Significance
This article fills an important gap in the literature on structural changes in the brain that are induced by speaking two languages. It has been suggested that early lifelong bilingualism affects the structure of white matter (WM) of the brain and preserves its integrity in older age. Here we show that similar WM effects are also found in bilingual individuals who learn their second language (L2) later in life and are active users of both languages. This finding presents a strong argument for the general benefits of additional language learning and the importance of language learning and use in a naturalistic environment.
Keywords: bilingualism, immersion, white matter, second language acquisition, TBSS
Abstract
Recent studies suggest that learning and using a second language (L2) can affect brain structure, including the structure of white matter (WM) tracts. This observation comes from research looking at early and older bilingual individuals who have been using both their first and second languages on an everyday basis for many years. This study investigated whether young, highly immersed late bilinguals would also show structural effects in the WM that can be attributed to everyday L2 use, irrespective of critical periods or the length of L2 learning. Our Tract-Based Spatial Statistics analysis revealed higher fractional anisotropy values for bilinguals vs. monolinguals in several WM tracts that have been linked to language processing and in a pattern closely resembling the results reported for older and early bilinguals. We propose that learning and actively using an L2 after childhood can have rapid dynamic effects on WM structure, which in turn may assist in preserving WM integrity in older age.
There is accumulating evidence that the experience of actively using two or more languages in everyday life can introduce benefits in cognitive functioning beyond language processing, most notably to executive functioning (see ref. 1 for a review). This has been particularly evidenced in populations of older bilinguals, leading to the suggestion that the experience of bilingualism results in a cognitive “reserve” in older age (2). At the same time, a growing number of neuroimaging studies have suggested that the cognitive benefits in bilinguals are often accompanied by, and possibly related to, structural changes in their brains (for recent reviews, see refs. 3 and 4). This article focuses on the effects of bilingualism on the structure and integrity of the white matter (WM) of the brain and the factors that have been shown to affect it.
The observed advantages for executive functioning performance in older bilinguals led Luk and colleagues (5) to investigate whether these advantages are related to significant effects in the WM structure of older bilinguals. This would explain the cognitive benefits of bilingualism as a result of better connectivity between brain areas. Luk and colleagues performed a diffusion tensor imaging (DTI) scan on 14 healthy older lifelong bilinguals and compared them with scans from 14 healthy age-matched monolingual participants. The researchers analyzed their data by using Tract-Based Spatial Statistics (TBSS) (6) and reported higher fractional anisotropy (FA) values for bilinguals in the corpus callosum (CC), extending both posteriorly in the bilateral superior longitudinal fasciculi and anteriorly in the right inferior frontooccipital fasciculus (IFOF) and uncinate fasciculus. Because high FA values have been related to greater WM integrity (6), Luk and colleagues suggested that lifelong bilingual experience preserves the WM integrity in older adults. However, the groups in Luk and colleagues had comparable cognitive performance in a series of standardized neuropsychological tasks, so no evidence for a cognitive advantage for bilinguals was provided. The structural findings by Luk and colleagues were challenged by Gold and colleagues (7), who tested 20 older lifelong bilinguals and 63 age-matched monolinguals and who reported lower FA values for bilinguals in several tracts, including the left IFOF, the fornix, and the CC. Gold and colleagues noted that this difference may be a result of the higher prevalence of Alzheimer’s disease among their bilinguals, however, and despite the observed reduction in WM integrity, the authors point out that their bilinguals were comparable or even more efficient than the monolinguals in a series of executive tasks, as reported in a separate study (8), which is in accordance with previous findings on bilinguals with Alzheimer’s disease (2).
It is important to note that the participants in the study by Luk and colleagues reported starting using both their languages daily at a range of ages, from birth to 11 y old. Because this description includes both early and late learners of a second language (9), it is important to further investigate whether this effect is driven by an early start of bilingualism or simply by the concurrent use of two languages. In other words, it would be interesting to investigate whether the proposed critical periods for second language acquisition (10) apply to the WM reorganization and subsequent benefits to the executive system or whether simply learning and effectively using an L2 can have significant effects on WM structure, even in late learners.
The effects of early and simultaneous language learning on WM structure were demonstrated by Mohades and colleagues (11), who compared FA values in three groups of children: 15 simultaneous bilinguals, 15 sequential bilinguals (L2 learned at the age of 3 y), and 10 monolinguals. Mohades and colleagues examined four specific bundles of fibers that have been associated with language processing and reported significant effects in two of them, including in the left IFOF, which has been suggested to play a role in semantic processing (12). In this bundle, simultaneous bilinguals demonstrated higher FA values than the other two groups, signifying, according to the authors, more effective semantic processing for this group. In addition, the simultaneous group demonstrated decreased FA values compared with monolinguals only, in the anterior third of the CC, an area implicated in the lateralization of brain function (13). Mohades and colleagues attributed this difference to left hemispheric dominance in their monolingual and sequential bilingual groups compared with the bilateral language patterns that have been reported for early bilinguals (14).
Further evidence of the effect of simultaneous language learning on the WM of early bilinguals was recently provided by García-Pentón and colleagues (15), who compared a group of 13 Spanish-Basque bilinguals and a group of 13 age-matched Spanish monolinguals. García-Pentón and colleagues used a network-based statistics approach (16) and revealed that two WM subnetworks provided more efficient connections among GM structures in bilinguals than monolinguals: first, a network connecting several language-related areas, including the left inferior frontal gyrus (IFG), pars triangularis, superior temporal gyrus, superior frontal gyrus, and the insula, and second, a network connecting the left angular gyrus, superior parietal gyrus, superior temporal pole, superior occipital gyrus, and right superior frontal gyrus. These areas have all been implicated in language processing and control (17, 18), with the first network having been particularly implicated in bilingualism (19). On the basis of these findings, García-Pentón and colleagues suggested that the additional cognitive demands that are imposed by the simultaneous use of two languages result in neural subnetworks that are more capable of transferring information between different language-related brain areas, especially networks that are involved in tackling phonological, semantic, and syntactic competition between languages, as well as in word recognition and semantic processing.
Although these studies have focused on WM effects as a result of early and/or lifelong bilingualism, findings from VBM studies on gray matter changes in late bilinguals (20, 21) bring into question whether similar effects on WM structure could be also observable in late bilingual populations. This was investigated by Schlegel and colleagues (22), who ran a longitudinal DTI study on 11 English L2 learners of Modern Standard Chinese and compared them with 16 monolingual control participants. Schlegel and colleagues collected eight monthly DTI scans per participant across a period of 9 mo and reported increased FA values for the language learning group in tracts connecting language areas in the left hemisphere and in right hemisphere tracts, as well as in the bilateral genu of the CC. Schlegel and colleagues interpreted these findings as evidence for the structural plasticity that underlies language learning, which can be explained as increased myelination across the tracts under investigation.
Further evidence for the effects of late L2 learning was presented by Hosoda and colleagues (23), who reported a cross-sectional and a longitudinal study on Japanese learners of English. Participants in the cross-sectional study demonstrated a positive correlation between the size of their L2 vocabulary and the FA values in the right hemisphere, including the subcortical WM beneath IFG, the arcuate fasciculus, and the IFG-caudate nucleus and IFG-superior temporal gyrus pathways. In the longitudinal study, a bilingual group was initially compared with an age-matched monolingual group (the “Pre” scanning point), and no between-groups differences were observed in the WM integrity. The same groups were scanned after 16 wk (the “Post-1” scanning point) after the bilingual group had undergone intensive L2 vocabulary training, and the results revealed significantly increased FA values in the regions that emerged in the cross-sectional study in the bilingual group only. Importantly, the bilingual group was scanned again after a year of no structured L2 training (the “Post-2” scanning point) and demonstrated that the WM structure of the above-studied pathways was not statistically different from the “Pre” condition. Hosoda and colleagues interpreted these results as indicative of neural “elasticity” and suggested that the L2 learning-induced plastic changes are dynamic and use-dependent.
Additional evidence against the increase of FA values as a result of late bilingualism has been provided by Cummine and Boliek (24), who tested 13 late bilingual Chinese–English speakers and 13 monolingual English speakers. Contrary to previous findings (5, 11, 22), Cummine and Boliek reported increased FA values for monolingual participants, compared with bilinguals, in the right IFOF and the anterior thalamic radiation bilaterally. The authors did not provide an explanation for the increased FA values in monolinguals; however, they attributed the absence of bilinguals > monolinguals effects to the fact that their bilingual group was younger than the corresponding group in Luk and colleagues (5), suggesting that the increased WM integrity reported by Luk and colleagues may only appear in late adulthood.
The review of the available literature reveals that it is unclear what the causes of the WM effects in bilinguals are. Although it appears that WM changes are to be expected in early bilinguals, the evidence on late bilinguals is still inconclusive. There is evidence that linguistic training can indeed affect WM structure (22), but it also appears that these effects might be temporary if the training is terminated (23). In this study, we scanned a group of highly proficient and highly immersed late L2 speakers of English who did not receive any linguistic training at the time of testing and compared them with a group of monolinguals with a TBSS analysis. This permitted us to investigate the effects of bilingualism in mature adult brains that are not otherwise affected structurally by either normal development (11) or aging (5). At the same time, we could investigate whether the previously observed WM effects are a temporary result of intensive linguistic training, or a more permanent outcome of everyday use of two languages, which also contributes to a sustained WM integrity in older age (5). If WM changes as a result of the continuous dynamic juggling of two languages, then we would expect to demonstrate increased FA values for our bilinguals in a pattern similar to what has been reported by Luk and colleagues, but in a young group of adult participants who can be assumed to be at their highest level of linguistic performance.
Results
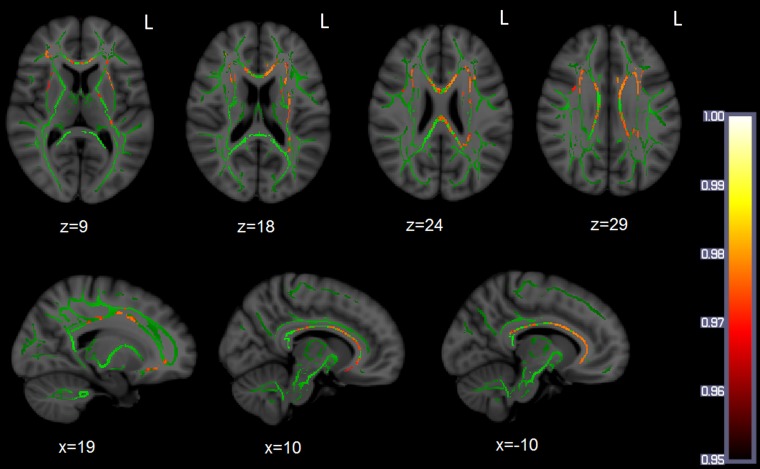
The TBSS analysis revealed higher FA values for the L2 group in the CC bilaterally, including the genu, the body, and the anterior part of the splenium. This extended bilaterally to the IFOF, uncinate fasciculi, and superior longitudinal fasciculi. The affected areas are illustrated in Fig. 1. The native speakers (NS) group did not reveal higher FA values than the L2 group in any voxel.
Fig. 1.
Significant L2 > NS differences in FA values (red/yellow), expressed in 1-P values (P < 0.05, corrected) and overlaid onto a standard space WM skeleton (green).
To investigate the effects of L2 immersion in WM structure, we reran the above analysis on the L2 group only, with the amount of immersion in months added as a regressor. This analysis produced no significant effects.
Discussion
This article investigated whether the effects of bilingualism on the WM structure of the brain of early and older bilinguals can also be observed in late bilinguals who are active users of their L2. Our results revealed significant effects for bilinguals vs. monolinguals in several WM tracts and in a pattern resembling the one reported by Luk and colleagues (5). This discussion examines the proposed role of the affected tracts in L2 learning and argues that bilingualism-induced WM effects are related to L2 immersion.
One of the tracts affected in our bilingual participants was the IFOF, bilaterally. These tracts have been heavily implicated in L2 learning, with Luk and colleagues (5) reporting increased FA values in the right IFOF and Mohades and colleagues (11) in the left IFOF for bilinguals vs. monolinguals. Because this bundle has been implicated in semantic processing (12), the observed effect may signify more efficient semantic processing in bilinguals, which can be related to the fact that bilinguals need to constantly select between L1 and L2 naming alternatives (19).
Another region that was found to be affected by bilingualism is the genu of the CC, also reported in Luk and colleagues (5). Schlegel and colleagues (22) also reported increased FA values in the genu of the CC for their participants in intensive language training courses, which they attributed to increased myelination of the CC fibers as a result of increased language switching demands. Although the role of the CC in language processing is not fully understood (22), it has been heavily implicated in effective interhemispheric communication and in executive functioning (25, 26). The explanation provided by Schlegel and colleagues also applies to the bilingual participants in our study, as well as in Luk and colleagues, with the difference being that our participants face similar switching demands as a result of linguistic immersion, and not language training.
The final two tracts that appeared to be affected by bilingualism in our study, as well as in Luk and colleagues (5), were the superior longitudinal fasciculus and uncinate fasciculus, bilaterally. According to Friederici (27), these tracts constitute, respectively, a dorsal and a ventral pathway connecting Broca’s area to temporal areas, notably the superior temporal gyrus and the middle temporal gyrus, which have been linked to phonological, semantic, and syntactic processing in various, and sometimes competing, theoretical models (see ref. 27 for a review). Differentiating the exact involvement of these WM pathways in language acquisition and in language processing is beyond the scope of this article and cannot be achieved with our current dataset; however, effective connectivity between the same areas has also been recently proposed in early bilinguals (15).
Despite the proposed links between WM structure and cognitive benefits in bilingualism, these hypotheses have not been tested systematically. Cummine and Boliek (24), for example, reported a negative correlation between FA values and reaction times in a task involving reading aloud words with regular and irregular phonology. Similarly, Hosoda and colleagues (23) showed a positive correlation between FA values and L2 vocabulary size in several WM tracts. Given the significant effects we found for our L2 group in the language-related WM tracts described here, we would also expect significant correlations between the FA values in the reported tracts and the participants’ performance in tasks tapping on semantics and syntax, signifying more efficient processing. In addition, the significant effects on the CC should predict enhanced executive functioning by the same group, as has also been proposed in ref. 1, highlighting further the effects of bilingualism on general cognition. However, as the appropriate behavioral tasks were not administered in our groups, we can only speculate about these effects.
It is important to highlight that our young late bilingual group demonstrated increased FA values in a widespread network of language-related WM tracts. This network resembles the networks identified in older bilinguals (5), as well as those in early bilinguals (15). In addition, and in contrast to language-training studies (22, 23), the WM effects persevere as a function of everyday use of L2, a suggestion that can account for the effects reported in Luk and colleagues, and can also explain the absence of significant effects in the linguistically trained but not immersed late learners (23, 24). The close mapping of the present results to those from Luk and colleagues suggests that everyday handling of more than one language functions as an intensive cognitive stimulation that benefits specific language-related brain structures by preserving their integrity, and therefore it protects them against deterioration in older age. In the light of these results, we propose that L2 learning, and the changes in WM connectivity that accompany it, is a dynamic process that relies heavily on L2 immersion. We also propose that the benefits of bilingualism on the WM structure that are observed in older age (5) may be independent of critical periods for L2 acquisition, but a direct consequence of lifelong active use of two languages. Future studies should focus on conducting longitudinal experiments on immersed language learners to identify how and when the WM restructuring takes place. These experiments should also include an extensive behavioral battery tapping on linguistic and general cognitive performance to demonstrate any links between WM structure and behavior.
Methods
Ethics Statement.
This research was approved by the University of Reading Research Ethics Committee. All participants provided written informed consent prior to participating.
Participants.
Twenty L2 speakers of English of various L1 backgrounds (female, 13; mean age, 31.85 y; SD, 8.06 y) formed the L2 group of this study. They had lived in the United Kingdom for a minimum of 13 mo (mean immersion, 91 mo; SD, 84 mo; range, 13–374 mo) and had started learning English around the age of 10 y (mean, 10.15 y; SD, 4.17 y), therefore classifying as sequential late learners (11). The full demographics of the L2 group are illustrated in Table S1. Their proficiency in English was assessed by the Quick Placement Tests (QPT) (28), a computerized assessment that has been previously used in L2 neuroimaging research (20, 29). The L2 group was highly proficient in English (mean QPT score, 82.3%; SD, 12.55%). In addition, they were asked to rate themselves in 1–7 Likert scales on their following linguistic skills: reading (mean rating, 6.15; SD, 1.04), writing (mean rating, 5.85; SD, 0.99), speaking (mean rating, 5.75; SD, 0.91), and listening (mean rating, 5.85; SD, 1.14). The self-ratings were highly correlated to each other (P < 0.001 for all correlations); in addition, the reading self-rating was positively correlated to their QPT score [r(18) = 0.436; P = 0.036], whereas the correlations between the QPT score and the rest of the ratings were in the same direction but did not reach statistical significance. Finally, they underwent a Backward Digit Span test (30) (mean score, 7.4; SD, 2.04).
Twenty-five native speakers of English (female, 14; mean age, 28.16 y; SD, 5.33 y) formed the NS group of this study. No one from the NS group reported speaking an L2. The demographics of the NS group are illustrated in Table S2. The NS group was also tested in the QPT test (mean score, 98.56; SD, 4), where they scored significantly higher than the L2 group [F(1,44) = 37.324; P < 0.001], and in the Backward Digit Span test (mean score, 8.12; SD, 2.14), where their performance did not differ from that of the L2 group [F(1,44) = 1.307; P = 0.259].
Data Acquisition, Preparation, and Analysis.
We used a 3.0-Tesla Siemens MAGNETOM Trio MRI scanner with Syngo software and 36-channel Head Matrix coil to acquire a whole-brain diffusion-weighted Echo-Plannar Imaging image (two averages, 30 directions, 60 axial slices; slice thickness, 2 mm, no interslice gap; field of view, 256 × 256 mm; acquisition matrix, 128 × 128; voxel size, 2 mm isotropic; echo time, 93 ms; repetition time, 8,200 ms; b-value, 1,000 s/mm2).
The images were preprocessed with FMRIB's Diffusion Toolbox, part of FSL (31), and were initially corrected for eddy-current distortion and head motion, and subsequently a diffusion tensor model was fit at each voxel of the corrected data with DTIFIT. This resulted in one FA image per participant. Preprocessing was followed by a TBSS (6) analysis in FSL as follows: The FA images for all participants were nonlinearly registered to a standard space FA target image and subsequently affine-transformed into the 1 × 1 × 1 Montreal Neurological Institute 152 space in a combined process avoiding sampling the images twice. This resulted in standard-space FA images for each participant that were merged and averaged to create a mean 4D image. The mean image was fed into the FA skeletonization program to create an FA skeleton that included voxels identified as WM across all participants. The mean skeleton image was thresholded at 0.2. We then analyzed the skeletonized data with a between-groups voxel-wise analysis with permutation-based nonparametric testing, corrected for multiple comparisons with TFCE (32). Sex was included in the model as a covariate of no interest, and two contrasts were examined: L2 > NS, and NS > L2. This resulted in corrected whole-brain t statistical images of the significant differences, which were thresholded at P < 0.05.
Supplementary Material
Acknowledgments
We thank Francesca Allerton for assisting in the recruitment of participants for this project. This study was internally funded by the Centre for Integrative Neuroscience and Neurodynamics, University of Reading.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414183112/-/DCSupplemental.
Data deposition: The data reported in this paper have been deposited in XNAT Central, https://central.xnat.org (Project ID code L2struc).
References
- 1.Bialystok E, Craik FIM. Cognitive and Linguistic Processing in the Bilingual Mind. Curr Dir Psychol Sci. 2010;19:19–23. [Google Scholar]
- 2.Schweizer TA, Ware J, Fischer CE, Craik FIM, Bialystok E. Bilingualism as a contributor to cognitive reserve: Evidence from brain atrophy in Alzheimer’s disease. Cortex. 2012;48(8):991–996. doi: 10.1016/j.cortex.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: Anatomical changes in the human brain. Cortex. 2014;58:301–324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Stein M, Winkler C, Kaiser A, Dierks T. Structural brain changes related to bilingualism: Does immersion make a difference? Front Psychol. 2014;5:1116. doi: 10.3389/fpsyg.2014.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luk G, Bialystok E, Craik FIM, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Gold BT, Johnson NF, Powell DK. Lifelong bilingualism contributes to cognitive reserve against white matter integrity declines in aging. Neuropsychologia. 2013;51(13):2841–2846. doi: 10.1016/j.neuropsychologia.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci. 2013;33(2):387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pliatsikas C, Marinis T. Processing of regular and irregular past tense morphology in highly proficient L2 learners of English: A self-paced reading study. Appl Psycholinguist. 2013;34:943–970. [Google Scholar]
- 10.Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92(1-2):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Mohades SG, et al. DTI reveals structural differences in white matter tracts between bilingual and monolingual children. Brain Res. 2012;1435:72–80. doi: 10.1016/j.brainres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Leclercq D, et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg. 2010;112(3):503–511. doi: 10.3171/2009.8.JNS09558. [DOI] [PubMed] [Google Scholar]
- 13.Putnam MC, Wig GS, Grafton ST, Kelley WM, Gazzaniga MS. Structural organization of the corpus callosum predicts the extent and impact of cortical activity in the nondominant hemisphere. J Neurosci. 2008;28(11):2912–2918. doi: 10.1523/JNEUROSCI.2295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hull R, Vaid J. Laterality and language experience. Laterality. 2006;11(5):436–464. doi: 10.1080/13576500600691162. [DOI] [PubMed] [Google Scholar]
- 15.García-Pentón L, Pérez Fernández A, Iturria-Medina Y, Gillon-Dowens M, Carreiras M. Anatomical connectivity changes in the bilingual brain. Neuroimage. 2014;84:495–504. doi: 10.1016/j.neuroimage.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 16.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 18.Abutalebi J, Green DW. Bilingual language production: The neurocognition of language representation and control. J Neurolinguist. 2007;20:242–275. [Google Scholar]
- 19.Parker Jones O, et al. Where, when and why brain activation differs for bilinguals and monolinguals during picture naming and reading aloud. Cereb Cortex. 2012;22(4):892–902. doi: 10.1093/cercor/bhr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pliatsikas C, Johnstone T, Marinis T. Grey matter volume in the cerebellum is related to the processing of grammatical rules in a second language: A structural voxel-based morphometry study. Cerebellum. 2014;13(1):55–63. doi: 10.1007/s12311-013-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein M, et al. Structural plasticity in the language system related to increased second language proficiency. Cortex. 2012;48(4):458–465. doi: 10.1016/j.cortex.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. J Cogn Neurosci. 2012;24(8):1664–1670. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- 23.Hosoda C, Tanaka K, Nariai T, Honda M, Hanakawa T. Dynamic neural network reorganization associated with second language vocabulary acquisition: A multimodal imaging study. J Neurosci. 2013;33(34):13663–13672. doi: 10.1523/JNEUROSCI.0410-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummine J, Boliek CA. Understanding white matter integrity stability for bilinguals on language status and reading performance. Brain Struct Funct. 2013;218(2):595–601. doi: 10.1007/s00429-012-0466-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, et al. Increased structural connectivity in corpus callosum in adolescent males with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2014;53:466–75.e1. doi: 10.1016/j.jaac.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friederici AD. Pathways to language: Fiber tracts in the human brain. Trends Cogn Sci. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Geranpayeh A. A quick review of the English Quick Placement Test. Res Notes. 2003;12:8–10. [Google Scholar]
- 29.Pliatsikas C, Johnstone T, Marinis T. FMRI evidence for the involvement of the procedural memory system in morphological processing of a second language. PLoS ONE. 2014;9(5):e97298. doi: 10.1371/journal.pone.0097298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. 4th Ed Pearson Asessment; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale. [Google Scholar]
- 31.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.