Abstract
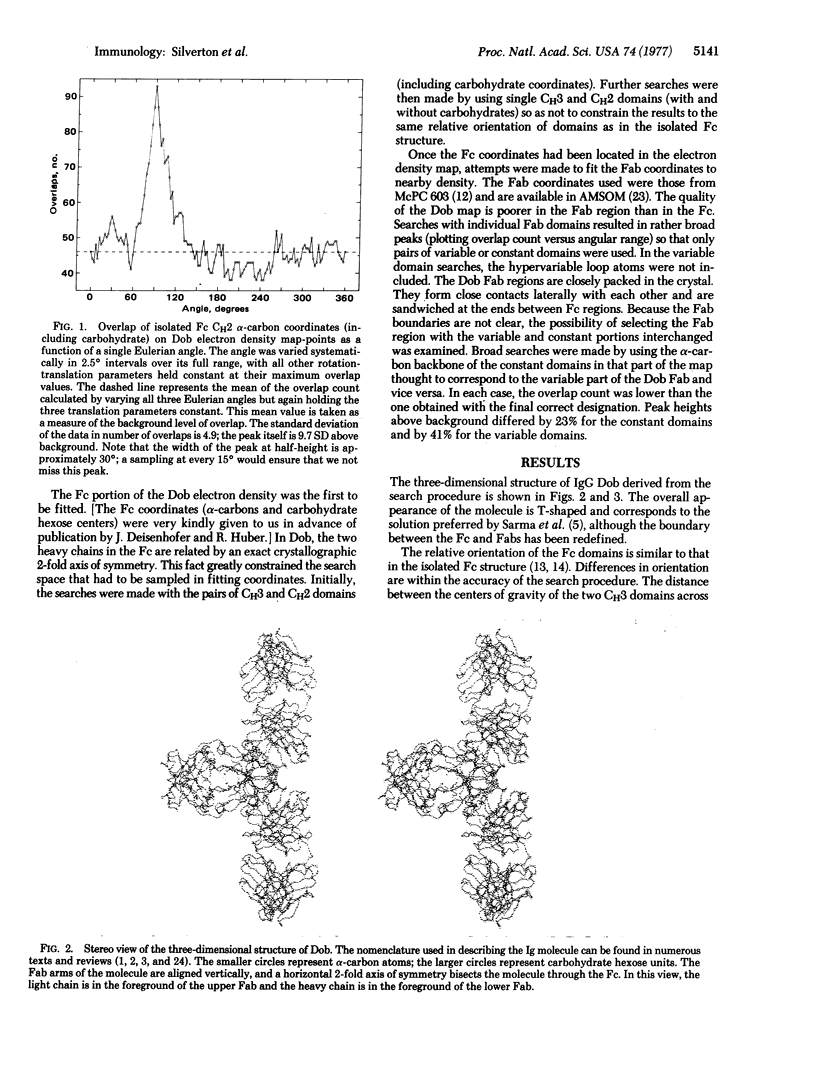
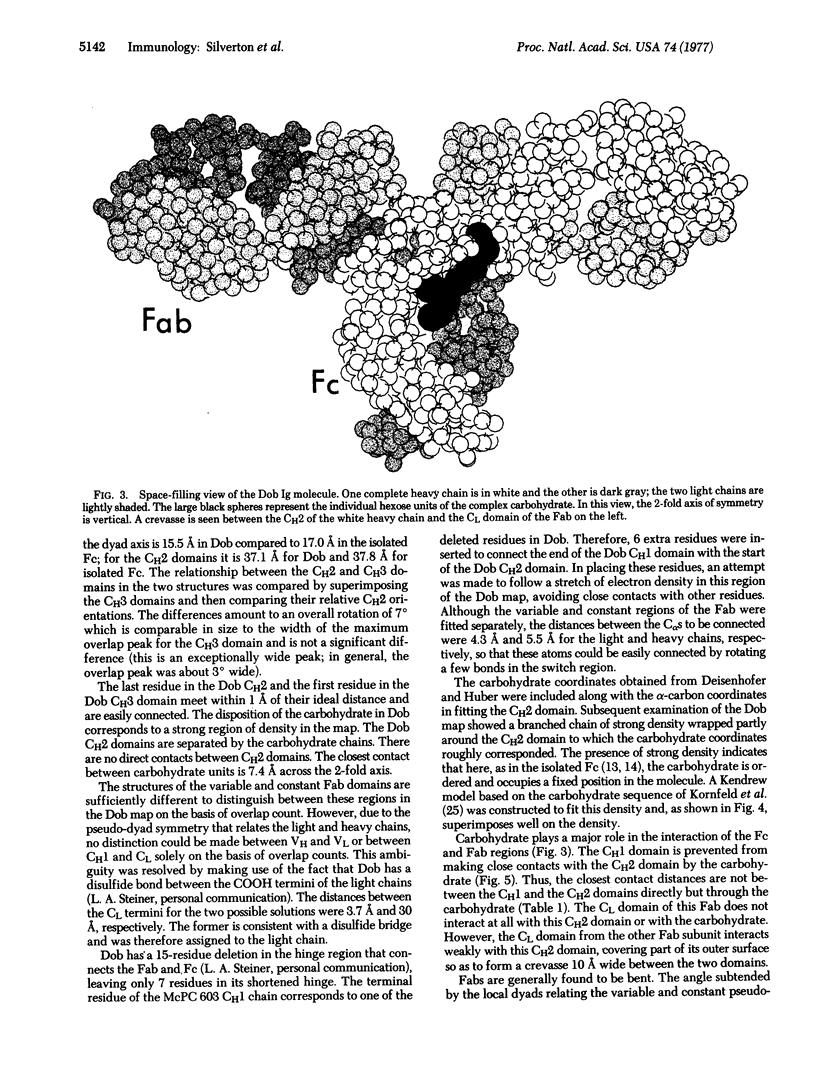
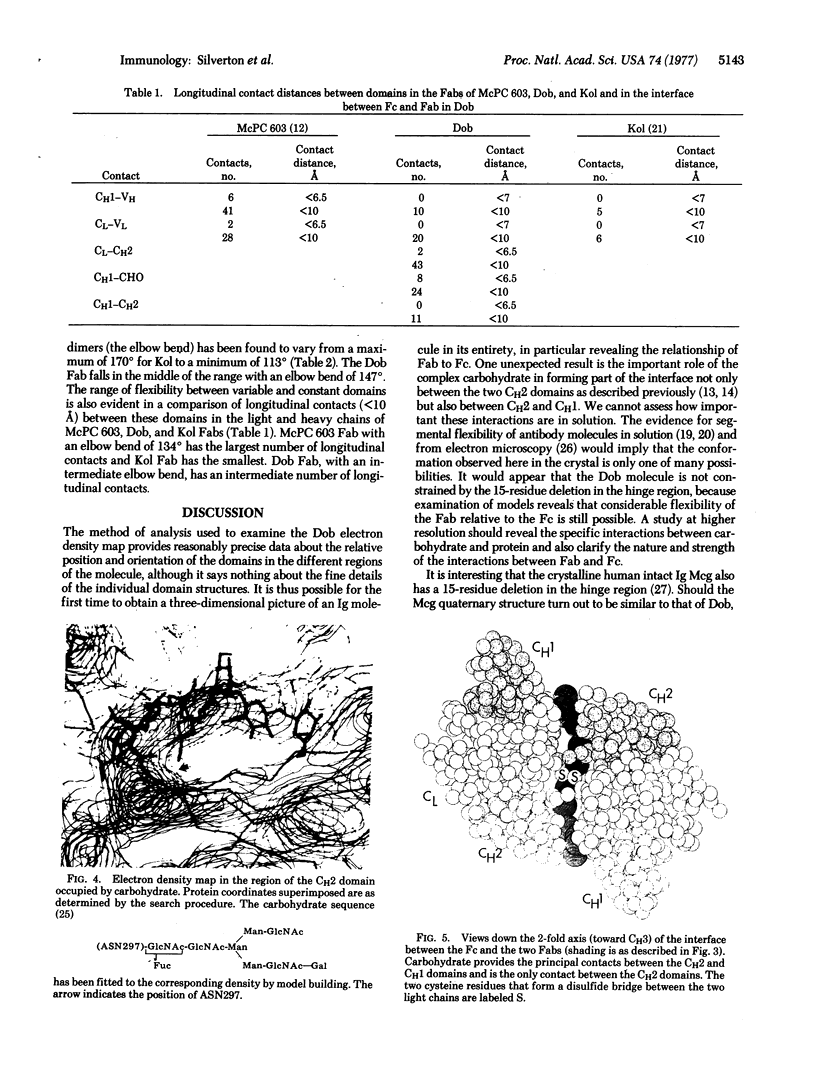
We have examined the low-resolution structure of a complete human IgG1 using known domain coordinates from crystallographic investigations of immunoglobulin fragment structures. Our results indicate that the Fc portion of this molecule has a structure similar to that of an isolated Fc fragment, with the carbohydrate moiety playing a central role as the principal contact between the CH2 domains. Carbohydrate also forms a large part of the interface between the Fc and Fab regions. The relative orientations of the variable and constant portions of the Fab regions are intermediate between those reported previously, emphasizing the flexibility of the switch region. These data do not support a two-state allosteric model such as has been proposed for antibody effector functions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colman P. M., Deisenhofer J., Huber R. Structure of the human antibody molecule Kol (immunoglobulin G1): an electron density map at 5 A resolution. J Mol Biol. 1976 Jan 25;100(3):257–278. doi: 10.1016/s0022-2836(76)80062-9. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Segal D. M. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1975;44:639–667. doi: 10.1146/annurev.bi.44.070175.003231. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Colman P. M., Epp O., Huber R. Crystallographic structural studies of a human Fc fragment. II. A complete model based on a Fourier map at 3.5 A resolution. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1421–1434. doi: 10.1515/bchm2.1976.357.2.1421. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Colman P. M., Huber R., Haupt H., Schwick G. Crystallographic structural studies of a human Fc-fragment. I. An electron-density map at 4 A resolution and a partial model. Hoppe Seylers Z Physiol Chem. 1976 Mar;357(3):435–445. doi: 10.1515/bchm2.1976.357.1.435. [DOI] [PubMed] [Google Scholar]
- Deutsch H. F., Suzuki T. A crystalline G1 human monoclonal protein with an excessive H chain deletion. Ann N Y Acad Sci. 1971 Dec 31;190:472–486. doi: 10.1111/j.1749-6632.1971.tb13557.x. [DOI] [PubMed] [Google Scholar]
- Edmundson A. B., Wood M. K., Schiffer M., Hardman K. D., Ainsworth C. F., Ely K. R. A crystallographic investigation of a human IgG immunoglobulin. J Biol Chem. 1970 May 25;245(10):2763–2764. [PubMed] [Google Scholar]
- Epp O., Colman P., Fehlhammer H., Bode W., Schiffer M., Huber R., Palm W. Crystal and molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI. Eur J Biochem. 1974 Jun 15;45(2):513–524. doi: 10.1111/j.1432-1033.1974.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Fehlhammer H., Schiffer M., Epp O., Colman P. M., Lattman E. E., Schwager P., Steigemann W., Schramm H. J. The structure determination of the variable portion of the Bence-Jones protein Au. Biophys Struct Mech. 1975 Feb 19;1(2):139–146. doi: 10.1007/BF00539775. [DOI] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- Labaw L. W., Davies D. R. An electron microscopic study of human gamma Gl immunoglobulin crystals. Preliminary results. J Biol Chem. 1971 Jun 10;246(11):3760–3762. [PubMed] [Google Scholar]
- Labaw L. W., Davies D. R. The molecular outline of human gamma G1 immunoglobulin from an EM study of crystals. J Ultrastruct Res. 1972 Aug;40(3):349–365. doi: 10.1016/s0022-5320(72)90106-2. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R. Variability of three-dimensional structure in immunoglobulins. Proc Natl Acad Sci U S A. 1975 Mar;72(3):819–823. doi: 10.1073/pnas.72.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W., Colman P. M. Letter: Preliminary x-ray data from well-ordered crystals of a human immunoglobulin G molecule. J Mol Biol. 1974 Feb 5;82(4):587–588. doi: 10.1016/0022-2836(74)90250-2. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Phizackerley R. P. Studies on the three-dimensional structure of immunoglobulins. Prog Biophys Mol Biol. 1976;31(1):67–93. doi: 10.1016/0079-6107(78)90005-6. [DOI] [PubMed] [Google Scholar]
- Sarma R., Zaloga G. Structure studies on styrene-treated immunoglobulin crystals. J Mol Biol. 1975 Nov 5;98(3):479–484. doi: 10.1016/s0022-2836(75)80081-7. [DOI] [PubMed] [Google Scholar]
- Sarma V. R., Davies D. R., Labaw L. W., Silverton E. W., Terry W. D. Crystal structure of an immunoglobulin molecule by x-ray diffraction and electron microscopy. Cold Spring Harb Symp Quant Biol. 1972;36:413–419. doi: 10.1101/sqb.1972.036.01.053. [DOI] [PubMed] [Google Scholar]
- Sarma V. R., Silverton E. W., Davies D. R., Terry W. D. The three-dimensional structure at 6 A resolution of a human gamma Gl immunoglobulin molecule. J Biol Chem. 1971 Jun 10;246(11):3753–3759. [PubMed] [Google Scholar]
- Schiffer M., Girling R. L., Ely K. R., Edmundson A. B. Structure of a lambda-type Bence-Jones protein at 3.5-A resolution. Biochemistry. 1973 Nov 6;12(23):4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry W. D., Matthews B. W., Davies D. R. Crystallographic studies of a human immunoglobulin. Nature. 1968 Oct 19;220(5164):239–241. doi: 10.1038/220239a0. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Green N. M. Electron microscopy of an antibody-hapten complex. J Mol Biol. 1967 Aug 14;27(3):615–617. doi: 10.1016/0022-2836(67)90063-0. [DOI] [PubMed] [Google Scholar]
- Yguerabide J., Epstein H. F., Stryer L. Segmental flexibility in an antibody molecule. J Mol Biol. 1970 Aug;51(3):573–590. doi: 10.1016/0022-2836(70)90009-4. [DOI] [PubMed] [Google Scholar]