Abstract
Background
A review of the effectiveness and outcomes in liver abscess drainage performed by different operators using percutaneous aspiration (PA) and catheter drainage (PCD), respectively, from 2008–2013 at Sir Charles Gairdner Hospital, a tertiary hospital in Australia.
Methods
Forty-two patients (29 males and 13 females; aged between 28–93 years; median age of 67 years) with liver abscesses underwent either ultrasound or CT-guided PA (n=22) and PCD (n=20) in conjunction with appropriate antimicrobial therapy. A median of 18 Gauge needle and 10 French catheters were utilised.
Results
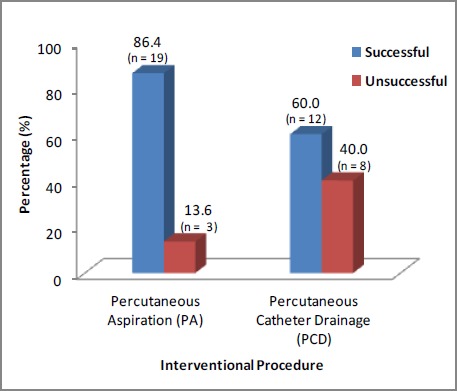
Nineteen (86.4 per cent) PA cases and 12 (60 per cent) PCD cases were successfully drained on a single attempt (p=0.08). More male patients (69 per cent) than females (31 per cent) were observed. Portal sepsis (42.9 per cent) was the most common cause identified. Fever (47.6 per cent) was the most frequent clinical presentation on admission. Thirty-two patients (76.2 per cent) had solitary abscesses with a right lobe (59.5 per cent) predilection. CRP was significantly raised. The PCD group observed a significantly larger abscess size (p=0.01). Klebsiella pneumoniae was the most common organism isolated in both pus (33.3 per cent) and blood cultures (11.9 per cent). Five procedure-related complications were noted, all in the PCD group. Thirty-day mortality was 2.4 per cent. No difference was observed in clinical and treatment outcomes in both groups.
Conclusion
The null hypothesis that both PA and PCD are equally effective in the drainage of liver abscess cannot be rejected. Apart from PA being simpler and safer to perform, the higher incidence of indwelling catheter-associated complications suggests that a trial of PA should always be attempted first.
Keywords: Aspiration, catheter drainage, interventional radiology, liver abscess
What this study adds:
-
What is known about this subject?
Liver abscess is a surgical dilemma with substantial morbidity and mortality. The treatment modalities include the use of potent broad-spectrum antibiotics in conjunction with minimally invasive percutaneous drainage under imaging guidance or surgical drainage.
-
What new information is offered in this study?
There is an increasing change from benign biliary pathologies to more malignant causes of liver abscess. The emergence of Klebsiella pneumoniae replacing Escherichia coli as an important pathogen is now being recognised with virulent strains reported worldwide. Dissimilarities and inconsistencies between blood and pus cultures reflecting the possible causative liver abscess microbe were noted. The management of six prospective studies was also reviewed.
-
What are the implications for research, policy, or practice?
Percutaneous intervention, either aspiration (PA) or catheter drainage (PCD), has become the preferred first therapeutic choice for liver abscess drainage. However, to date, which should be the first-line treatment remains debatable.
Background
Liver abscess is a rare but potentially fatal disease.1–8 Historically, liver abscess has been managed exclusively by surgery.2,9–11 Advances in imaging technology have advocated the shift to minimally invasive interventional procedures.1,8,10–13 The advent and delivery of potent antimicrobials and improved ICU care have also improved patient outcomes.2,4,14,15 Surgery is now reserved for selected cases.1,2,12,16–19 Despite these advances, mortality rates remain high.1,5,9,11–13,17 The percutaneous intervention can either be a percutaneous aspiration (PA) or a percutaneous catheter drainage (PCD), but to date which should be the first-line treatment remains debatable.
Subjects and methods
Clinical data was retrieved from medical records, the hospital computer system, and the Picture Archiving and Communication System (PACS) for patients who underwent percutaneous aspiration and/or catheter drainage with the Interventional Radiology team at our tertiary medical centre from 2008–2013.
Forty-two patients underwent a total of 57 procedures. Out of 22 PA patients, 19 had a single (n=19) successful aspirate (nine out of 10 cases as successful needle aspiration and 10 out of 12 cases as successful catheter aspiration). In the unsuccessful arm, two patients had two (n=4) procedures and one had three (n=3) procedures done, respectively.
PCD was performed in 20 patients. In the successful arm, nine patients had a single (n=9) drainage, two patients had two abscesses drained separately on the same setting on a single attempt each (n=4), and one had three drainage procedures for three different abscesses (n=3) on a single attempt each. In the unsuccessful arm, one patient had a single drainage (n=1), while seven others had two (n=14) procedures done.
A procedure was considered successful if there was no change in the initial procedure from aspiration to drainage or vice-versa, or subsequent surgery and successful drainage of the abscess allowing for clinical discharge. Procedural-related complications were noted independently but were not considered as a reason for failed treatment as the known risks were discussed with the patient prior to the procedure.
Intervention
Written consent, coagulation profile assessment, and coagulopathy correction were performed prior to the procedure. Ultrasonographic (US) guidance used the Phillips iU22 system with 3.5- or 5-MHz convex transducers, while CT guidance was carried out with the Phillips Brilliance 64 row detector CT unit. Lignocaine 1 per cent was the choice for local anaesthesia and conscious sedation with Fentanyl and Midazolam was sometimes used.
Aspiration
PA was performed using either needle (size range 17–21 Gauge, median 18 Gauge) or pig-tail catheters (size range 6– 10 French, median 10 French). Once the needle tip or catheter is within the abscess cavity, it is aspirated until no more pus can be aspirated. This is followed by needle or catheter removal.
Drainage
PCD was performed using self-locking pig-tail catheters (size range 6–14 French, median 10 French). Under image guidance, the catheter was placed within the abscess cavity using the modified Seldingers technique. Aspiration of the abscess material was then performed until no more pus could be aspirated. The catheter was secured to the skin for continuous external drainage. Follow-up imaging was performed only in patients who were not improving clinically. The attending physician made the decision for drain removal. Dislodged catheters were either repositioned or removed with subsequent PA being performed. Procedure details including technique, and number and size of needle and catheter were recorded. Patients who subsequently required PA, PCD, or surgery after the initial procedure were also documented.
Statistical analysis
Quantitative variables (patient demographics, laboratory findings, abscess characteristics, and length of stay) were analysed using Welch t-test for normally distributed data or a non-parametric test (Kruskal-Wallis rank sum test). Test of normality was performed using Shapriro-Wilk test. Categorical variables (clinical features, mortality, treatment success, and complications) were analysed using the Fisher’s exact test. The level of significance was set at p value two-sided test <0.05. All statistical tests were done using the open source R language. Furthermore, the aetiologies of the abscesses were not uniform and formed a heterogeneous group. In addition, the type of interventional procedure performed was governed simply by operator preference and was certainly not random.
Results
Patients demographics
Forty-two patients aged between 28–93 years with a median age of 67 years underwent interventional procedures. There were more males (69 per cent) than females (31 per cent). There was no significant difference noted (Table 1) in both the age and sex when both groups were compared.
Table 1: Patient demographics.
| Parameters | PA (n=22) | PCD (n=20) | P value (NS: Not significant) |
|---|---|---|---|
| 1. Age (years) | 28–83 | 35–93 | * NS; † NS |
| Mean +/- SD | 66.3 ± 13.3 | 63.2 ± 17.6 | |
| Median | 65.5 | 68.5 | |
| 2. Sex | 15:7 | 14:6 | ‡NS |
| (Male:Female) |
* Shapiro-Wilk normality test
† Welch t-test
‡ Fisher’s Exact Test
Aetiology
Table 2 shows the frequency of aetiologies in our series. Portal sepsis is the most common aetiology noted in our series with diabetics prevalently susceptible. Interestingly, five parasitic infections with three amoebiasis (Entamoeba histolytica serology Indirect Hemagglutinin serology of 128, 1024 and >4096, respectively), one Strongyloides infection (Strogyloides IgE Enzyme Immunoassay 0.73), and one Fasciola hepatica infection (Fasciola antibody IgG ratio > 2.5 with evidence of hepatic trematode eggs noted in the biopsied sample) were observed. All five were treated successfully. All three amoebiasis patients were Caucasians, while the Strongyloides infection involved an Asian. The Caucasian patient with the Fasciola hepatica infection had recently travelled to Southeast Asia in the last three months prior to presentation. The aetiology findings of both groups when compared were statistically not significant.
Table 2: Aetiology of liver abscess.
| Aetiology | Total, n (%) |
|---|---|
| 1. Portal sepsis | 18 (42.9) |
| (a) Pancreatitis | 3 (7.1) |
| (b) Hepatitis | 3 (7.1) |
| (c) Peptic ulcer/gall bladder/small bowel perforation | 7 (16.7) |
| (d) Diverticular disease | 2 (4.8) |
| (e) Active Crohns disease (on steroids) | 2 (4.8) |
| (f) Endometritis | 1 (2.4) |
| 2. Diabetes mellitus | 15 (35.7) |
| 3. Underlying malignancy | 10 (23.8) |
| (a) Pancreatic carcinoma | 4 (9.5) |
| (b) Colorectal carcinoma with liver metastasis | 4 (9.5) |
| (c) Hepatocellular carcinoma | 1 (2.4) |
| (d) Malignant liver sarcoma | 1 (2.4) |
| 4.Septicaemia/Haematogenous spread | 8 (19.0) |
| 5. Biliary pathology | 6 (14.3) |
| (a) Cholecystitis | 3 (7.1) |
| (b) Cholelithiasis | 2 (4.8) |
| (c) Cholangitis | 1 (2.4) |
| 6. Recent abdominal surgery/ Instrumentation | 6 (14.3) |
| 7. Cryptogenic | 6 (14.3) |
| 8. Parasitic | 5 (11.9) |
Clinical features
Fever (47.6 per cent), abdominal pain (40.0 per cent), and septic shock (28.6 per cent) were the three main clinical presentations documented at admission. However, there was no significant difference observed between both groups when compared Table 3).
Table 3: Clinical presentations at admission.
| Clinical features | Fisher Exact test p value | 95% confidence interval | Odds ratio |
|---|---|---|---|
| Fever | 0.54 | 0.44–7.14 | 1.74 |
| Abdominal pain | 0.74 | 0.32–6.58 | 1.42 |
| Sepsis / Shock | 0.32 | 0.08–2.12 | 0.45 |
| Lethargy | 0.48 | 0.37–11.05 | 1.9 |
| Chills/Rigors | 0.45 | 0.34–15.56 | 2.07 |
| Loss of weight | 0.67 | 0.04–4.07 | 0.51 |
| Diarrhoea | 0.66 | 0.18–23.156 | 1.74 |
| Nausea/Vomiting | 0.60 | 0.11–143.97 | 2.29 |
| Confusion | 1.00 | 0.01–90.77 | 1.1 |
Laboratory results
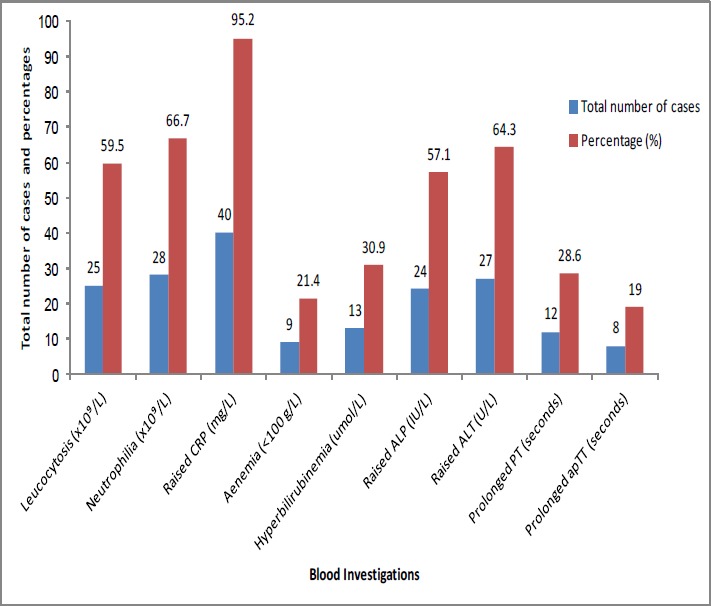
Apart from the significantly raised C-reactive protein (CRP) with a mean of 199.5mg/L, no difference was noted in the other blood parameters between both groups (Figure 1). Despite 14 anaemic patients, only 9 (21.4 per cent) had a haemoglobin less than 100g/L.
Figure 1. Laboratory results.
Imaging
A total of 48 ultrasound and nine CT-guided procedures were carried out.
Abscess characteristics
Abscess characteristics are detailed in Table 4.
Table 4: Abscess characteristics.
| Characteristics | PA, n=22 | PCD, n=20 | Total, n (%) | P value (NS: Not significant) |
|---|---|---|---|---|
| Number | *NS | |||
| Single | 16 | 16 | 32 (76.2) | |
| Multiple | 6 | 4 | 10 (23.8) | |
| Site | * NS | |||
| Right lobe | 15 | 10 | 25 (59.5) | |
| Left lobe | 1 | 6 | 7 (16.7) | |
| Bi-lobar | 6 | 4 | 10 (23.8) | |
| Size | †<0.001 | |||
| Diameter of abscess (mm) | ‡0.012 | |||
| Mean | 53.7 | 72.4 | – | |
| ± SD | ± 20.1 | ± 36.3 | ||
| Median | 50 | 64.5 | – |
* Fisher’s Exact Test
† Shapiro-Wilk normality test
‡ Kruskal-Wallis rank sum test
Microbiological Data
Out of the 16 patients who had both positive blood and pus cultures (Table 5), seven (43.75 per cent) positive blood cultures had completely different microbes isolated from the pus specimen, while another three (18.75 per cent) had additional organisms cultured. The most common first-line antimicrobial therapies commenced were intravenous tazobactem plus pipercillin in combination with metronidazole. Data regarding detailed antimicrobial therapy were not available.
Table 5: Microbiological data.
| Characteristics | PA, n=22 | PCD, n=20 | Total, n (%) |
|---|---|---|---|
| Growth culture | |||
| Total number of: | |||
| 1. Positive pus culture | 18 | 15 | 33 (78.6) |
| 2. Monomicrobial culture | 13 | 10 | 23 (54.8) |
| 3. Polymicrobial culture | 5 | 5 | 10 (23.8) |
| 4. Positive blood culture | 9 | 7 | 16 (38.1) |
| 5. Positive pus and blood culture | 9 | 7 | 16 (38.1) |
| Organism cultured (pus) | |||
| 1. Klebsiella pneumonia | 10 | 4 | 14 (33.3) |
| 2. Streptococcus anginosus group | 10 (23.8) | ||
| – Strep. anginosus | 2 | 4 | 6 |
| – Strep. intermedius | 1 | 2 | 3 |
| – Strep. constellatus | 1 | 0 | 1 |
| 3. Escherichia coli | 3 | 3 | 6 (14.3) |
| 4. Enterobacter faecium | 2 | 3 | 5 (11.9) |
| 5. Entamoeba histolytica | 1 | 2 | 3 (7.1) |
| 6. Candida species | 0 | 3 | 3 (7.1) |
| 7. Klebsiella oxytoca | 0 | 2 | 2 (4.8) |
| 8. Pseudomonas aeruginosa | 0 | 2 | 2 (4.8) |
| 9. Aeromonas species | 1 | 0 | 1 (2.4) |
| 10. Bacteroides ovatus | 1 | 0 | 1 (2.4) |
| 11. Enterobacter cloacae | 0 | 1 | 1 (2.4) |
| 12. Fasciola hepatica | 0 | 1 | 1 (2.4) |
| 13. Kluyvera species | 1 | 0 | 1 (2.4) |
| 14. Lactococcus lactis | 1 | 0 | 1 (2.4) |
| 15. Mixed anaerobic bacteria | 0 | 1 | 1 (2.4) |
| 16. Nocardia farcinica | 1 | 0 | 1 (2.4) |
| 17. Serratia marcescens | 1 | 0 | 1 (2.4) |
| 18. Streptococcus sanguinis | 1 | 0 | 1 (2.4) |
| 19. Strongyloides species | 0 | 1 | 1 (2.4) |
| 20. No growth | 4 | 5 | 9 (21.4) |
| Organism cultured (blood) | 3 | ||
| 1. Klebsiella pneumonia | 2 | 5 (11.9) | |
| 2. Streptococcus anginosus group | 4 (9.5) | ||
| – Strep. anginosus | 2 | 0 | 2 |
| – Strep. intermedius | 0 | 2 | 2 |
| 3. Escherichia coli | 1 | 1 | 2 (4.8) |
| 4. Staphylococcus species | 1 | 1 | 2 (4.8) |
| 5. Enterobacter faecium | 0 | 1 | 1 (2.4) |
| 6. Bacteroides fragillis | 1 | 0 | 1 (2.4) |
| 7. Candida species | 1 | 0 | 1 (2.4) |
| 8. Streptococcus sanguinis | 1 | 0 | 1 (2.4) |
| 9. No growth | 12 | 10 | 22 (52.4) |
| 10. Not done | 1 | 3 | 4 (9.5) |
Clinical and treatment outcomes
The clinical summary is detailed in Table 6.
Table 6: Clinical and treatment outcome.
| Parameters | PA (n=22) | PCD (n=20) | P value (NS: Not significant N/A: Not applicable) |
|---|---|---|---|
| 1. Length of hospital stay (days) | *0.001; †NS | ||
| – Mean ± SD | 32.8 ± 22.1 | 26.2 ± 19.3 | |
| – Median | 28.5 | 23.5 | |
| 2. Duration of catheter drain in-situ (days) | |||
| – Mean ± SD | N/A | 14.4 ± 17.6 | N/A |
| – Median | N/A | 6 | N/A |
| 3. No. of procedure related complications | 0 | 5 | ‡0.02 |
| 4. No. of patients requiring subsequent surgery | 1 | 2 | ‡NS |
| 5. No. of patient death | 5 | 5 | ‡NS |
| 6. No. of patients with successful treatment (Figure 3) | 19 | 12 | ‡NS |
| – needle aspiration | 9 / 10 | N/A | |
| – catheter | 10 / 12 | N/A | |
| 7. Unsuccessful Cases | |||
| Procedure | |||
| First | Second | Third | Reason for initial treatment failure |
| PA | PCD | – | Abscess remains unchanged |
| PA | PCD | PCD | Abscess remains unchanged, PCD re-inserted due to catheter dislodgement |
| PA | Surgery | – | Emergency cholecystectomy and surgical evacuation of the re-accumulated pus |
| PCD | PA | – | Catheter dislodgements (n=2) |
| PCD | PA | – | No clinical or radiological improvement (n=3) |
| PCD | Surgery | – | Non-resolving complex abscess |
| PCD | Surgery | – | Surgical resection of a liver mass, radiologically seen to be an abscess initially |
| PCD | – | – | Inpatient death secondary to severe sepsis |
* Shapiro-Wilk normality test
† Kruskal-Wallis rank sum test
‡ Fisher’s Exact Test
Complications
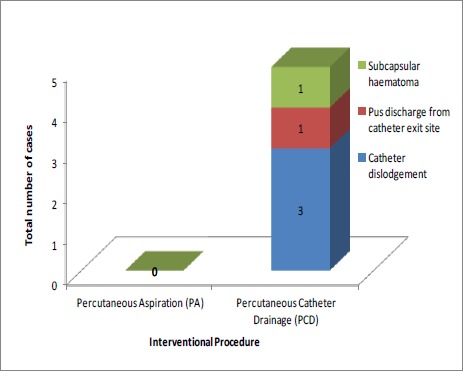
Five procedure-related complications (Figure 2) were observed, all five in the PCD group. These were three catheter drain dislodgements, one subcapsular haematoma, and one pus discharge from the catheter exit site. All three dislodged catheters were self-locking catheters.
Figure 2. Procedural-related complications.
Mortality
There were 10 (23.8 per cent) mortalities with two (4.8 per cent) inpatient deaths (Table 7). Thirty-day mortality was recorded at 2.4 per cent (one case). None of these mortalities were related to the procedures.Figure 3
Table 7: Patient death characteristics.
| Patient age and sex | Underlying pathology | Abscess site | Interventional procedure | Cause and time of death (time of death after liver abscess drainage) |
|---|---|---|---|---|
| 75 / M | Pancreatic cancer | Right lobe | US PA | Sepsis post tumour resection (15 months) |
| 62 / F | Type 2 Diabetes | Right lobe | US PA | Myocardial infarction (24 months) |
| 78 / F | Metastatic colon carcinoma | Right lobe | US PA | Metastatic disease (Six months) |
| 56 / F | Metastatic colon carcinoma | Right lobe | US PA | Pulmonary embolism (48 days) |
| 43 / M | Type 2 Diabetes | Right lobe | US PCD | Sepsis (Three days) |
| 78 / F | Peptic ulcer | Left lobe | US PCD | Unknown (Three months) |
| 71 / M | Pancreatic carcinoma | Right lobe | US PCD | Metastatic disease (23 months) |
| 61 / M | Pancreatic colon carcinoma | Right lobe | US PCD | Sepsis post tumour resection (Two months) |
| 64 / F | Pancreatic carcinoma | Right lobe | US PA (had PCD subsequently) | Underlying malignancy (10 months) |
| 71 / M | Nil | Right lobe | US PCD (had surgery subsequently) | Malignant liver sarcoma (Three months) |
Figure 3. Outcome of interventional procedure.
Discussion
Consistent with previous reports, our series saw a large elderly population.1,3,5–9,11,13,16–18,20–24 The peak average age has increased markedly.9,13 A slight male predominance was also observed.3,5–7,9,11,14,15,17–25 Portal sepsis has been recognised as a common aetiology in the past.11 Other reports have shown that biliary pathology is increasing in prevalence.1,3,4,6,7,9,11,13,16,17,19–21,23,26 Some authors have classified both benign and malignant biliary pathologies together to show this prevalence.4,6,7,13 Conversely, we classified them separately to illustrate an increasing change from benign biliary pathologies to more malignant causes.1,22,26 Similarly, reports have shown that liver metastasis has resulted in the rise of liver abscess incidences from one per cent to seven per cent.1 Due to poor prognosis and patient outcomes, other authors have excluded underlying malignancies from their series.2,5,15,16,23
We observed diabetes mellitus as an equally important aetiology with some authors describing it as the most common co-existing disease in their series.2,4,7,11,20,22,27 Several reports also have found that majority of the liver abscesses were cryptogenic.5,7,9,11,19,24,25,27 The classical triad of fever, right upper-quadrant pain, and jaundice is often described in literature but is rarely seen clinically2,16,27 as the symptomatology is often non-specific.19,20,24,25,27 As a result, a high index of suspicion is often required for early diagnosis of liver abscess.24,25 Apart from reflecting an acute inflammatory response and liver anomalies, laboratory findings are non-specific to diagnose liver abscess.24,27 Reports have shown raised C-reactive protein (CRP)3,20,24,25 and leucocytosis9,11,20,21,23 as common laboratory findings, but nearly all were statistically insignificant.
Our findings are in agreement with the suggestion that in view of the non-specific symptomology, diabetics with fever and raised CRP, without a potential focus of infection should undergo abdominal imaging to rule out intra-abdominal infections due to the large blood flow to the right lobe15,17,25 and the fact that the majority of the hepatic volume is in the right lobe.15 The association of liver abscess size and treatment outcomes has been discussed with some authors suggesting that the initial abscess size does not affect the eventual outcome,10,16 while others found large abscesses more difficult to drain completely on a single attempt.14
We did not study the characteristics of abscess septations or loculations, which may have influenced the choice of drainage method employed by each operator as not all the performed imaging’s reported on the same.
The emergence of Klebsiella pneumoniae replacing Escherichia coli as an important pathogen is now being recognised with virulent strains reported worldwide. 1,7,9,13,15,20,21,28,29 The exact cause for the change in bacteriology is not known, but it was suggested that the increased use of indwelling biliary stents and broadspectrum antibiotics in the management hepatobiliary and pancreatic neoplasms could be a possible cause.1 We found 12 monomicrobial and two polymicrobial isolates of Klebsiella pneumoniae with eight out of the 14 (57 per cent) isolates from diabetic patients.28–30 Eleven of the isolates were obtained from solitary abscesses. This association has been reported previously.28–29
The ethnic breakdown in our series observed nine Caucasians, three Asians, one Aboriginal, and one African patient each who positively cultured for Klebsiella pneumoniae. Klebsiella pneumoniae has also been recognised for its extrahepatic manifestations, including the dreaded invasive liver abscess syndrome,30 which was seen in two of our patients.31 One manifested with cerebritis, endophthalmitis, pneumonia, liver, and calf abscesses occurring on a background of undiagnosed type 2 diabetes mellitus who had a stormy hospital admission and lost vision in the right eye, but was eventually treated successfully, while the other died within three days of admission following severe septicaemia secondary to the liver abscess, pneumonia, and meningitis. We found dissimilarities and inconsistencies between blood and pus cultures reflecting the possible causative liver abscess microbe as reported previously.9 In some series the majority of abscess cultures were sterile.12,14,15,25
The management of six prospective studies were reviewed (Table 8). Success rates have been reported between 90– 100 per cent for PA10,12,16,25 and between 69–100 per cent for PCD.1,11,13–15,18,21,23 Some of these findings are from retrospective series and may be subject to selection bias. Average catheter drain remained in-situ is reported between five to eight days.26,27 Previous reports have described minor complications particularly involving in-situ catheter drainage.4,7,12–16,23,24 Regardless, we have to acknowledge that this is an important concern often requiring further treatment. Generally, mortality rates are recorded between 1.5–31 per cent1,3,5,9,11–13,17,19–22,24,26 with our series observing an overall mortality of 23.8 per cent due to various underlying pathologies and a 30-day mortality of 2.4 per cent as a direct result of the liver abscess complications. Contrasting reports on the duration of hospital stay was observed2,10,12–15,23,25 suggesting that there is no particular procedure superior to the other in terms of recovery and hospitalisation.
Table 8: Summary of six prospective studies reviewed.
| Author/ Study type | Sample size | Success rates | Findings | Complications | Limitations |
|---|---|---|---|---|---|
| Rajak et al. / Randomised | 50 |
PA:
15 of 25 patients (60%) PCD: All 25 patients (100%) |
Average time needed for a 50 per cent reduction in the abscess cavity size
was significantly greater in the aspiration group. The average time for total resolution of abscess, clinical improvement, and the mean hospital stay were similar in the two treatment groups. Needle aspiration, if limited to two attempts, has a high failure rate. Repeated aspirations could improve success rates, but would be a traumatic experience for the patient. This, too, did not guarantee a cure. Hence, percutaneous catheter drainage is more effective than needle aspiration in the treatment of liver abscesses. |
PA:
Haemorrhage within the abscess cavity (n=1) PCD: Severe pain at the catheter entry site (n=1) Pericatheter leak (n=1) Pericatheter leak (n=1) |
The data did not allow the authors to predict the type of abscesses likely to respond to needle aspiration alone. |
| Yu et al. / Prospective randomised trial | 64 |
PA:
30 of 32 patients (94%) PCD: 27 of 32 patients (84%) |
Although not achieving statistical significance, all three outcome measures
of this study; hospital stay duration, treatment success rate, and mortality
rate, favoured the intermittent needle aspiration group. PNA was as effective as PCD. PNA should be considered as first-line management as the technique is easier, simpler, less time consuming, and cheaper. The current study and authors’ previous work have shown no significant increase in morbidity or mortality from the repeat aspiration sessions. |
PA:
Nil PCD: Sepsis (n=1) |
Limitations of sample size in this study. |
| Zerem et al. / Randomised | 60 |
PA:
20 of 30 patients (67%) PCD: All 30 patients (100%) |
Continuous PCD is more efficient than intermittent PNA. PCD is more
efficient for multiloculated liver abscesses . Percutaneous needle aspiration can be used as a valid alternative for simple abscesses ≤5cm in diameter or smaller. Hospital stay did not differ significantly between the groups. |
Nil | Exclusion of patients with coexisting malignant biliary disease, which is a poor prognostic factor and the leading cause of death among patients with pyogenic liver abscess. |
| Singh S et al. / Prospective randomied comparative study | 60 |
PA:
23 of 30 patients (77%) PCD: All 30 patients (100%) |
PCD was a better modality than PNA when draining large abscesses, which are partially liquefied or with thick pus. Earlier clinical improvement and less time for 50 per cent reduction in abscess cavity in the percutaneous catheter drainage group. No significant difference in time required for abscess resolution or duration of hospital stay was noted between both groups. Each repeated aspiration improved the success of treatment by percutaneous needle aspiration. The chances of failure of percutaneous needle aspiration increased with the increase in size of abscess cavity to be aspirated. |
Nil | Exclusion of all abscess cavities smaller than 5cm in their greatest dimension, prior interventions and
concomitant biliary tract malignancies. Aetiology of abscesses was not uniform and formed a heterogeneous group with abscesses of both amoebic and pyogenic aetiology existing in both groups. |
| Deenari et al. / Prospective analytic | 40 |
PA:
Not assessed PCD: All 40 patients (100%) |
PCD should be used in the management of pyogenic liver abscesses >5cm with effective outcome in terms of success, hospital stay, mortality, and morbidity when compared to the surgical group. |
PA:
Nil PCD: Catheter blockage (n=1) Dislodged catheterq (n=2) Sepsis (n=1) |
The patients with abscess size ≤5cm and those who responded to medical therapy were excluded. |
| Singh O et al. / Randomised | 72 |
PA:
31 of 36 (86%) patients PCD: 35 of 36 patients (97%) |
PCD was a better option when managing large liver abscesses typically those ≥10cm in terms of duration to attain clinical relief and intravenous antibiotic treatment. Duration of hospital stay was similar in the two groups. Multiple attempts of PNA needed for large abscesses may be uncomfortable and perceived as more traumatic by patients. |
PA:
Subcapsular hematoma (n=1) PCD: Bile leakage (n=1) |
Patients with liver abscess <10cm, coexisting malignant biliary disease were excluded. |
Conclusion
In conclusion, our findings could not reject the null hypothesis that both PA and PCD are equally effective in the drainage of liver abscess.23 The question is which modality should be the first-line treatment and to date that still remains debatable. Our retrospective review supports that PA should always be attempted first in the management of liver abscess due to its simplicity, effectiveness, and fewer procedural-related complications.12,13,16,19 Multiple abscess cavities can also be aspirated in the same setting.10,16,19 However, when draining abscesses larger than 5cm, PCD is deemed more effective15,18,23 taking into account that the mortality and morbidity rates are equal or less than the surgical group.7,11,18,19 Finally, surgery is recognised as the treatment of choice for liver abscess drainage with concurrent intra-abdominal pathology, multiloculated abscess with biliary communication and failure of PCD.4,19,22 In view that the choice of therapy relies heavily on abscess factors,6,8 routine reporting of abscess characteristics, including loculations and septations should be emphasised. Research into predictors of mortality and treatment failure is timely.3,5,11,21
In summary:
PA and PCD are equally effective in liver abscess drainage.
PA should always be attempted first in the management of liver abscess due to its simplicity, effectiveness, and fewer procedural-related complications.
Operator choice of therapy relies heavily on abscess factors, thus routine reporting of abscess characteristics, including loculations and septations should be emphasised.
Repeated aspirations improved the success of treatment, and if done correctly, no significant increase in morbidity or mortality from the repeat aspiration sessions as previously reported.
No difference in time required for abscess resolution or duration of hospital stay was noted between both groups.
ACKNOWLEDGEMENTS
The authors would like to express their appreciation and gratitude to Cao Nguyen, senior data management officer, Health System Improvement Unit, and Fiona Bowden, data analyst, Clinical Improvement and Innovations Unit, Sir Charles Gairdner Hospital, Department of Health, WA for the data and statistical analysis review.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
ETHICS COMMITTEE APPROVAL
This retrospective research has been approved and registered with a Quality Improvement Activity (QIA) number of 4490 by the Governance Knowledge Evidence Outcomes (GEKO) ethics committee, Sir Charles Gairdner Hospital, Western Australia.
Please cite this paper as: Dulku G, Mohan G, Samuelson S, Ferguson J, Tibballs J. Percutaneous aspiration versus catheter drainage of liver abscess: A retrospective review. AMJ 2015;8(1):7-18.http://dx.doi.org/10.4066/AMJ.2015.2240
References
- 1.Huang CJ, Pitt HA, Lipsett PA, Osterman Jr FA, Lillemoe KD, Cameron JL, Zuidema GD. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600–9. doi: 10.1097/00000658-199605000-00016. PMCID: PMC1235191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. AJR. Am J Roentgenol. 2007;3:W138–2. doi: 10.2214/AJR.07.2173. doi:10.2214/ajr.07.2173. [DOI] [PubMed] [Google Scholar]
- 3.Alkofer B, Dufay C, Parienti JJ, Lepennec V, Dargere S, Chiche L. Are pyogenic liver abscesses still a surgical concern? A Western experience. HPB Surgery. doi: 10.1155/2012/316013. 2012:316013. doi:10.1155/2012/316013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CH, Gervais DA, Hahn PF, Arellano RS, Uppot RN, Mueller PR. Percutaneous hepatic abscess drainage: do multiple abscesses or multiloculated abscesses preclude drainage or affect outcome? Vasc Interv Radiol. 2009;8:1059–65. doi: 10.1016/j.jvir.2009.04.062. doi: 10.1016/j.jvir.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 5.Ng WC, Li WH, Cheung MT. Audit of management of pyogenic liver abscess in a tertiary referral hospital. Surgical Practice. 2008;1:7–10. doi:10.1111/j.1744- 1633.2007.00385.x. [Google Scholar]
- 6.Petri A, Hohn J, Hodi Z, Wolfard A, Balogh A. Pyogenic liver – 20 years' experience. Comparison of results of treatment in two periods. Langenbecks Arch Surg. 2002;1:27–31. doi: 10.1007/s00423-002-0279-9. doi:10.1007/s00423-002-0279-9. [DOI] [PubMed] [Google Scholar]
- 7.Tan YM, Chung YFA, Chow KHP, Cheow PC, Wong WK, Ooi LL. et al. An Appraisal of Surgical and Percutaneous Drainage for Pyogenic Liver Abscesses Larger Than 5 cm. Ann Surg. 2005;3:485–90. doi: 10.1097/01.sla.0000154265.14006.47. doi:10.1097/01.sla.0000154265.14006.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung YFA, Tan YM, Lui HF, Tay KH, Lo RHG, Kurup A. et al. Management of pyogenic liver abscesses– percutaneous or open drainage? Singapore Med J. 2007;12:1158–65. 18043848. [PubMed] [Google Scholar]
- 9.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;11:1654–9. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 10.Baek SY, Lee MG, Cho KS, Lee SC, Sung KB, Auh YH. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. AJR. Am J Roentgenol. 1993;4:799–802. doi: 10.2214/ajr.160.4.8456667. doi: 10.2214/ajr.160.4.8456667. [DOI] [PubMed] [Google Scholar]
- 11.Chu KM, Fan ST, Lai EC, Lo CM, Wong J. Pyogenic liver abscess. An audit of experience over the past decade. Arch Surg. 1996;2:148–52. doi: 10.1001/archsurg.1996.01430140038009. [DOI] [PubMed] [Google Scholar]
- 12.Yu CHS, Ho SMS, Lau WY, Yeung TKD, Yuen HYE, Lee SFP. et al. Treatment of pyogenic liver abscess: prospective randomised comparison of catheter drainage and needle aspiration. Hepatology. 2004;4:932–8. doi: 10.1002/hep.20133. doi:10.1002/hep.20133. [DOI] [PubMed] [Google Scholar]
- 13.Bertel CK, van Heerden JA, Sheedy PF. II. Treatment of pyogenic hepatic abscesses. Surgical vs percutaneous drainage. Arch Surg. 1986;5:554–8. doi: 10.1001/archsurg.1986.01400050072009. PMID: 3707333. [DOI] [PubMed] [Google Scholar]
- 14.Rajak CL, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR. Am J Roentgenol. 1998;4:1035–9. doi: 10.2214/ajr.170.4.9530055. doi:10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Chaudhary P, Saxena N, Khandelwal S, Poddar DD, Biswal UC. Treatment of liver abscess: prospective randomised comparison of catheter drainage and needle aspiration. Ann Gastroenterol. 2013;4:332–9. PMCID: PMC3959473. [PMC free article] [PubMed] [Google Scholar]
- 16.Giorgio A, de Stefano G, Di Sarno A, Liorre G, Ferraioli G. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. AJR. Am J Roentgenol. 2006;6:1585–90. doi: 10.2214/AJR.05.1104. doi:10.2214/ajr.05.1104. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez JA, Gonzalez JJ, Baldonedo RF, Sanz L, Carreno G, Jorge JI. Single and multiple pyogenic liver abscesses: etiology, clinical course, and outcome. Dig Surg. 2001;4:283–8. doi: 10.1159/000050153. doi:10.1159/000050153. [DOI] [PubMed] [Google Scholar]
- 18.Deenari RA, Jalbani MH, Abro MA, Shaikh SM, Bhatti Y, Baloch I. et al. Percutaneous Catheter Drainage Under Ultrasound Guide For Pyogenic Liver Abscess Larger Than 05cm: Experience at Chandka Medical College Hospital Larkana. Medical Channel. 2010;1:75–7. [Google Scholar]
- 19.Barakate MS, Stephen MS, Waugh RC, Gallagher PJ, Solomon MJ, Storey DW, Sheldon DM. Pyogenic liver abscess: A review of 10 years' experience in management. ANZ J Surg. 1999;3:20–9. doi: 10.1046/j.1440-1622.1999.01523.x. [DOI] [PubMed] [Google Scholar]
- 20.Chan KS, Chen CM, Cheng KC, Hou CC, Lin HJ, Yu WL. Pyogenic liver abscess: a retrospective analysis of 107 patients during a 3-year period. Jpn J Infect Dis. 2005;6:366–8. PMID: 16377869. [PubMed] [Google Scholar]
- 21.Chou FF, Sheen-Chen SM, Chen YS, Chen MC. Single and multiple pyogenic liver abscesses: clinical course, etiology, and results of treatment. World J Surg. 1997;4:384–9. doi: 10.1007/pl00012258. doi: 10.1007/PL00012258. [DOI] [PubMed] [Google Scholar]
- 22.Ferraioli G, Garlaschelli A, Zanaboni D, Gulizia R, Brunetti E, Tinozzi FP. et al. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liver Dis. 2008;8:690–6. doi: 10.1016/j.dld.2008.01.016. doi:10.1016/j.dld.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Singh O, Gupta S, Moses S, Jain DK. Comparative study of catheter drainage and needle aspiration in management of large liver abscesses. Indian J Gastroenterol. 2009;3:88–92. doi: 10.1007/s12664-009-0032-1. doi: 10.1007/s12664- 009-0032-1. [DOI] [PubMed] [Google Scholar]
- 24.Pang TC, Fung T, Samra J, Hugh TJ, Smith RC. Pyogenic liver abscess: an audit of 10 years' experience. World J Gastroenterol. 2011;12:1622–30. doi: 10.3748/wjg.v17.i12.1622. doi: 10.3748/wjg.v17.i12. 1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangukiya DO, Darshan JR, Kanani VK, Gupta ST. A prospective series case study of pyogenic liver abscess: recent trands in etiology and management. Indian J Surg. 2012;5:385–90. doi: 10.1007/s12262-011-0397-0. doi:10.1007/s12262-011-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyorffy EJ, Frey CF, Silva J Jr, McGahan J. Pyogenic liver abscess: Diagnostic and therapeutic strategies. Ann Surg. 1987;6:699–705. doi: 10.1097/00000658-198712000-00003. PMCID: PMC1493323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannsen EC, Sifri CD, Madoff LC. Pyogenic Liver Abscesses. Infect Dis Clin North Am. 2000;3:547–63. doi: 10.1016/s0891-5520(05)70120-3. PMID: 10987109. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Wang JY, Jiang W. An Increasing Prominent Disease of Liver Abscess: Etiology, Diagnosis, and Treatment. Gastroenterol Res Pract. doi: 10.1155/2013/258514. 2013:258514. doi:10.1155/2013/258514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu S. Klebsiella pneumoniae: An Emerging Pathogen of Pyogenic Liver Abscess. OMJ. 2009;2:131–3. doi: 10.5001/omj.2009.28. doi:10.5001/omj.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdul-Hamid A, Bailey SJ. Klebsiella pneumoniae liver abscess and endophthalmitis. BMJ Case Rep. 2013 Apr 3; doi: 10.1136/bcr-2013-008690. doi:10.1136/bcr-2013-008690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dulku G, Tibballs J. Cryptogenic Invasive Klebsiella pneumoniae liver abscess syndrome (CIKPLA) in Western Australia? Australas Med J. 2014;7(11):436–440. doi: 10.4066/AMJ.2014.2188. doi:10.4066/AMJ.2014.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]


