-control in order to avoid starving to death [2]. However, it has also been hypothesized that self-control is vital for a society to function in harmony [3,4], which we refer to as the social hypothesis. These two alternatives present an interesting paradox for an animal such as the honeybee, which has a high metabolic rate, but yet is also highly social.
Free-flying honeybee foragers have been shown to exhibit self-control, an observation interpreted as being consistent with the social hypothesis, because individual bees work for the ‘good of the colony’, and as evidence against the metabolic hypothesis [3]. However, such studies with free-flying foraging bees may not be well equipped to distinguish between the two alternative hypotheses as they are unable to control for the energetic state of an individual bee, which is normally tied to that of its colony. Some recent work has shown that individual bees can modulate their behavioural decisions based on their own energetic state, independent of the colony state [5,6]. This therefore presents an opportunity to test if self-control in a social animal is exclusively guided by the animal's own energetic state or its social environment, or by an interaction between the two. We therefore tested whether impulsivity displayed by an individual bee is a function of its own energetic state once it is isolated from the regulatory cues of its colony environment. To see whether the possible neurological mechanism underlying any observed impulsivity can be generalized across taxa, we also quantified the brain biogenic amines of individuals at different energetic states.
2. Material and methods
We collected the brood from three different colonies, hatched them in an incubator and introduced the newly emerged bees into a nursery colony after paint marking them on their thorax. Four week old marked foragers were captured individually, immobilized on ice and were randomly assigned to either the impulsivity or the biogenic amine experiment.
(a). Impulsivity experiment
We harnessed these bees individually and starved them for 2 h in an incubator maintained at 25°C and 70% relative humidity to increase their motivation for appetitive learning with the proboscis extension reflex (PER) assay [7]. Using only those bees that extended their proboscis to 50% sucrose solution upon touching their antennae, 10 bees were conditioned at a time with the PER assay. Each conditioning trial consisted of exposing the bee to a 1-octanol, eugenol, hexanol or octanone odour (a conditioned stimulus, CS) partially overlapping in time with a sucrose reward (an unconditioned stimulus, US), allowing the bee an opportunity to associate the two (see electronic supplementary material for details). Bees were conditioned to associate two different odours with two different rewards: either a large (1 µl) or a small (0.2 µl) volume of 50% sucrose solution. In one set of conditioning trials, the two rewards were not linked with any time delays, whereas in another, the small reward was delivered to the bee after 1 s of the CS presentation and the large reward after a 5 s delay. Bees were able to show acquisition of the delayed rewards, preferring the large reward with a longer delay (electronic supplementary material, figure S1).
Following these conditioning trials, we fed the bees until satiation with 30% sucrose solution and placed them in the incubator. After starving the bees for 6, 18 and 24 h, a time period during which their energetic state is known to decline progressively [5], we gave a choice test to each bee at each of these time points, using the two-alternative forced choice PER assay [5,8]. This assay consists of presenting the two previously learned odours (CS) to the bee from two different directions, on either side of its head, and recording the side (and therefore the CS) to which the bee turns its head and extends its proboscis (see the electronic supplementary material for details). The final direction of its head was taken as a measure of choice towards that particular CS–US pairing.
(b). Biogenic amine quantification
Harnessed bees were fed 30% sucrose solution ad libitum and were starved for periods matching the same time points for the choice test in the impulsivity experiment. At the end of the starvation period, we flash froze these bees in liquid nitrogen and removed their heads, and stored them at −80°C. We dissected the bee brains on dry ice to minimize amine degradation, and three brains were pooled together. High-performance liquid chromatography (HPLC) was used to measure the amount of biogenic amines in these samples (see electronic supplementary material for details). We restricted the quantification of biogenic amines only to bees starved for 18 and 24 h, when they displayed a significant preference for one of the two rewards.
3. Results
Preference for the large reward depends on whether or not a delay is associated with it. When the large reward was associated with a delay, preference for it decreased as bees were more starved and when starved for 24 h, bees displayed a significant preference for the small reward (repeated measures GLMM: time × treatment interaction: F5,331 = 7.64, p < 0.0001; intercept: 0.77, p = 0.001, figure 1). There were no significant differences in the amount of brain biogenic amines octopamine, tyramine and serotonin at the two different levels of starvation (multivariate GLM: octopamine: F1,58 = 0.43, p = 0.51; tyramine: F1,58 = 1.80, p = 0.18; serotonin: F1,58 = 0.28, p = 0.59), but there was a significantly higher amount of dopamine in the brains of bees that were starved for 24 h (dopamine: F1,58 = 5.83, p = 0.01, figure 2).
Figure 1.
Preference for the large reward in a choice test when the two rewards are either associated or not with a delay. Each bar represents the mean (±s.e.), with letters denoting significant differences between pairwise comparisons, asterisks indicating a significant difference from random choice represented by the dashed line, and the corresponding sample sizes are indicated above each bar.
Figure 2.
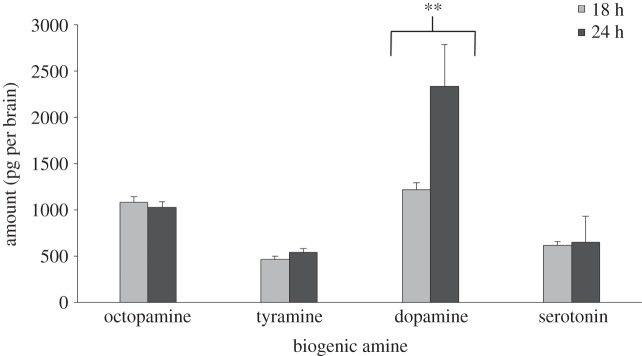
Biogenic amine levels in bees at different levels of starvation presented as means (±s.e.) with the asterisks indicating a significant difference at α = 0.05 level. Data consist of 30 replicates in each of which three brains were pooled together for analysis.
4. Discussion
These results show that honeybees are able to maintain self-control when their energetic state is relatively high but they become more impulsive with starvation, which corresponds to an increase in dopamine levels found in the brain. To the best of our knowledge, this is the first time dopamine has been linked to impulsive behaviour in an invertebrate, and the results are consistent with what is known about the general link between dopamine and impulsivity [9,10]. The relationship among dopamine, starvation and impulsivity is, however, fairly complex, and more direct experimental manipulations would be required to establish a causal connection between dopamine and the modulation of impulsivity as a result of starvation.
Our results show that that even in a eusocial animal such as the honeybee, individual energetic state drives impulsivity in a manner consistent with the metabolic hypothesis. The reason a bee can be expected to display self-control under normal circumstances is not necessarily because it is for the ‘good of the society’, but because it is generally satiated in those settings and is not at a risk of starvation despite its high metabolic rate. The observed plasticity of self-control at an individual level is especially meaningful in such a context. These results are also consistent with the idea that animals foraging to provision should exhibit a higher level of self-control because they are ‘foraging for the future’ and therefore following a shallower temporal discounting function than animals foraging to satisfy their own hunger [1,3], although this interpretation has been questioned [11].
In contrast to a large body of research showing that energetic state has a strong effect on temporal discounting and self-control [4,12], some recent human studies found no effect of energetic depletion on self-control, thus arguing against the metabolic hypothesis [13,14]. These studies instead offer a motivational model for how carbohydrates regulate self-control, which nevertheless involves an effect on the dopaminergic pathway. A lack of deprivation effect on self-control could also arise if choices are controlled by the relative, rather than the absolute, value of the reinforcers on a discounting task [15]. In this case, deprivation would result in proportionally equivalent changes in the value of the two alternative reinforcers, leaving preference between these alternatives unchanged.
The concept of impulsivity covers a wide range of actions which can be defined as poorly conceived, prematurely expressed, unduly risky or inappropriate to the situation, which often result in undesirable outcomes [16]. In a related idea about impulsivity that involves fast and inaccurate choices over slow and accurate ones, the degree of impulsive behaviour in honeybees and other social insects has been suggested to be positively correlated to the energetic need the animal is facing [17–19]. In honeybees, a metabolic regulation of impulsivity could mean that individual foragers can become more impulsive owing to an energetic stress resulting from ecological factors such as a parasitic disease [20], or resource scarcity [21], and cause them to not take advantage of the entire floral landscape.
The results of this study most importantly show that the social and metabolic hypotheses for impulsivity may not be mutually exclusive. These results add to the growing body of evidence that the mechanisms driving altruistic foraging in eusocial animals are fundamentally similar to those found in solitary animals [6,22]. One can speculate that having a continuous access to food and energy from a build-up of communal stores as seen in many social insects has promoted self-control in individuals and has facilitated the evolution of social behaviour. The observed plasticity in impulsivity, associated with changing hunger levels, however, shows that metabolic rate and the consequent energetic state of an individual are critical drivers of this trait, and this can disrupt and threaten social harmony when the individual energetic state is in conflict with that of the group.
Supplementary Material
Supplementary Material
Acknowledgements
C.M. thanks Frank Hirche and Gabriele Stangl for access and assistance with the HPLC machine, and Ying Wang and Julie Mustard for showing how to perform brain dissections.
Data accessibility
All data underlying the findings described in this manuscript are fully available without restriction in the electronic supplementary material.
Author contributions
C.M. and D.N. conceived the study and its experimental design. C.M. conducted the experiments and analysed the data. C.M. and D.N. wrote the manuscript.
Funding statement
This work was supported by an NSF CAREER award to D.N. and an Alexander von Humboldt Foundation Fellowship award to C.M.
Competing interests
The authors declare no competing financial interests.
References
- 1.Stevens JR, Stephens DW. 2008. Patience. Curr. Biol. 18, R11–R12. ( 10.1016/j.cub.2007.11.021) [DOI] [PubMed] [Google Scholar]
- 2.Tobin H, Logue AW. 1994. Self-control across species (Columba livia, Homo sapiens, and Rattus norvegicus). J. Comp. Psychol. 108, 126–133. ( 10.1037/0735-7036.108.2.126) [DOI] [PubMed] [Google Scholar]
- 3.Cheng K, Pena J, Porter MA, Irwin JD. 2002. Self-control in honeybees. Psychonom. Bull. Rev. 9, 259–263. ( 10.3758/BF03196280) [DOI] [PubMed] [Google Scholar]
- 4.Gailliot MT, Baumeister RF. 2007. The physiology of willpower: linking blood glucose to self-control. Pers. Soc. Psychol. Rev. 11, 303–327. ( 10.1177/1088868307303030) [DOI] [PubMed] [Google Scholar]
- 5.Mayack C, Naug D. 2011. A changing but not an absolute energy budget dictates risk-sensitive behaviour in the honeybee. Anim. Behav. 82, 595–600. ( 10.1016/j.anbehav.2011.06.022) [DOI] [Google Scholar]
- 6.Mayack C, Naug D. 2013. Individual energetic state can prevail over social regulation of foraging in honeybees. Behav. Ecol. Sociobiol. 67, 929–936. ( 10.1007/s00265-013-1517-6) [DOI] [Google Scholar]
- 7.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119. ( 10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 8.Shafir S, Yehonatan L. 2014. Comparative evaluations of reward dimensions in honey bees: evidence from two-alternative forced choice proboscis-extension conditioning. Anim. Cogn. 17, 633–644. ( 10.1007/s10071-013-0694-z) [DOI] [PubMed] [Google Scholar]
- 9.Berridge KC. 2004. Motivation concepts in behavioral neuroscience. Physiol. Behav. 81, 179–209. ( 10.1016/j.physbeh.2004.02.004) [DOI] [PubMed] [Google Scholar]
- 10.Pine A, Shiner T, Seymour B, Dolan RJ. 2010. Dopamine, time, and impulsivity in humans. J. Neurosci 30, 8888–8896. ( 10.1523/JNEUROSCI.6028-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fawcett TW, McNamara JM, Houston AI. 2012. When is it adaptive to be patient? A general framework for evaluating delayed rewards. Behav. Process. 89, 128–136. ( 10.1016/j.beproc.2011.08.015) [DOI] [PubMed] [Google Scholar]
- 12.Wang XT, Dvorak RD. 2010. Sweet future: fluctuating blood glucose levels affect future discounting. Psychol. Sci. 21, 183–188. ( 10.1177/0956797609358096) [DOI] [PubMed] [Google Scholar]
- 13.Molden DC, Hui CM, Scholer AA, Meier BP, Noreen EE, D'Agostino PR, Martin V. 2012. Motivational versus metabolic effects of carbohydrates on self-control. Psychol. Sci. 23, 1137–1144. ( 10.1177/0956797612439069) [DOI] [PubMed] [Google Scholar]
- 14.Inzlicht M, Schmeichel BJ, Macrae CN. 2014. Why self-control seems (but may not be) limited. Trends Cogn. Sci. 18, 127–133. ( 10.1016/j.tics.2013.12.009) [DOI] [PubMed] [Google Scholar]
- 15.Oliveira L, Calvert A, Green L, Myerson J. 2013. Level of deprivation does not affect degree of discounting in pigeons. Learn. Behav. 41, 148–158. ( 10.3758/s13420-012-0092-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evenden JL. 1999. Varieties of impulsivity. Psychopharmacology 146, 348–361. ( 10.1007/PL00005481) [DOI] [PubMed] [Google Scholar]
- 17.Chittka L, Dyer AG, Bock F, Dornhaus A. 2003. Psychophysics: bees trade off foraging speed for accuracy. Nature 424, 388 ( 10.1038/424388a) [DOI] [PubMed] [Google Scholar]
- 18.Franks NR, Dornhaus A, Fitzsimmons JP, Stevens M. 2003. Speed versus accuracy in collective decision making. Proc. R. Soc. Lond. B 270, 2457–2463. ( 10.1098/rspb.2003.2527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns JG. 2005. Impulsive bees forage better: the advantage of quick, sometimes inaccurate foraging decisions. Anim. Behav. 70, e1–e5. ( 10.1016/j.anbehav.2005.06.002) [DOI] [Google Scholar]
- 20.Mayack C, Naug D. 2009. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 100, 185–188. ( 10.1016/j.jip.2008.12.001) [DOI] [PubMed] [Google Scholar]
- 21.Naug D. 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142, 2369–2372. ( 10.1016/j.biocon.2009.04.007) [DOI] [Google Scholar]
- 22.Toth AL, Robinson GE. 2007. Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341. ( 10.1016/j.tig.2007.05.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying the findings described in this manuscript are fully available without restriction in the electronic supplementary material.



