Abstract
Toxicity from accidental and intentional ingestion of yellow phosphorus, ubiquitously present in fireworks and rodenticides, has recently become more frequent. Gastrointestinal, renal, neurologic, and cardiovascular manifestations are common, with mortality of 23 per cent to 73 per cent. Reports of haematological abnormalities are rare. We report only the second case of severe neutropenia secondary to selective myelosuppression in a 14-year-old girl following intentional ingestion of yellow phosphorus. Leucocyte counts recovered spontaneously without further complications. Our case indicates that, besides hepatic and renal function monitoring, physicians should meticulously monitor blood counts in such cases for early detection of marrow suppression. Further studies are required to elucidate the complex mechanisms and significance of this unusual toxicity of yellow phosphorus.
Keywords: yellow phosphorus, myelosuppression, neutropenia
Implications for Practice:
-
What is known about this subject?
Yellow phosphorus toxicity results in gastrointestinal and renal dysfunction, with fulminant liver failure being the most dreaded manifestation.
-
What new information is offered in this study?
Yellow phosphorus poisoning may lead to severe neutropenia with sparing of other cell lines due to selective suppression of myeloid series in the bone marrow.
-
What are the implications for research, policy, or practice?
Apart from hepatic and renal functions, serial monitoring of blood counts may provide clues leading to early detection of myelosuppression from yellow phosphorus toxicity.
Background
Yellow phosphorus is available as a paste with concentrations varying from two to five per cent.1 In the past, the main application of this chemical was in firecrackers and military ammunition, however, it also has a role as an animal poison. Following reports of declining efficacy of warfarins as rodenticides,2 interest in yellow phosphorus as a rodenticide was re-ignited. This has paved the way for increasing numbers of intentional and accidental ingestions of this toxin in humans, resulting in high mortality and morbidity. The lethal dose has been calculated to be 1mg/kg body weight.3 We present a rare case, in which the ingestion of yellow phosphorus paste led to severe bone marrow toxicity selectively affecting the myeloid series and spontaneous remission over a one-week period.
Case details
A 14-year-old girl was brought to the emergency room by her parents 18 hours after intentional ingestion of approximately 5g of yellow phosphorus (three per cent). The compound was being used at home as a paste for killing rodents. She received gastric lavage from a nearby hospital before arriving at our hospital. Apart from occasional nausea, she was asymptomatic. She had no past personal or family history of psychiatric illness or attempts of deliberate self-harm.
On examination, she was conscious, oriented, and cooperative. Her pulse was 78 beats/minute and blood pressure was 110/70mmHg in the supine position. She was mildly dehydrated; there was no postural drop in blood pressure. She had neither pallor nor jaundice. There were no petechiae, purpurae, or ecchymosis. Signs of liver cell failure were absent. Systemic examination was otherwise unremarkable.
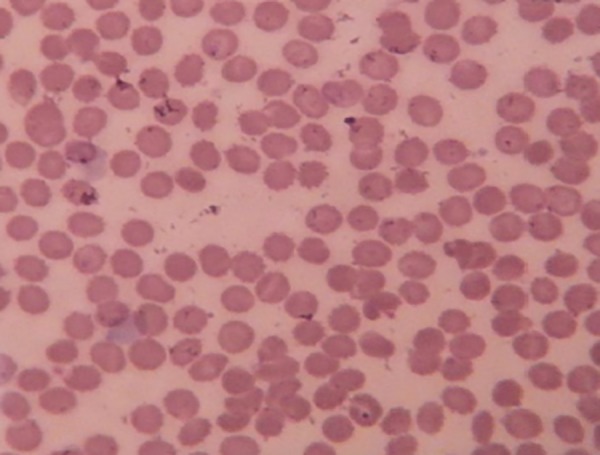
Baseline investigations revealed leucopenia [total count (TLC) 1.8x109/L; ref: 4–11x109/L)] with 11 per cent neutrophils [absolute neutrophils count (ANC); 0.198x109/L], 86 per cent lymphocytes, and three per cent monocytes. Haemoglobin (Hb) (114g/L, ref.; 120–140) and platelet counts (TPC) (190x109/L, ref.; 150–450) were within normal range. Liver function tests were within normal limits for the first two days. Prothrombin time and activated partial thromboplastin time were also normal. The leucocyte count dropped further on the next two days to 1.2x109 and 0.7x109/L, respectively (Table 1). Peripheral smear revealed normocytic, normochromic anaemia with marked leucopenia, neutropenia, and relative lymphocytosis but no abnormal cells (Figure 1).
Table 1: Serial haemoglobin and blood counts during hospitalisation.
| Day1 | Day 2 | Day 3 | Day 5 | Day6 | Day 7 | |
|---|---|---|---|---|---|---|
| Haemoglobin (g/L) [ref: 120–140] | 114 | 116 | 112 | 117 | 117 | 120 |
| Total WBC (cellsx109/L) [ref: 4–11] | 1.8 | 1.2 | 0.7 | 1.3 | 2.4 | 5.2 |
| Neutrophils (%) | 11 | 10 | 11 | 14 | 18 | 25 |
| Lymphocyte (%) | 86 | 87 | 82 | 79 | 76 | 70 |
| Monocytes (%) | 3 | 2 | 6 | 4 | 2 | 1 |
| Eosinophils (%) | 0 | 1 | 1 | 3 | 4 | 4 |
| Platelets (cells x109/L) [ref: 150–400] | 190 | 195 | 192 | 191 | 191 | 200 |
Figure 1. Peripheral smear.
Peripheral smear showing marked leucopenia with normal erythrocytes, platelets, and no abnormal cells (Leishman stain, 100X).
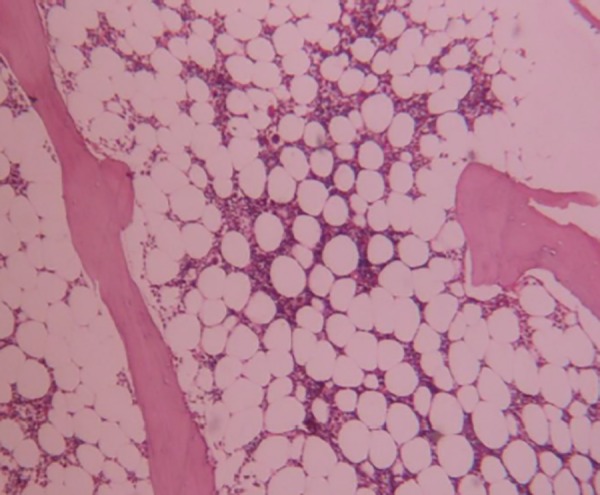
In view of the declining leucocyte count, bone marrow examination was performed, which showed markedly decreased cellularity for her age (average cellularity of 30 per cent) with focal interstitial oedema. There was marked suppression of myeloid series with preserved normoblastic erythroid maturation, normal megakaryocytes, lymphocytes, and plasma cells (Figure 2). There was no evidence of dyspoiesis, increased histiocytes (one per cent), malignancy, or granulomas. On the fourth day post-admission, her hepatic transaminases showed significant elevation (AST–360IU/L and ALT–138IU/L). Serum transaminases continued to rise over the next two days and then steadily fell to near normal levels in the subsequent three to four days. Renal functions remained normal throughout her hospital stay. Serological tests for HIV, Hepatitis B, Hepatitis C, and antinuclear antibodies (ANA) were negative.
Figure 2. Bone marrow biopsy.
Bone marrow biopsy showing marked hypocellularity and selective suppression of myeloid series with preserved erythroid and megakaryocyte lineages (Hematoxylin eosin 200X).
Meanwhile, the patient was managed with adequate hydration, proton pump inhibitors for gastritis, and neutropenic precautions. Over a one-week period, her complete blood count revealed Hb of 120g/L, TLC of 5.2x109/L with differential of neutrophils 25 per cent, lymphocytes 70 per cent, eosinophils 4 per cent, monocytes 1 per cent, ANC of 1.3x109/L; and TPC of 200x109/L (Table 1). She was discharged from the hospital after psychiatric counselling. The patient presented for follow-up after two weeks and was found to have normal haemoglobin (123g/L), leucocyte count (5.7x109/L), and platelet count (214x109/L). Her mood was euthymic and she is currently under follow-up with the psychiatrist.
Discussion
Yellow phosphorus, also known as white phosphorus, is a protoplasmic toxin that causes arrest of cellular replication in the metaphase stage of mitosis.4 The clinical features of yellow phosphorus toxicity are mostly related to the gastrointestinal system.3 The kidneys and the central nervous system may be affected to a lesser extent. Garlic odour, vomiting, mucosal erosions, and phosphorescent faeces are well-described features. Since more than 60 per cent of an ingested dose is concentrated in the liver after rapid absorption from the gastrointestinal tract,5 the liver is particularly vulnerable to damage. In fact, a systematic review on acute liver failure in Turkey reports that yellow phosphorus is the second most common cause for toxin-induced liver failure.6
Among the gastrointestinal manifestations, acute liver failure with coagulopathy is the most dreaded complication. When present alone, this complication may be managed by liver transplantation.7 However, it is often accompanied by acute tubular necrosis, hepato-renal syndrome, or cardiovascular complications, in which case it is almost always fatal. The course of events following yellow phosphorus poisoning is usually spread out over three stages.8 Gastrointestinal symptoms like nausea and vomiting predominate in the first stage in the absence of any laboratory abnormalities. After 24–48 hours, this is followed by a stage of hepatitis characterised by rising transaminases, although the patient may be asymptomatic. In a few cases, this progresses to the third stage of acute liver failure, which can be fatal. Variations in these stages have been reported, such as haematologic abnormalities in the second stage,9 and cholestatic presentation during the third stage.10
Our patient presented with selective myeloid series suppression leading to leucopenia after ingestion of yellow phosphorus. The transaminase elevation occurred after 48–72 hours, which followed the usual pattern of liver injury seen in yellow phosphorus toxicity. However, it did not progress to acute liver failure or coagulopathy. The leucopenia worsened over the first couple of days, only to steadily improve and become normal by the end of one week (ANC=1.3x109/L).
Haematological manifestations have not been described as a classical feature of yellow phosphorus toxicity.11 Taskesen reported low haemoglobin in a paediatric victim of yellow phosphorus toxicity.12 Only one case of neutropenia due to acute yellow phosphorus poisoning has been reported to date in the literature.9 Tafur et al. reported the case of a young girl who presented nine hours after ingestion of yellow phosphorus.9 Her leucocyte count was normal at baseline, but started declining after 24 hours with a nadir of 3.5x109/L. The counts improved spontaneously after 48 hours. In contrast, our patient presented with severe leucopenia within 24 hours of toxin ingestion. Her leucocyte count dropped to as low as 0.7x109/L with a nadir ANC of 0.098x109/L and returned to normal over a period of 5–7 days.
Comparison of bone marrow features of this and the previously reported cases9 reveals interesting observations. Interstitial oedema and haemorrhages in the marrow and the suppression of myeloid series were common to both cases. However, the severity of myelosuppression was more marked in the present case (30 per cent) compared to the previous one (50 per cent). The myelosuppression selectively involving the myeloid series and sparing other lineages as seen in our case, was not reported by Tafur et al.9 A more recent study from Ecuador reported that apart from vomiting, diarrhoea, and abdominal cramps, abnormal liver enzymes and coagulation times were the major clinical features of yellow phosphorus toxicity.13 However, no abnormalities of blood counts or bone marrow suppression were reported by the authors.
Though metaphase arrest of cells by yellow phosphorus might explain its effects on the leucocytes, it is not sufficient to unravel the pathogenesis of selective suppression of granulocytes, especially neutrophils. In fact, experiments in animals have shown that yellow phosphorus induces an increase in leucocyte count with destruction of erythrocytes9. Though studies on humans are scarce, one by Taussie et al. has reported a temporary fall in leucocyte count following yellow phosphorus toxicity.14
In general, drug-induced neutropenia results from either direct bone marrow suppression or immune-mediated destruction. The former mechanism operates in cases of drugs like chloramphenicol, whereas penicillins and cephalosporins produce neutropenia by the latter mechanism.15 Given the limited literature on yellow phosphorus-induced neutropenia, it is difficult to hypothesise which of these mechanisms were involved in our case. Considering the marked hypocellularity of marrow in our patient, direct suppression of myeloid precursors in the marrow seems more likely. Besides, the rarity of marrow involvement with concomitant relative sparing of liver in these two cases indicates that pharmacogenetic factors involved in metabolism of yellow phosphorus might be crucial in determining its toxicity profile. Studies on the role of HLA haplotypes in agranulocytosis caused by clozapine and methimazole further justify this possibility.16,17
Spontaneous recovery of granulocyte counts in both cases suggests that yellow phosphorus causes reversible direct marrow toxicity. Though our patient had severe neutropenia, she did not develop life-threatening infections that are likely to occur in neutropenic patients, especially following cancer chemotherapy. Mortality from yellow phosphorus toxicity ranges from 23 per cent among those presenting with gastrointestinal symptoms, to 73 per cent among those with neurological symptoms.8 Early attendance (within 12 hours) at a medical care facility may be associated with better prognosis.12 No deaths have been reported following haematologic abnormalities. The relatively mild hepatic involvement and preserved haemoglobin and platelets might have contributed to the good outcome in our patient.
Conclusion
Toxicity from intentional and accidental ingestion of yellow phosphorus is becoming increasingly common, as it is replacing superwarfarins as a freely available effective rodenticide. Though rare, physicians need to be aware of the hematologic complications of this toxin. Apart from liver functions, regular monitoring of complete blood counts during the first week after ingestion would help detect bone marrow toxicity and institute therapy. Effects of yellow phosphorus on the bone marrow seem complex and further research is needed to shed light on the mechanisms involved in selective myelosuppression observed in this case. Surveillance of blood counts and evaluation of bone marrow specimens from similar cases might contribute to the understanding of this uncommon toxicity.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
Dr Aneesh Basheer discloses that he is on the editorial board of the Australasian Medical Journal.
PATIENT CONSENT
The authors, Basheer A, Mookkappan S, Padhi S, and Iqbal N declare that:
- They have obtained written, informed consent for the publication of the details relating to the patient(s) in this report.
- All possible steps have been taken to safeguard the identity of the patient(s).
- This submission is compliant with the requirements of local research ethics committees.
Please cite this paper as: Basheer A, Mookkappan S, Padhi S, Iqbal N. Selective myelosuppression following yellow phosphorus ingestion. AMJ 2015;8(1):19–23. http//dx.doi.org/10.4066/AMJ.2014.2241
References
- 1.Brent J, Wallace KL, Burkhart KK. Phosphorus. Brent J, Wallace KL, Burkhart KK, Phillips SD, Donovan JW, editors. Critical Care Toxicology–Diagnosis and Management of the Critically Poisoned Patient. Philadelphia, PA: Elsevier Mosby. 2005:851–61. [Google Scholar]
- 2.Simon FA, Pickering LK. Acute yellow phosphorus poisoning. "Smoking stool syndrome.". JAMA. 1976;235:1343–4. [PubMed] [Google Scholar]
- 3.Fernandez OU, Fernandez LL. Acute hepatotoxicity from ingestion of yellow phosphorus-containing fireworks. J Clin Gastroenterol. 1995;21:139–42. doi: 10.1097/00004836-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Li MF, Traxler GS, Langille LM. Toxic effects of elemental phosphorus on L-cells cultivated suspension. Canad J Zoology. 1970;48:133–8. doi: 10.1139/z70-016. [DOI] [PubMed] [Google Scholar]
- 5.Ghoshal AK, Porta EA, Hartroft WS. Isotopic studies on the absorption and tissue distribution of white phosphorus in rats. Exp Mol Pathol. 1971;14:212–19. doi: 10.1016/0014-4800(71)90066-9. [DOI] [PubMed] [Google Scholar]
- 6.Cuneyt K, Veysel E, Sezai Y. Acute liver failure in Turkey: A systematic review. Turk J Gastroenterol. 2014;25:35–40. doi: 10.5152/tjg.2014.4231. [DOI] [PubMed] [Google Scholar]
- 7.Santos O, Restrepo JC, Velásquez L, Castaño J, Correa G, Sepúlveda E. et al. Acute liver failure due to white phosphorus ingestion. Ann Hepatol. 2009;8:162–5. [PubMed] [Google Scholar]
- 8.McCarron MM, Gaddis GP, Trotter AT. Acute yellow phosphorus poisoning from pesticide pastes. Clin Toxicol. 1981;18:693–712. doi: 10.3109/15563658108990295. [DOI] [PubMed] [Google Scholar]
- 9.Tafur AJ, Zapatier JA, Idrovo LA, Oliveros JW, Garces JC. Bone marrow toxicity after yellow phosphorus ingestion. Emerg Med J. 2004;21:259–60. doi: 10.1136/emj.2003.007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshmi CP, Goel A, Basu D. Cholestatic presentation of yellow phosphorus poisoning. J Pharmacol Pharmacother. 2014 Jan;5(1):67–9. doi: 10.4103/0976-500X.124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taskesen M, Adıguzel S. A rare cause of poisoning in childhood: yellow phosphorus. J Emerg Med. 2012 Aug;43(2):270–2. doi: 10.1016/j.jemermed.2011.05.083. [DOI] [PubMed] [Google Scholar]
- 12.Beloskurskaia GI, Balmakhaeva RM. Functional and morphologic characteristics of leukocytes in patients with chronic phosphorus poisoning. Gig Tr Prof Zabol. 1982;9:46–9. [PubMed] [Google Scholar]
- 13.González-Andrade F, López-Pulles R. White phosphorus poisoning by oral ingestion of firecrackers or little devils: current experience in Ecuador. Clin Toxicol (Phila). 2011 Jan;49(1):29–33. doi: 10.3109/15563650.2010.547860. doi: 10.3109/15563650.2010.547860. [DOI] [PubMed] [Google Scholar]
- 14.Taussie O. Blood count results in cases of acute phosphorus intoxication. Archives fur Experimentelle Pathologie und Pharmakologie. 1992;30:161–79. [Google Scholar]
- 15.Price MM, Dale DC. The selective neutropenias. Clin Haematol. 1978;7:501–21. [PubMed] [Google Scholar]
- 16.Yunis JJ, Lieberman J, Yunis EJ. Major histocompatibility complex associations with clozapine-induced agranulocytosis. The USA experience. Drug Saf. 1992;(7 Suppl):1–7. doi: 10.2165/00002018-199200071-00005. [DOI] [PubMed] [Google Scholar]
- 17.Tamai H, Sudo T, Kimura A, Mukuta T, Matsubayashi S, Kuma K. et al. Association between the DRB1*08032 histocompatibility antigen and methimazole-induced agranulocytosis in Japanese patients with Graves disease. Ann Intern Med. 1996;124:490. doi: 10.7326/0003-4819-124-5-199603010-00005. [DOI] [PubMed] [Google Scholar]


