Abstract
The dynamics of leaf nitrogen (N) and phosphorus (P) have been intensively explored in short-term experiments, but rarely at longer timescales. Here, we investigated leaf N : P stoichiometry over a 27-year interval in an Inner Mongolia grassland by comparing leaf N : P concentration of 2006 with that of 1979. Across 80 species, both leaf N and P increased, but the increase in leaf N lagged behind that of leaf P, leading to a significant decrease in the N : P ratio. These changes in leaf N : P stoichiometry varied among functional groups. For leaf N, grasses increased, woody species tended to increase, whereas forbs showed no change. Unlike leaf N, leaf P of grasses and forbs increased, whereas woody species showed no change. Such changes may reflect N deposition and P release induced by soil acidification over the past decades. The interannual effect of precipitation may somewhat have reduced the soil available N, leading to the more modest increase of leaf N than of leaf P. Thus, leaf N : P stoichiometry significantly responded to long-term environmental changes in this temperate steppe, but different functional groups responded differently. Our results indicate that conclusions of plant stoichiometry under short-term N fertilization should be treated with caution when extrapolating to longer timescales.
Keywords: N : P stoichiometry, N deposition, soil acidification, Chinese grassland
1. Introduction
The status and dynamics of leaf nitrogen (N) and phosphorus (P) are closely linked to many aspects of biological processes, from cellular physiology to ecosystem productivity [1]. Therefore, leaf N : P stoichiometry has been studied intensively to understand the relationships between plants and the external environment [2]. Many studies have shown that both leaf N and P often respond to short-term environmental changes. However, there are few data on how leaf N and P respond to long-term environmental changes [3]. It is important to fill this knowledge gap to better understand ecosystem functions and how plants respond to environmental changes.
The semi-arid Inner Mongolia grassland, which is an important component of the Eurasian grassland biome, is predicted to be sensitive to environmental changes [4], such as increased temperature, decreased precipitation, accelerated N deposition and soil acidification [5,6]. Given the long-term continuous scientific survey of this region, this grassland biome is ideal for investigating the stoichiometric responses of leaf N and P to these long-term environmental changes. Here, we compared recently measured leaf N and P concentrations with published data from 1979 [7] to investigate plant stoichiometric responses to long-term environmental changes and their underlying mechanisms.
2. Material and methods
(a). Study area and field sampling
This study was conducted in the Xilin River Basin (43.43–44.48° N, 115.53–117.20° E), Inner Mongolia of northern China. This area has a semi-arid continental temperate steppe climate. The mean annual temperature is 2.4°C and mean annual precipitation is 278 mm (1953–2011). The dominant soil types are Chernozem and Kastanozems [7].
Samples were collected during the peak growing season (late July) in 2006. The sampling sites were chosen based on the survey in 1979 and were subjected to minimal grazing and anthropogenic disturbances. Eighty species from 28 families including three functional groups were measured in 1979 [7] and resampled in this study. Thirty-one species, referred to as ‘matched species’ hereafter, were collected from the same sites as in 1979. The remaining species were sampled from similar plant communities nearby, because they were not found at the same places as in 1979 (electronic supplementary material, table S1). For each species, leaves from five to 10 individuals were collected randomly following a protocol detailed previously [8]. Soil property measurements were conducted during a soil investigation campaign in 2007 (electronic supplementary material, table S2).
(b). Leaf chemical analysis
Leaf N concentrations of 2006 were determined by combustion method using an elemental analyser (2400 II CHNS/O Elemental Analyzer, Perkin-Elmer, Boston, MA, USA). Since leaf N concentrations in 1979 were determined by the Kjeldahl N determination method, leaf N concentrations of 11 randomly selected species were measured simultaneously by both the Kjeldahl and combustion methods (electronic supplementary material, figure S1). Regression between leaf N measured by the two methods showed a ratio of 1.115, which is similar to many previous studies [9]. Thus, leaf N concentrations of 1979 were transformed using this ratio to eliminate methodological bias. Leaf P concentrations were measured in both years by the molybdate/stannous chloride method after H2SO4-H2O2-HF digestion [8].
(c). Statistical analysis
We performed linear mixed-effects model analysis using year and functional group as fixed factors and using site and species as random factors. We performed this analysis both for the 80 species overall and 31 matched species. For each functional group, we performed linear mixed-effects model analysis using year as the fixed factor and using site and species as random factors to examine the difference of leaf N : P stoichiometry between 1979 and 2006. All statistical analyses were performed in R v. 3.1.1.
3. Results
Compared with 1979, leaf N of 2006 was 5.0% (relative percentage change, hereafter) and 8.9% higher for overall and matched species, respectively (tables 1 and 2, and figure 1a). Compared with 1979, grass leaf N of 2006 was 38.3% and 26.7% higher, and woody species leaf N was 8.4% and 15.3% higher for overall and matched species, respectively, whereas forbs showed no significant change (figure 2a).
Table 1.
Results of linear mixed-effects models on the effects of year (Y), functional group (FG) and their interaction on leaf N, P and N : P ratio. Italicized values indicate significance at p < 0.05.
| leaf N |
leaf P |
N : P ratio |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| numDF | denDF | F | p | F | p | F | p | ||
| overall (n = 80) | Y | 1 | 77 | 2.85 | 0.096 | 15.78 | <0.001 | 14.89 | <0.001 |
| FG | 2 | 68 | 7.42 | 0.001 | 16.59 | <0.001 | 14.02 | <0.001 | |
| Y × FG | 2 | 77 | 6.29 | 0.003 | 1.31 | 0.276 | 1.26 | 0.289 | |
| grass (n = 13) | Y | 1 | 12 | 28.15 | <0.001 | 29.60 | <0.001 | 3.73 | 0.078 |
| forb (n = 48) | Y | 1 | 47 | 0.19 | 0.664 | 5.63 | 0.022 | 10.22 | 0.003 |
| woody (n = 19) | Y | 1 | 18 | 3.52 | 0.077 | 2.40 | 0.139 | 1.04 | 0.321 |
| matched species (n = 31) | Y | 1 | 28 | 4.27 | 0.048 | 15.61 | <0.001 | 14.8 | <0.001 |
| FG | 2 | 19 | 3.33 | 0.058 | 9.08 | 0.002 | 8.35 | 0.003 | |
| Y × FG | 2 | 28 | 1.67 | 0.206 | 0.88 | 0.427 | 2.7 | 0.084 | |
| grass (n = 6) | Y | 1 | 5 | 21.70 | 0.006 | 5.06 | 0.074 | 2.12 | 0.205 |
| forb (n = 15) | Y | 1 | 14 | 0.02 | 0.896 | 9.44 | 0.008 | 19.10 | <0.001 |
| woody (n = 10) | Y | 1 | 9 | 8.54 | 0.017 | 2.42 | 0.154 | 0.13 | 0.723 |
Table 2.
Leaf N, P and N : P ratios for the overall species, matched species and different functional groups in 1979 and 2006.
| leaf N (mg g−1) |
leaf P (mg g−1) |
N : P ratio |
||||
|---|---|---|---|---|---|---|
| 1979 | 2006 | 1979 | 2006 | 1979 | 2006 | |
| overall (n = 80) | ||||||
| mean | 26.44 | 27.77 | 1.50 | 1.74 | 19.28 | 16.63 |
| median | 24.91 | 26.48 | 1.42 | 1.72 | 18.06 | 16.72 |
| CV | 0.35 | 0.26 | 0.41 | 0.26 | 0.37 | 0.27 |
| grass (n = 13) | ||||||
| mean | 18.94 | 26.20 | 0.84 | 1.29 | 25.00 | 20.56 |
| median | 18.97 | 25.81 | 0.78 | 1.31 | 22.14 | 21.72 |
| CV | 0.13 | 0.17 | 0.34 | 0.19 | 0.36 | 0.16 |
| forb (n = 48) | ||||||
| mean | 29.66 | 29.14 | 1.57 | 1.78 | 19.69 | 16.87 |
| median | 27.81 | 27.65 | 1.48 | 1.76 | 19.44 | 17.14 |
| CV | 0.34 | 0.28 | 0.34 | 0.26 | 0.31 | 0.25 |
| woody (n = 19) | ||||||
| mean | 23.41 | 25.37 | 1.77 | 1.94 | 14.35 | 13.36 |
| median | 23.75 | 25.50 | 1.63 | 1.94 | 14.38 | 13.52 |
| CV | 0.21 | 0.23 | 0.36 | 0.19 | 0.31 | 0.26 |
| matched species | ||||||
| mean | 25.15 | 27.39 | 1.39 | 1.74 | 19.83 | 16.36 |
| median | 24.16 | 26.18 | 1.34 | 1.79 | 19.83 | 15.66 |
| CV | 0.35 | 0.28 | 0.43 | 0.26 | 0.35 | 0.28 |
| grass (n = 6) | ||||||
| mean | 18.97 | 24.03 | 0.83 | 1.16 | 25.65 | 21.09 |
| median | 18.61 | 23.67 | 0.76 | 1.09 | 24.30 | 22.00 |
| CV | 0.16 | 0.11 | 0.43 | 0.20 | 0.37 | 0.14 |
| forb (n = 15) | ||||||
| mean | 29.04 | 29.31 | 1.40 | 1.87 | 21.07 | 16.03 |
| median | 27.68 | 27.58 | 1.30 | 1.90 | 20.76 | 15.19 |
| CV | 0.36 | 0.33 | 0.40 | 0.24 | 0.21 | 0.29 |
| woody (n = 10) | ||||||
| mean | 23.01 | 26.52 | 1.71 | 1.90 | 14.46 | 14.02 |
| median | 24.35 | 26.77 | 1.71 | 1.85 | 14.91 | 14.95 |
| CV | 0.25 | 0.24 | 0.32 | 0.14 | 0.35 | 0.24 |
Figure 1.
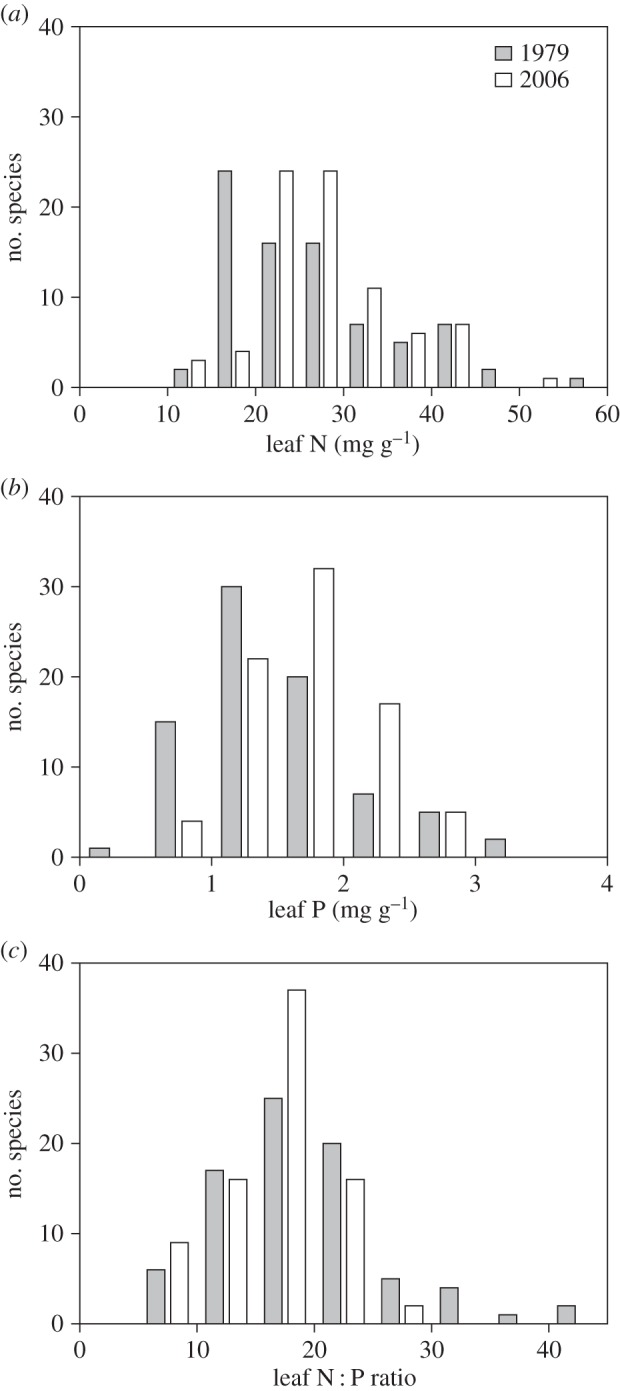
Frequency histograms of (a) leaf N, (b) leaf P and (c) leaf N : P ratios for overall 80 species of 1979 (grey bars) and 2006 (white bars).
Figure 2.
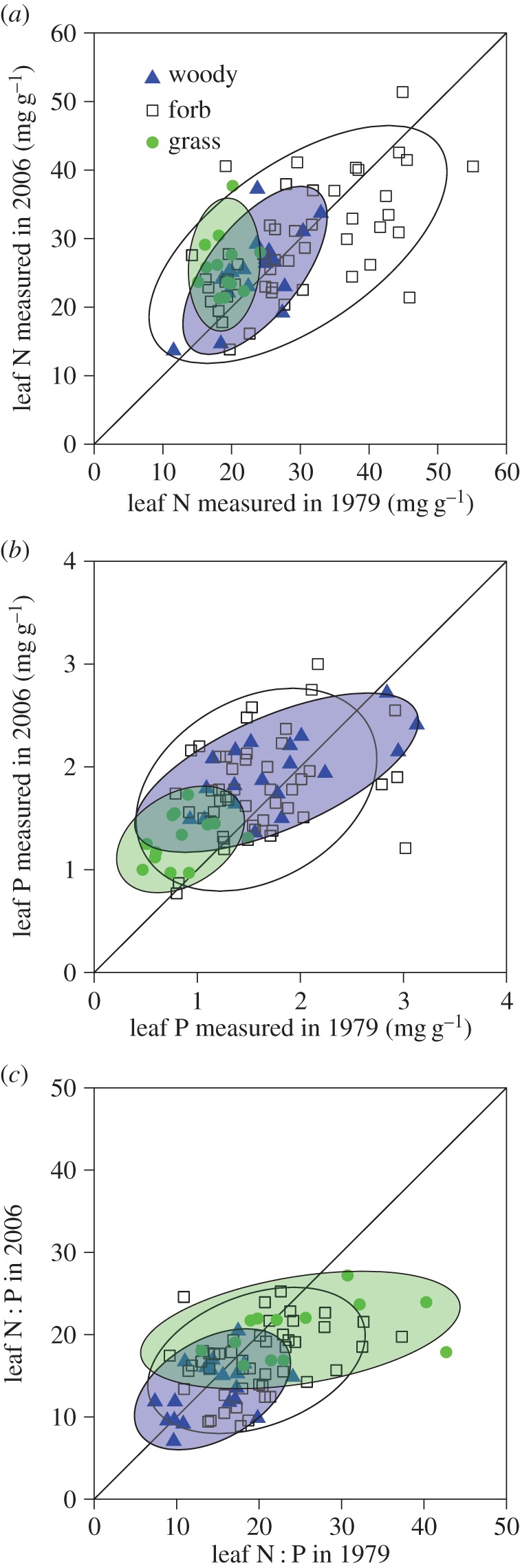
Relationships between leaf N and P stoichiometry of the 80 species of 1979 and 2006. Each circle includes 90% of the data for each functional group. The 1 : 1 line is shown. (Online version in colour.)
Leaf P of 2006 was 16.0% and 25.2% higher than that of 1979 for overall and matched species, respectively (tables 1 and 2, and figure 1b). Compared with 1979, grass leaf P of 2006 was 53.6% and 39.8% higher, and forbs leaf P of 2006 was 13.4% and 33.6% higher for overall and matched species, respectively, whereas woody species showed no significant change (figure 2b).
Compared with 1979, leaf N : P ratios were 13.7% and 17.5% lower for overall and matched species, respectively (tables 1 and 2, and figure 1c). Among the three functional groups, only forbs showed a significant decrease in leaf N : P ratio (figure 2c).
4. Discussion
This study provides the first evidence of the stoichiometric changes of leaf N and P over a 27-year interval in the Inner Mongolia grassland, which could be representative for semi-arid temperate grasslands.
It is likely that the changes in leaf stoichiometry over the 27-year interval were mainly induced by the alteration in soil nutrient availability. There are two possible processes influencing soil N availability. On one hand, accelerated N deposition at a rate of 10–15 kg ha−1 a−1 in the Inner Mongolia Steppe tends to increase N availability [5]. On the other hand, the below-average precipitation of 2006 may decrease soil N availability compared with 1979, in which the above-average precipitation may increase soil N availability in the study area (electronic supplementary material, figure S2) [10]. The increased N deposition may overwhelm the reduction due to the lower precipitation and result in an increase in soil N availability, leading to a higher leaf N in 2006 than in 1979.
Soil P availability is mainly controlled by the chemical balance between insoluble and soluble forms of phosphate, which is largely influenced by soil pH [11]. During the past decades, China's grasslands have suffered severe acidification with soil pH decreasing by 0.67 units in the typical steppe, driven largely by the significant atmospheric N and sulfur deposition [6]. Therefore, the acidification process would release phosphate radicals, increasing soil P availability, thus enhancing the plant phosphate uptake and leaf P concentration [12]. The below-average precipitation of 2006 may have increased soil P availability relative to 1979, in which the above-average precipitation may not influence soil P availability [13]. The relatively lower increase of leaf N than of P resulted in a lower leaf N : P ratio of 2006 than of 1979. The lower N : P ratio of 2006 may reflect the alleviation of P limitation of this region [14].
The changes in leaf stoichiometry varied among functional groups, reflecting the varying abilities of species to take advantage of the changes in available nutrients in particular traits related to the ability of plant nutrient uptake and resorption. For instance, herbaceous plants (grasses and forbs) have greater specific root length [11] and higher nutrient resorption [15] than woody plants. Previous studies found that N addition benefits grasses at the expense of forbs [16], indicating a competitive advantage of N for grasses to some extent. This competitive advantage and the greater mobility of soil inorganic available N than of P [11] may explain the significant increase of leaf N in grasses but relatively stability in forbs, while both grasses and forbs showed a significant increase in leaf P. Therefore, only forbs showed a significant decrease in leaf N : P ratio. Our results diverge somewhat from those of short-term manipulative experiments [16,17]. The results of short-term experiments may be due to the same or larger total amount of N added manually once or several times per year which may generate pulse effects, compared with N deposition in long-term natural conditions. Therefore, different functional groups respond in divergent ways to these changes, which may be attributed to the various inherent nutrient acquisition abilities of individual species [18–20].
Our study suggests that environmental changes will result in significant changes in plant stoichiometry. Different functional groups showed differential responses to long-term environmental changes, reflecting their different nutrient acquisition strategies [21]. The different plant N : P stoichiometry between our study and artificial N addition experiments indicates that conclusions drawn from studies of short-term N fertilization should be treated with caution when extrapolating to longer timescales.
Supplementary Material
Acknowledgements
We thank Cunzhu Liang for plant species identification in the field, Yuxi Cheng and Bing Xu for field sampling assistance, Jacob Weiner for language editing and two anonymous reviewers for valuable comments.
Data accessibility
All stoichiometric data used for analysis are available in the electronic supplementary material.
Authors contributions
Z.M., Y.H., H.G. and D.F. carried out the data analysis and drafted the manuscript; H.G. and W.Z. collected field data; J-S.H. designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Funding statement
This research was supported by the National Basic Research Program of China (grant no. 2014CB954004) and the National Natural Science Foundation of China (grant nos. 31025005 and 31321061).
Conflict of interests
We have no competing interests.
References
- 1.Elser JJ, et al. 2000. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550. ( 10.1046/j.1461-0248.2000.00185.x) [DOI] [Google Scholar]
- 2.Güsewell S. 2004. N : P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266. ( 10.1111/j.1469-8137.2004.01192.x) [DOI] [PubMed] [Google Scholar]
- 3.Sardans J, Rivas-Ubach A, Peñuelas J. 2012. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111, 1–39. ( 10.1007/s10533-011-9640-9) [DOI] [Google Scholar]
- 4.Christensen L, Coughenour MB, Ellis JE, Chen ZZ. 2004. Vulnerability of the Asian typical steppe to grazing and climate change. Clim. Change 63, 351–368. ( 10.1023/B:Clim.0000018513.60904.Fe) [DOI] [Google Scholar]
- 5.Jia YL, Yu GR, He NP, Zhan XY, Fang HJ, Sheng WP, Zuo Y, Zhang DY, Wang QF. 2014. Spatial and decadal variations in inorganic nitrogen wet deposition in China induced by human activity. Sci. Rep. 4, 3763 ( 10.1038/srep03763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YH, Ji CJ, Ma WH, Wang SF, Wang SP, Han WX, Mohammat A, Robinson D, Smith P. 2012. Significant soil acidification across northern China's grasslands during 1980s–2000s. Glob. Change Biol. 18, 2292–2300. ( 10.1111/j.1365-2486.2012.02694.x) [DOI] [Google Scholar]
- 7.Chen ZZ, Huang DH, Zhang HF. 1985. The feature of chemical elements of 122 plant species in Xilin River Basin, Inner Mongolia, China. In Research on grassland ecosystem (ed. Inner Mongolia Grassland Ecosystem Research Station, Chinese Academy of Sciences), pp. 112–131. Beijing, China: Science Press. [Google Scholar]
- 8.He JS, Wang L, Flynn DF, Wang XP, Ma WH, Fang JY. 2008. Leaf nitrogen : phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155, 301–310. ( 10.1007/s00442-007-0912-y) [DOI] [PubMed] [Google Scholar]
- 9.Dhaliwal GS, Gupta N, Kukal SS, Meetpal-Singh 2014. Standardization of automated Vario EL III CHNS analyzer for total carbon and nitrogen determination in plants. Commun. Soil Sci. Plant Anal. 45, 1316–1324. ( 10.1080/00103624.2013.875197) [DOI] [Google Scholar]
- 10.Wang CH, Wan SQ, Xing XR, Zhang L, Han XG. 2006. Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biol. Biochem. 38, 1101–1110. ( 10.1016/j.soilbio.2005.09.009) [DOI] [Google Scholar]
- 11.Chapin FS, III, Chapin MC, Matson PA, Vitousek P. 2011. Principles of terrestrial ecosystem ecology, pp. 241–290, 2nd edn New York, NY: Springer. [Google Scholar]
- 12.Chen DM, Lan ZC, Bai X, Grace JB, Bai YF. 2013. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 101, 1322–1334. ( 10.1111/1365-2745.12119) [DOI] [Google Scholar]
- 13.Lü XT, Kong DL, Pan QM, Simmons M, Han XG. 2012. Nitrogen and water availability interact to affect leaf stoichiometry in a semi-arid grassland. Oecologia 168, 301–310. ( 10.1007/s00442-011-2097-7) [DOI] [PubMed] [Google Scholar]
- 14.Koerselman W, Meuleman AFM. 1996. The vegetation N : P ratio: a new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 33, 1441–1450. ( 10.2307/2404783) [DOI] [Google Scholar]
- 15.Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. 2012. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 82, 205–220. ( 10.1890/11-0416.1) [DOI] [Google Scholar]
- 16.Song L, Bao X, Liu X, Zhang Y, Christie P, Fangmeier A, Zhang F. 2011. Nitrogen enrichment enhances the dominance of grasses over forbs in a temperate steppe ecosystem. Biogeosciences 8, 2341–2350. ( 10.5194/bg-8-2341-2011) [DOI] [Google Scholar]
- 17.Drenovsky RE, Richards JH. 2004. Critical N : P values: predicting nutrient deficiencies in desert shrublands. Plant Soil 259, 59–69. ( 10.1023/B:PLSO.0000020945.09809.3d) [DOI] [Google Scholar]
- 18.Bai YF, Wu JG, Clark CM, Naeem S, Pan QM, Huang JH, Zhang LX, Han XG. 2010. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from Inner Mongolia grasslands. Glob. Change Biol. 16, 358–372. ( 10.1111/j.1365-2486.2009.01950.x) [DOI] [Google Scholar]
- 19.Ronnenberg K, Wesche K. 2011. Effects of fertilization and irrigation on productivity, plant nutrient contents and soil nutrients in southern Mongolia. Plant Soil 340, 239–251. ( 10.1007/s11104-010-0409-z) [DOI] [Google Scholar]
- 20.Kinugasa T, Tsunekawa A, Shinoda M. 2012. Increasing nitrogen deposition enhances post-drought recovery of grassland productivity in the Mongolian steppe. Oecologia 170, 857–865. ( 10.1007/s00442-012-2354-4) [DOI] [PubMed] [Google Scholar]
- 21.Yu Q, Chen QS, Elser JJ, He NP, Wu HH, Zhang GM, Wu JG, Bai YF, Han XG. 2010. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 13, 1390–1399. ( 10.1111/j.1461-0248.2010.01532.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All stoichiometric data used for analysis are available in the electronic supplementary material.


