Abstract
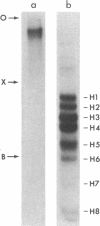
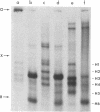
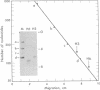
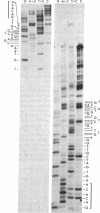
The complete sequences of the untranslated 5' regions of human alpha- and beta-globin mRNAs were determined by sequence analysis of full-length cDNAs. The single-stranded cDNAs were digested with the restriction endonuclease Hae III, and the two 3'-terminal fragments of 75 and 132 nucleotides, complementary to the 5' termini of the alpha- and beta-globin mRNAs, respectively, were isolated and sequenced. Including the initiation codon AUG, the untranslated 5' regions of human alpha- and beta-globin mRNAs contain 41 and 54 nucleotides, respectively, and exhibit striking homologies with the corresponding sequences in the rabbit. Human alpha- and beta-globin mRNAs have five bases in the region of the initiation codon that may form base pairs with the 3' terminus of 18S rRNA. Stable secondary structures with hairpin loops can be constructed in the untranslated 5' regions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baralle F. E. Complete nucleotide sequence of the 5' noncoding region of rabbit beta-globin mRNA. Cell. 1977 Apr;10(4):549–558. doi: 10.1016/0092-8674(77)90088-5. [DOI] [PubMed] [Google Scholar]
- Baralle F. E. Structure-function relationship of 5' non-coding sequence of rabbit alpha- and beta-globin mRNA. Nature. 1977 May 19;267(5608):279–281. doi: 10.1038/267279a0. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Browne J. K., Paddock G. V., Liu A., Clarke P., Heindell H. C., Salser W. Nucleotide sequences from the rabbit beta globin gene inserted into Escherichia coli plasmids. Science. 1977 Jan 28;195(4276):389–391. doi: 10.1126/science.318762. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal M., Forget B. G., Prensky W., Marotta C. A., Weissman S. M. Human beta-globin messenger RNA. II. nucleotide sequences derived from 125I-labeled globin messenger RNA. J Biol Chem. 1977 Jul 25;252(14):5032–5039. [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Marotta C. A., Weissman S. M., Verma I. M., McCaffrey R. P., Baltimore D. Nucleotide sequences of human globin messenger RNA. Ann N Y Acad Sci. 1974 Nov 29;241(0):290–309. doi: 10.1111/j.1749-6632.1974.tb21888.x. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Paddock G. V., Wall R., Salser W. A general method for cloning eukaryotic structural gene sequences. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3146–3150. doi: 10.1073/pnas.73.9.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Site-specific cleavage of single-stranded DNA by a Hemophilus restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2555–2558. doi: 10.1073/pnas.72.7.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M., Varmus H. E., Taylor J. M., Holland J. P., Lie-Injo L. E., Ganesan J., Todd D. Deletion of alpha-globin genes in haemoglobin-H disease demonstrates multiple alpha-globin structural loci. Nature. 1975 May 15;255(5505):255–256. doi: 10.1038/255255a0. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Varmus H. E. Demonstration of non-functional beta-globin mRNA in homozygous beta (0) thalassemia. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5140–5144. doi: 10.1073/pnas.72.12.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S. Characterization of the ribosome-protected regions of 125I-labelled rabbit globin messenger RNA. J Mol Biol. 1976 Sep 5;106(1):37–53. doi: 10.1016/0022-2836(76)90299-0. [DOI] [PubMed] [Google Scholar]
- Legon S., Robertson H. D. The binding of 125I-labelled rabbit globin messenger RNA to reticulocyte ribosomes. J Mol Biol. 1976 Sep 5;106(1):23–36. doi: 10.1016/0022-2836(76)90298-9. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Paddock G. V., Heindell H. C., Salser W. Nucleotide sequences from a rabbit alpha globin gene inserted in a chimeric plasmid. Science. 1977 Apr 8;196(4286):192–195. doi: 10.1126/science.847466. [DOI] [PubMed] [Google Scholar]
- Lockhard R. E., Rajbhandary U. L. Nucleotide sequences at the 5'termini of rabbit alpha and beta globin mRNA. Cell. 1976 Dec;9(4 Pt 2):747–760. doi: 10.1016/0092-8674(76)90138-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Forget B. G., Cohne-Solal M., Wilson J. T., Weissman S. M. Human beta-globin messenger RNA. I. Nucleotide sequences derived from complementary RNA. J Biol Chem. 1977 Jul 25;252(14):5019–5031. [PubMed] [Google Scholar]
- Marotta C. A., Wilson J. T., Forget B. G., Weissman S. M. Human beta-globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. J Biol Chem. 1977 Jul 25;252(14):5040–5053. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R., Paddock G. V., Heindell H., Whitcome P., Salser W., Kacian D., Bank A., Gambino R., Ramirez F. Nucleotide sequence analysis of RNA synthesized from rabbit globin complementary DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3502–3506. doi: 10.1073/pnas.71.9.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Longley J. I. The 3' terminal sequences of human alpha and beta globin messenger RNAs: comparison with rabbit globin messenger RNA. Cell. 1976 Dec;9(4 Pt 2):733–746. doi: 10.1016/0092-8674(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Sequence analysis of the 3' non-coding regions of rabbit alpha- and beta-globin messenger RNAs. J Mol Biol. 1976 Nov 15;107(4):491–525. doi: 10.1016/s0022-2836(76)80080-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Wahba A. J., Laughrea M., Moore P. B. Differential requirements for polypeptide chain initiation complex formation at the three bacteriophage R17 initiator regions. Nucleic Acids Res. 1977 Jan;4(1):1–15. doi: 10.1093/nar/4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]
- Wilson J. T., Forget B. G., Wilson L. B., Weissman S. M. Human globin messenger RNA: importance of cloning for structural analysis. Science. 1977 Apr 8;196(4286):200–202. doi: 10.1126/science.847468. [DOI] [PubMed] [Google Scholar]