Abstract
Identifying group members and individuals' status within a group are fundamental tasks in animal societies. For ants, this information is coded in the cuticular hydrocarbon profile. We manipulated profiles of the ant Odontomachus brunneus to examine whether the releaser and primer effects of fertility signals are dependent on chemical context. Fertility status is signalled through increased abundance of (Z)-9-nonacosene (Z9 : C29). Across the ant's distribution, populations have distinct hydrocarbon profiles but the fertility signal is conserved. Foreign queens and fertility-signal-treated workers from the same population, sharing a similar chemical background, elicited releaser effects from workers, whereas queens and fertility-signal-treated workers from different populations did not. Z9 : C29 presented without chemical background did not elicit releaser effects. A primer-effect experiment found that Z9 : C29, presented without a chemical background, did not inhibit worker reproduction. Our results demonstrate that a familiar chemical background is necessary for appropriate responses to fertility signals.
Keywords: fertility signal, cuticular hydrocarbon, queen pheromone
1. Introduction
Social behaviour depends on identifying group members who benefit from mutual cooperation [1,2]. In large societies where unfamiliar individuals encounter one another, phenotypic ‘tags’ can be used to indicate relatedness or group membership [3]. For example, in human evolution, hunter–gatherer bands may have used tags such as shared accents of identifiable vocalizations or proto-languages [4,5]. To interpret group membership, the receiver of these signals must first recognize the vocalization and then the accent nested within. Like humans, social insects often form large societies, wherein individual-level recognition is not possible. Instead, colony members share a gestalt nestmate chemical profile [6,7]. This cuticular hydrocarbon profile consists of approximately 10–40 long-chained (C25–C35) hydrophobic lipids that coat the insect cuticle. Within this profile, a subset of compounds signal an individual's fertility status [8]. Social insects, therefore, compose model systems for studying nested signals of group membership and individual status.
Social insects are thought to first assess group membership, then make more detailed assessments related to division of labour or reproductive status ([9], but see [10]). This hypothesis remains largely untested owing to the small number of experimentally identified signals of caste or reproductive status. Experimental studies identifying the primer effect of queen hydrocarbon fertility signals in which a single compound is introduced to workers suggest that compounds without chemical background can mimic the presence of a queen by inhibiting worker ovarian development [11–13]. However, these compounds occur naturally only in the context of the entire chemical profile of an individual or an egg. By contrast, experimental studies of the releaser effect of fertility signals have supplemented the cuticular profile of non-reproductive workers with single compounds correlated with fertility [14,15]. These treatments evoke aggressive reactions (policing) from nestmate workers, inhibiting the potential reproductive efforts of their nestmates.
Cuticular hydrocarbon fertility signals have been experimentally identified in only seven species [13]. Of these, the trap-jaw ant Odontomachus brunneus is an ideal system to study the effect of chemical background on signal perception. This species is distributed throughout the southeastern United States, and populations have specific qualitative and quantitative differences in cuticular hydrocarbon compound presence and abundances [16]. Nevertheless, different populations signal fertility using the same compound, (Z)-9-nonacosene (Z9 : C29), which is relatively more abundant in the profile of reproductive queens and reproductive workers than in non-reproductives [15,16]. Population variation of the overall profile and the conserved fertility signal allow us to examine the influence of a group membership signal on the perception of a signal of individual status. We measured the releaser-effect reaction of workers to nestmate and non-nestmate queens of differing hydrocarbon profiles, non-reproductive nestmate and non-nestmate workers treated with Z9 : C29, and Z9 : C29 without a hydrocarbon profile background. We also tested for primer effects by introducing Z9 : C29 to isolated groups of workers and monitoring their reproductive activity, relative to various controls.
2. Material and methods
Colonies were collected from three populations in Florida: Archbold Biological Station in Venus, West Palm Beach and Withlacoochee State Forest in Lecanto. (See the electronic supplementary material for expanded methods.)
(a). Releaser-effect experiments
Test workers originated from 12 Archbold colonies and were harnessed in a 5 × 5 cm paper restraint. Stimuli were presented to restrained workers by contacting their antennae and measuring antennal retraction. Antennal retraction is a releaser-response, a stereotypical submissive posture of O. brunneus worker display in proximity to a reproductive queen or worker [15–17]. Each worker was presented with a stimulus three times and the consensus reaction of that worker (submissive or non-submissive reaction in at least two of the three trials) was recorded. Experiment 1 exposed workers to nestmate queens, non-nestmate same-population queens, non-nestmate different population queens and nestmate non-reproductive workers as a control. Experiment 2 exposed workers to Z9 : C29-treated (i) non-reproductive nestmate workers, (ii) non-reproductive non-nestmate workers from the same population and (iii) non-reproductive non-nestmate workers from a different population. Experiment 3 exposed workers to nestmate workers whose hydrocarbon profiles had been removed (through successive hexane washes) and which had been treated with Z9 : C29 or a hydrocarbon control (heptacosane, C27). Thirty microlitres of a hydrocarbon working solution (0.125 mg ml−1 in hexane; 3.75 μg of hydrocarbon) were used for all experiments as per previous bioassays with this species [15]. Each experiment used a different set of workers, and stimuli were presented in random order. (See the electronic supplementary material for an additional releaser-effect experiment.)
Trials were video recorded, with videos given a coded title assuring that the data recorder was blind to the treatments. Data were analysed using Cochran's Q-test, and sign tests were used for pairwise comparisons with Holm–Bonferroni adjusted significance levels for pairwise comparisons. All statistics were performed using statistica v. 7 software (StatSoft, USA).
(b). Primer-effect experiment
Thirteen colonies from the Archbold population were split into three equal-sized queenless groups (mean group size 21; min. 12, max. 34), which were housed in a single 60 × 15 mm Petri dish nest with a moistened dental plaster floor, within a 19 × 13.5 cm arena. Treatments consisted of adding Z9 : C29 or heptacosane (3.75 µg doses), or hexane (control) to glass coverslips. A single coverslip was added to each colony and replaced daily. Groups were fed sugar water ad libitum and 2–3 termites per day. Each nest was inspected daily for the presence of worker-laid eggs. Successful worker egg-laying was classified according to the date eggs first appeared and remained present in the nest for at least 48 h. This ensured that the eggs were not trophic (shared as food between nestmates) or policed (destroyed). All groups were given 45 days to lay eggs. The likelihood that worker groups successfully laid eggs was compared between treatments. For colonies in which all treatment groups laid eggs (8/13), the number of days until egg-laying was compared, within colonies, by a Friedman ANOVA test.
3. Results
(a). Releaser-effect experiments
A larger percentage of workers showed submissive reactions when they were presented with nestmate queens and non-nestmate queens from the same population than when presented queens from different populations or nestmate workers (figure 1a). Worker submissive reactions were more frequent in response to nestmate and foreign workers from the same population treated with the fertility signal, than to fertility-signal-treated workers from a different population (figure 1b). By contrast, submissive reactions of workers to nestmates stripped of hydrocarbons and then treated with Z9 : C29 were not different from the responses displayed to the C27-treated controls (figure 1c; electronic supplementary material contains similar results from an additional experiment with isolated compounds).
Figure 1.
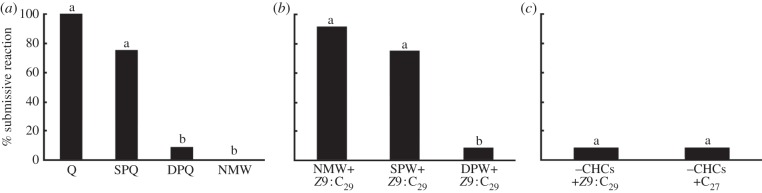
Releaser-effect experimental results showing submissive reactions of test workers to (a) queens, (b) workers treated with the fertility signalling compound Z9 : C29 and (c) workers stripped of their hydrocarbon profiles and treated with Z9 : C29 or C27. (a: Cochran's Q-test, Q = 24.64, p < 0.001; same-population queen (SPQ) versus different population queen (DPQ) p = 0.013, SPQ versus nestmate worker (NMW) p = 0.007, nestmate queen (Q) versus DPQ p = 0.002, Q versus NMW p = 0.008, Q versus SPQ p = 0.24, DPQ versus NMW p = 1). (b: Cochran's Q-test, Q = 15.27, p < 0.001; NMW versus same-population worker (SPW) p = 0.61, SPW versus different population worker (DPW) p = 0.013, NMW versus DPW p = 0.004). (c: z = 0, p = 1). N = 12 for all groups.
(b). Primer-effect experiment
Treatment type did not influence the probability that queenless worker groups would lay eggs (number of groups egg-laying per treatment out of 13 possible: Z9 : C29 = 10, C27 = 12, hexane = 10; Cochran Q-test Q = 4, p = 0.13). Across the entire experiment, the average number of days until worker egg-laying was 23 (min. = 10, max. = 44). Survival of initial worker populations up to the point of egg-laying averaged 98% (min. = 87%, max. = 100%). A total of eight of 13 colonies had all worker groups lay eggs and were therefore directly comparable across all treatments. There was no effect of treatment on differences in days until worker egg-laying (figure 2).
Figure 2.

Tests of primer effects of the pure compounds C27 (hydrocarbon control) and Z9 : C29 showing days to egg-laying, relative to the hexane control group (zero). Dotted lines connect data from the same colonies, Friedman ANOVA χ2 = 1.6, p = 0.46.
4. Discussion
Our results indicate that the fertility signal of O. brunneus requires a familiar or near-nestmate chemical background to be perceived properly as a fertility signal. This result contrasts with other primer-effect experiments, wherein a compound presented without the normal chemical background inhibited worker ovarian development [11–13].
Z9 : C29 is present on the cuticles of non-reproductive O. brunneus workers and brood [16,18]. The numbers of workers and brood vary seasonally and throughout the colony life cycle [19]. Thus, assessing queen fertility by the total amount of fertility signal present in the colony is unreliable. Instead, encountering a complete hydrocarbon profile in which the fertility signalling compound is present in a relative abundance unique to reproductive individuals is likely to be a more reliable indicator of queen presence and fertility.
Our results are in agreement with the hypothesis of hierarchical assessments of hydrocarbon profiles, wherein recognition of nestmate or near-nestmate signals precedes caste and task-specific recognition [9]. Additional evidence of the importance of a chemical background comes from social parasites that invade colonies to usurp the role of the queen. For example, the bumblebee, Bombus terrestris, can be parasitized by congeners B. vestalis and B. bohemicus, whose hydrocarbon profiles mimic the complete chemical profile of the host queen and suppress host worker ovarian development [20,21]. If a single compound were sufficient for mimicking a queen, social parasites would not need to replicate or obtain more complete profiles and an individual with a profile lacking nestmate signals but displaying the correct fertility signals would be an omnipotent parasite of the host species. However, resistance to parasitism in ants and wasps is thought to evolve by increasing the diversity and complexity of the nestmate recognition signals coded in the cuticular profiles, supporting the notion that a complex chemical background is advantageous because it inhibits mimicry [22].
Interpreting nested signals is not a challenge unique to social organisms. For example, a male parasitoid wasp chooses mates based on one or a small subset of female-specific cuticular hydrocarbons. Bioassays have shown that courtship behaviour is not elicited by single, sex-specific compounds, but rather requires other non-sex-specific compounds as a chemical background [23]. Further research in other systems examining context-specific signals of individual status will broaden our understanding of the evolution of cooperation.
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to L. M. Hanks for comments on a draft of the manuscript and Anne Curé for assistance in data collection.
Data accessibility
All data are available in the electronic supplementary material.
Author contributions
A.A.S. designed and performed the experiments, analysed data and wrote the manuscript. J.G.M. synthesized the chemicals and wrote the manuscript. A.V.S. designed the experiments, funded the project and wrote the manuscript.
Funding statement
This project was partially funded by Hatch Act project CA-R*-5181-H to J.G.M.
Conflict of interests
The authors have no competing interests.
References
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour I. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 3.Axelrod R, Hammond RA, Grafen A. 2004. Altruism via kin-selection strategies that rely on arbitrary tags with which they coevolve. Evolution 58, 1833–1838. ( 10.1111/j.0014-3820.2004.tb00465.x) [DOI] [PubMed] [Google Scholar]
- 4.Cohen E. 2012. The evolution of tag-based cooperation in humans. Curr. Anthropol. 53, 588–616. ( 10.1086/667654) [DOI] [Google Scholar]
- 5.Moffett M. 2013. Human identity and the evolution of societies. Hum. Nat. 24, 219–267. ( 10.1007/s12110-013-9170-3) [DOI] [PubMed] [Google Scholar]
- 6.Crozier RH, Dix MW. 1979. Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav. Ecol. Sociobiol. 4, 217–224. ( 10.1007/BF00297645) [DOI] [Google Scholar]
- 7.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères AG.), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Liebig J. 2010. Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères AG.), pp. 254–281. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Le Conte Y, Hefetz A. 2008. Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 53, 523–542. ( 10.1146/annurev.ento.52.110405.091434) [DOI] [PubMed] [Google Scholar]
- 10.Moore D, Liebig J. 2010. Mixed messages: fertility signaling interferes with nestmate recognition in the monogynous ant Camponotus floridanus. Behav. Ecol. Sociobiol. 64, 1011–1018. ( 10.1007/s00265-010-0916-1) [DOI] [Google Scholar]
- 11.Holman L, Jorgensen CG, Nielsen J, d'Ettorre P. 2010. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. B 277, 3793–3800. ( 10.1098/rspb.2010.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman L, Lanfear R, D’ Ettorre P. 2013. The evolution of queen pheromones in the ant genus Lasius. J. Evol. Biol. 26, 1549–1558. ( 10.1111/jeb.12162) [DOI] [PubMed] [Google Scholar]
- 13.Van Oystaeyen A, et al. 2014. Conserved class of queen pheromones stops social insect workers from reproducing. Science. 343, 287–290. ( 10.1126/science.1244899) [DOI] [PubMed] [Google Scholar]
- 14.Smith AA, Hölldobler B, Liebig J. 2009. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr. Biol. 19, 78–81. ( 10.1016/j.cub.2008.11.059) [DOI] [PubMed] [Google Scholar]
- 15.Smith AA, Millar JG, Hanks LM, Suarez AV. 2012. Experimental evidence that workers recognize reproductives through cuticular hydrocarbons in the ant Odontomachus brunneus. Behav. Ecol. Sociobiol. 66, 1267–1276. ( 10.1007/s00265-012-1380-x) [DOI] [Google Scholar]
- 16.Smith AA, Millar JG, Hanks LM, Suarez AV. 2013. A conserved fertility signal despite population variation in the cuticular chemical profile of the trap-jaw ant Odontomachus brunneus. J. Exp. Biol. 216, 3917–3924. ( 10.1242/jeb.089482) [DOI] [PubMed] [Google Scholar]
- 17.Powell S, Tschinkel WR. 1999. Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: a new organizational mechanism in ants. Anim. Behav. 58, 965–972. ( 10.1006/anbe.1999.1238) [DOI] [PubMed] [Google Scholar]
- 18.Smith AA, Vanderpool W, Millar JG, Hanks LM, Suarez AV. 2014. Conserved male-specific cuticular hydrocarbon patterns in the trap-jaw ant Odontomachus brunneus. Chemoecology 24, 29–34. ( 10.1007/s00049-013-0143-0) [DOI] [Google Scholar]
- 19.Hart LM, Tschinkel WR. 2012. A seasonal natural history of the ant, Odontomachus brunneus. Insect Soc. 59, 45–54. ( 10.1007/s00040-011-0186-6) [DOI] [Google Scholar]
- 20.Kreuter K, Bunk E, Luckemeyer A, Twele R, Francke W, Ayasse M. 2012. How the social parasitic bumblebee Bombus bohemicus sneaks into power of reproduction. Behav. Ecol. Sociobiol. 66, 475–486. ( 10.1007/s00265-011-1294-z) [DOI] [Google Scholar]
- 21.Martin SJ, Carruthers JM, Williams PH, Drijfhout FP. 2010. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. 36, 855–863. ( 10.1007/s10886-010-9805-3) [DOI] [PubMed] [Google Scholar]
- 22.Martin SJ, Helantera H, Drijfhout FP. 2011. Is parasite pressure a driver of chemical cue diversity in ants? Proc. R. Soc. B 278, 496–503. ( 10.1098/rspb.2010.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syvertsen TC, Jackson LL, Blomquist GJ, Vinson SB. 1995. Alkadienes mediating courtship in the parasitoid Cardiochiles nigriceps (Hymenoptera: Braconidae). J. Chem. Ecol. 21, 1971–1989. ( 10.1007/BF02033856) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the electronic supplementary material.


