Significance
Many therapeutic proteins suffer from short plasma half-lives and, as a consequence, require frequent injections to be therapeutically effective; this in turn can adversely affect patient compliance and quality of life. In contrast, therapeutic antibodies typically have half-lives of weeks in humans. Consequently, there is considerable interest in generating functional antibodies with agonist or antagonist activities. Based on the structure of a natural bovine antibody with an ultralong, well-folded heavy-chain complementarity-determining region, we have developed a strategy for the generation of functional human antibody–hormone chimeras with biological activities comparable to native hormones and significantly enhanced pharmacological properties. This approach likely provides a general, relatively straightforward platform for generating antibody agonists and antagonists for a range of therapeutic applications.
Keywords: antibody, protein engineering, growth hormone, leptin, pharmacology
Abstract
On the basis of the 3D structure of a bovine antibody with a well-folded, ultralong complementarity-determining region (CDR), we have developed a versatile approach for generating human or humanized antibody agonists with excellent pharmacological properties. Using human growth hormone (hGH) and human leptin (hLeptin) as model proteins, we have demonstrated that functional human antibody CDR fusions can be efficiently engineered by grafting the native hormones into different CDRs of the humanized antibody Herceptin. The resulting Herceptin CDR fusion proteins were expressed in good yields in mammalian cells and retain comparable in vitro biological activity to the native hormones. Pharmacological studies in rodents indicated a 20- to 100-fold increase in plasma circulating half-life for these antibody agonists and significantly extended in vivo activities in the GH-deficient rat model and leptin-deficient obese mouse model for the hGH and hLeptin antibody fusions, respectively. These results illustrate the utility of antibody CDR fusions as a general and versatile strategy for generating long-acting protein therapeutics.
Many endocrine hormones are used therapeutically (1); however, they generally suffer from short circulating half-lives (2, 3) and therefore require high doses and frequent injections to achieve efficacious exposures. For example, human growth hormone (hGH) is a 22-kDa four-helix–bundle protein produced by the pituitary gland, and its recombinant form has long been used for the treatment of growth hormone deficiency (GHD). Due to the short in vivo half-life of hGH, which is on the timescale of minutes, current GHD therapy requires daily injection (4), resulting in lower patient compliance (5). Studies have shown that a continuous infusion of recombinant (r)hGH has a similar efficacy and safety profile as daily rhGH therapy (6), and thus there is considerable interest in the development of long-acting forms of hGH. The first and only approved long-acting hGH, Nutropin Depot, a sustained-release formulation based on a biodegradable polymer, was withdrawn due to manufacturing difficulties. Various forms of long-acting GH have since been generated: those currently in clinical development include LB03002 (microparticle suspension), NNC126-0083 (PEGylation), NNC0195-0092 (reversible albumin-binding GH derivative), MOD-4023 (CTP modification), ACP-001 (prodrug strategy), Albutropin (albumin fusion), and VRS-317 (XTEN fusion), and have been extensively reviewed elsewhere (5). However, challenges associated with heterogeneity, stability, immunogenicity, toxicity, and/or compromised potency could potentially limit their clinical utility (3, 7–10).
Another short-lived hormone in which there is considerable clinical interest is human leptin, a 16-kDa four-helix–bundle protein that plays a central role in the homeostasis of body weight. Recombinant leptin has been approved for the treatment of leptin-deficient lipodystrophy patients (11), and long-term leptin therapy is being evaluated in clinical and preclinical studies for the treatment of type 1 and type 2 diabetes, nonalcoholic steatohepatitis, and obesity (12–14). However, due to its short circulating half-life, frequent doses are again required to obtain a modest clinical efficacy. Therefore, long-acting forms of these and other hormones are desirable to reduce dose frequency while still providing sustained therapeutic effect.
We recently solved the X-ray crystal structure of an unusual bovine antibody in which the Ig domain and a disulfide cross-linked “knob domain” of heavy-chain complementarity-determining region 3 (CDR3H) are spatially separated by a rigid, solvent-exposed, antiparallel β-strand stalk (Fig. 1A) (15). Previously, we showed that replacement of the knob domain with the cytokines GCSF, EPO, or a CXCR4 peptide antagonist yields fusion proteins that retain their biological activity and have improved pharmacokinetic properties (16–18). However, one potential obstacle that could limit the therapeutic use of these engineered antibody agonists/antagonists is a human host immune response stimulated by the bovine antibody scaffold. Here we demonstrate that this same fusion strategy can be applied to a human antibody scaffold to generate antibody agonists for the growth hormone and leptin receptors. hGH and hLeptin were fused into multiple CDRs of the humanized antibody Herceptin with rigid linkers to afford chimeric proteins that retain the in vitro activities of the native hormones and have significantly enhanced in vivo pharmacological properties.
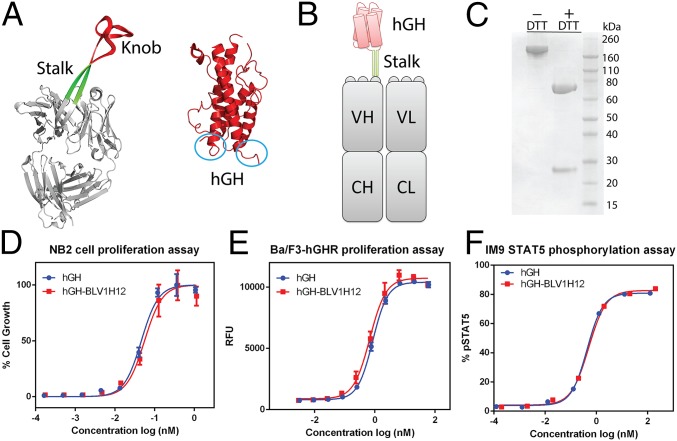
Fig. 1.
(A) X-ray crystal structures of bovine antibody BLV1H12 Fab fragment [Protein Data Bank (PDB) ID code 4K3D] and human growth hormone (PDB ID code 1HGU). The N and C termini of the four-helix–bundle protein are circled in blue. (B) A schematic representation of hGH–BLV1H12 fusion. The knob domain of BLV1H12 is replaced by hGH. (C) SDS/PAGE analysis of hGH–BLV1H12 fusion. (D) hGH–BLV1H12 fusion protein stimulates proliferation of rat NB2-11 cells in a dose-dependent manner. Cells were treated with various concentrations of hGH and hGH–BLV1H12 fusion proteins and cell viability was quantified using PrestoBlue reagent; assays were performed in triplicate. (E) hGH-dependent Ba/F3-hGHR cells proliferate in a dose-dependent manner upon treatment with hGH–BLV1H12. Cells were treated with various concentrations of hGH and hGH–BLV1H12 fusion proteins, and cell viability was quantified using PrestoBlue reagent; assays were performed in triplicate. RFU, relative fluorescence units. (F) hGH–BLV1H12 fusion protein stimulates STAT5 phosphorylation in IM9 cells. Serum-starved IM9 cells were treated with various concentrations of hGH and hGH–BLV1H12 for 10 min, and phosphorylation of STAT5 was quantified by flow cytometry analysis. Assays were performed in duplicate. Error bars represent the standard deviation.
Results and Discussion
An hGH–Bovine Antibody CDR Fusion Maintains Native hGH Activity.
Like many four-helix–bundle proteins, the X-ray crystal structure of hGH shows that its N and C termini are in close proximity to each other (Fig. 1A). In addition, both biochemical studies and the structure of hGH in complex with the extracellular domain of its receptor indicate that the key residues involved in binding the receptor reside in helices 1, 3, and 4, with the N and C termini of hGH distal to the binding interface (19, 20). Moreover, previous studies have shown that covalent cross-linking of the N and C termini of four-helix–bundle cytokines with a PEGylated short peptide sequence does not significantly affect the biological activities of the cytokines (21). Therefore, we reasoned that connecting the two termini of hGH with the CDR3H stalk of the bovine antibody BLV1H12 would likely yield a fusion protein with wild-type hGH activity but with the superior pharmacological properties of the Ig scaffold. To test this notion, we first replaced the knob domain of BLV1H12 with hGH by fusing the N and C termini of hGH with the ascending and descending β-strands of the stalk, respectively (Fig. 1B). Short flexible GGGGS linkers were included at the junctions to promote correct folding. The resulting hGH–BLV1H12 fusion protein was transiently expressed in 293F cells in a good yield of 20 mg/L. After protein G chromatography, the purified protein could be concentrated to over 10 mg/mL in PBS (pH 7.4) without aggregation. SDS/PAGE analysis revealed that the fusion protein migrates as a single band around 190 kDa. In the presence of DTT, the heavy and light (L) chains of the fusion protein migrate at 70 and 25 kDa, respectively, matching the calculated molecular weight of the hormone–antibody fusion (Fig. 1C).
Next, we determined whether the hGH antibody CDR fusion protein retains its biological activity in vitro. A rat NB2 cell proliferation assay was used to evaluate the mitogenic activity of hGH (22, 23); rhGH and the hGH–BLV1H12 fusion have nearly equal potency, with EC50 values of 0.046 ± 0.008 and 0.055 ± 0.011 nM, respectively (Fig. 1D). Because the NB2 proliferation assay relies on the cross-reactivity of hGH with rat lactogenic receptor, a second assay was used to confirm the potency of hGH–BLV1H12 in direct activation of hGH receptor (hGHR). An hGH-dependent cell line (Ba/F3-hGHR) was generated by stable expression of hGHR on the surface of Ba/F3 cells. Treatment with various concentrations of hGH or hGH–BLV1H12 resulted in dose-dependent growth of Ba/F3-hGHR cells, with EC50 values of 0.85 ± 0.06 and 0.68 ± 0.11 nM, respectively (Fig. 1E). Finally, hGH-induced signal transduction was examined with a STAT5 phosphorylation assay in IM9 cells, a human B lymphoblastoid cell line naturally expressing hGHR (24). Upon treatment with hGH or hGH–BLV1H12, the hGHR signaling pathway is activated and results in STAT5 phosphorylation, which was quantified by flow cytometry analysis to give EC50 values of 0.43 ± 0.02 and 0.49 ± 0.09 nM, respectively (Fig. 1F). These data indicate that the hGH–CDR3H fusion folds correctly and is functional. In contrast, many of the long-acting forms of growth hormone in development, such as PEGylated GH and the XTEN and GH–GHR fusions, have a significant loss of in vitro potency (24–26).
Generation of a Humanized hGH–Antibody Fusion.
The β-strand–forming sequence of antibody BLV1H12 is evolved during VDJ rearrangement and B-cell maturation and is conserved in most bovine antibodies with ultralong CDR3Hs (15). This observation suggests that a rigid-stalk motif is required for correct folding of the canonical Ig domain and the associated knob domain. However, the β-strand stalk in the bovine antibody appears to be stabilized by interactions with other residues in the variable region (15). We recently showed that the β-strand stalk of BLV1H12 can be replaced with a rigid, stable antiparallel coiled-coil stalk motif in a CDR3H fusion to the four-helix–bundle cytokine GCSF. The resulting fusion protein expressed well in mammalian cells and had similar activity to the corresponding CDR3H fusion using a β-strand linker (27). This result raises the question of whether a rigid-stalk motif is sufficient to allow the fusion of cytokines and growth factors to CDRs with antibodies from other species. To test this notion, we attempted to replace the bovine Ig domain in the hGH–BLV1H12 fusion with Herceptin. Herceptin is a Food and Drug Administration-approved fully humanized anti-HER2 receptor monoclonal antibody (28–31) that shows minimal immunogenicity in humans and has been optimized for industry-scale production. Therefore, a functional hGH–CDR3H fusion with Herceptin would likely be less immunogenic than the hGH–BLV1H12 fusion.
Sequence alignment of Herceptin and BLV1H12 suggested that hGH could be fused to the Trp99–Met107 loop of CDR3H in Herceptin (between the conserved “G” and “F” β-strands of the Ig fold) using an antiparallel coiled-coil linker to connect CDR3H to the N and C termini of hGH (Fig. 2 A and B). The sequences of the ascending and descending linkers are H2N-GGSGAKLAALKAKLAALKGGGGS-COOH and H2N-GGGGSELAALEAELAALEAGGSG-COOH, respectively (32). In addition to fusions to CDR3H of BLV1H12, we recently showed that CDR2H can also be used to generate functional CDR fusion proteins (18). Furthermore, the X-ray crystal structure of Herceptin reveals that CDR3H and CDR3L exhibit similar β-strand conformations and contain multiple intra- and interloop hydrogen bonds (Fig. 2A). Therefore, in addition to CDR3H, we also attempted to fuse hGH with a coiled-coil stalk into the Thr93–Pro95 loop of CDR3L and the Thr54–Asn55 loop of CDR2H. Finally, the hIgG1 constant regions of all three chimeric antibodies were modified with seven mutations (E233P, L234V, L235A, ΔG236, A327G, A330S, and P331S) to reduce complement-dependent and antibody-dependent cell-mediated cytotoxicity (33, 34).
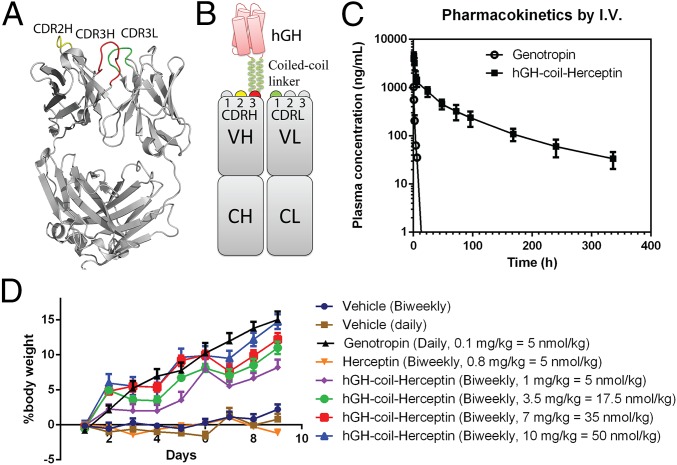
Fig. 2.
(A) X-ray crystal structure of humanized antibody Herceptin Fab fragment (PDB ID code 1N8Z). CDR loops used for hGH fusion are marked and highlighted in yellow, red, and green for CDR2H, CDR3H, and CDR3L, respectively. (B) A schematic representation of the hGH-CDR3H-coil-Herceptin fusion. The N and C termini of hGH are fused to Herceptin CDR3H with a coiled-coil linker (H2N-GGSGAKLAALKAKLAALK-COOH and H2N-ELAALEAELAALEAGGSG-COOH). CDR loops used for hGH fusion are highlighted in yellow, red, and green for CDR2H, CDR3H, and CDR3L, respectively. Fusions for CDR2H and CDR3L are assembled similarly onto the corresponding loops. (C) Pharmacokinetics of hGH-CDR3H-coil-Herceptin in rat by i.v. injection. A single dose (2 mg/kg) of Genotropin (rhGH) and hGH-CDR3H-coil-Herceptin was administered by i.v. injection into Sprague–Dawley rats (n = 3). The estimated terminal half-lives after i.v. injection are 34 min and 47 h for Genotropin and hGH-coil-Herceptin, respectively. (D) Pharmacodynamic study of hGH-coil-Herceptin in the hypophysectomized rat model. Animals matched by initial weights and pretreatment growth rates were sorted and randomized to receive s.c. injections according to one of the following protocols: daily injection of vehicle or Genotropin (0.1 mg/kg = 5 nmol/kg); a biweekly administration of vehicle, Herceptin (0.8 mg/kg = 5 nmol/kg); or increasing doses of the hGH–Herceptin fusion (1 mg/kg = 5 nmol/kg, 3.5 mg/kg = 17.5 nmol/kg, 7 mg/kg = 35 nmol/kg, and 10 mg/kg = 50 nmol/kg). Error bars represent the standard deviation.
All three fusion proteins were transiently expressed in FreeStyle 293F cells and purified as described above with final yields of ∼12 mg/L for hGH-CDR3H-coil-Herceptin, ∼18 mg/L for hGH–CDR3L, and ∼17 mg/L for hGH-CDR2H-coil-Herceptin (SI Appendix, Figs. S1–S3). SDS/PAGE analysis indicated that the masses of the heavy and light chains of the purified fusion proteins match the calculated molecular weights. The in vitro activities of the fusion proteins were measured by both the NB2 proliferation and IM9 pSTAT5 phosphorylation assays described above, and the results are summarized in SI Appendix, Table S1. In both assays, all fusion proteins maintain comparable activity to the native hGH hormone in vitro. These results suggest that functional antibody fusion proteins can be generated successfully from an antibody with a CDR of the appropriate geometry and a functional protein or peptide with juxtaposed N and C termini by simply using a coiled-coil motif to allow independent folding of the two domains.
Next, we examined whether the fusion of hGH abolishes the binding of Herceptin scaffold to HER2 receptor. Flow cytometry analysis was carried out by incubating 10 nM fusion protein with SKBR3 cells (HER2+++) and HER2 stably transfected MDA-MB-435 cells (HER2+++), and with the HER2-negative cells MDA-MB-468 (HER2−) as a control. No peak shift was observed in the flow cytometry histograms after incubation of hGH-CDR3H-coil-Herceptin with all three cell lines (SI Appendix, Fig. S4). Slight peak shifts were observed when hGH-CDR3L-coil-Herceptin was incubated with HER2-overexpressing cells but not with MDA-MB-468 cells (SI Appendix, Fig. S5). These results suggest that antigen binding by the original human Herceptin antibody scaffold can largely be abolished by fusion of a protein to CDR3H and CDR3L. However, HER2-positive cells treated with the hGH-CDR2H-coil-Herceptin showed a strong peak shift (which was not observed in HER2-negative cells), indicating that fusion of hGH to CDR2H does not abolish antibody binding to HER2 receptor (SI Appendix, Fig. S6). An examination of the cocrystal structure of the HER2–Herceptin complex indicates that the CDR3H and CDR3L regions of Herceptin interact with the HER2 extracellular domain, whereas the CDR2H loop makes no direct contact with the antigen (SI Appendix, Fig. S7). This observation is consistent with the flow cytometry analysis of HER2 receptor binding by the fusion proteins. Although the CDR2H of Herceptin may not be suitable for generating an hGH fusion due to undesired HER2 binding, this strategy can likely be applied to other human antibody scaffolds wherein a CDR2H fusion eliminates binding to the original antigen, or to antibody scaffolds that do not bind human antigens such as Synagis, an RSV-neutralizing antibody. In addition, the above results suggest that it may be possible to fuse multiple distinct proteins into different CDRs of a single antibody scaffold to create multifunctional therapeutic proteins.
Pharmacokinetic Analysis of the hGH-CDR3H-Coil-Herceptin Fusion.
Pharmacokinetic (PK) analysis was performed in rats to determine the circulating half-lives of the hGH-coil-Herceptin fusion proteins. Single doses (2 mg/kg) of Genotropin (recombinant hGH, a registered trademark of Pfizer) and hGH-CDR3H-coil-Herceptin in PBS (pH 7.4) were s.c. or i.v. injected into Sprague–Dawley (SD) rats (three per group). Plasma samples were collected from day 0 to day 14 and analyzed by a standard ELISA using an hGH-specific antibody. The estimated terminal t1/2 after i.v. injection is 34 min for Genotropin, which is consistent with published results (24), and 47 h for hGH-CDR3H-coil-Herceptin (Fig. 2C), based on data fit using a two-compartment model. The pharmacokinetics of hGH-CDR3H-coil-Herceptin were also analyzed following a single-dose s.c. injection into SD rats (SI Appendix, Fig. S8). Pharmacokinetic parameters were obtained by noncompartmental analysis using WinNonlin (Pharsight) and are summarized in Table 1. hGH has a rapid plasma clearance after s.c. administration, whereas hGH-CDR3H-coil-Herceptin had a slower absorption phase, greater Cmax (maximum concentration), longer residence time, and much slower plasma clearance. For comparison, examples of reported t1/2 values for other long-lasting hGHs in rat include ∼3 h for the hGH–albumin fusion (Albutropin) (35); ∼5 h for PEGylated GH (ARX201) (24); ∼6.8 h for the XTEN–hGH fusion; ∼15 h for the XTEN1-hGH-XTEN2 fusion (VRS-317) (25); and ∼21 h for the GH–GHR fusion (26). Thus, the hGH–antibody fusion significantly extends the half-life of the hormone in a rat model and, to the best of our knowledge, is the largest reported value to date. As mentioned earlier, fusion of XTEN, an unstructured polypeptide, to both ends of hGH greatly increases its t1/2 (15 h in rat) compared with fusion to only one terminus of the protein (6.8 h in rat) (25). Similarly, the antibody CDR fusion potentially protects both termini of hGH to increase the hormone’s half-life (in addition to the positive effect provided by neonatal Fc receptor-mediated recycling). However, unlike the double-XTEN fusion, the antibody fusion maintains in vitro activity comparable to the native hGH.
Table 1.
Pharmacokinetic parameters for single-dose s.c. administration of Genotropin and hGH-CDR3H-coil-Herceptin in Sprague–Dawley rats
| Parameter | Genotropin | hGH-CDR3H-coil-Herceptin |
| Cmax, ng/mL | 651 (±131) | 776 (±216) |
| Half-life, h | 0.8 (±0.2) | 37.0 (±-9.7) |
| AUC0→inf, h⋅µg−1⋅mL−1 | 2.70 (±0.54) | 49.9 (±13.9) |
| MRT, h | 2.9 (±0.3) | 58.5 (±7.7) |
| Cl/f, mL⋅h−1⋅kg−1 | 741 (±101) | 40 (±17) |
Single s.c. injection at a dosage of 2 mg/kg. Concentration vs. time curves were evaluated by noncompartmental analysis using WinNonlin. Values shown are averages of three rats in the group. AUC0→inf, area under the concentration–time curve extrapolated to infinity; Cl/f, plasma clearance; Cmax, maximum concentration; MRT, mean residence time.
Pharmacodynamic Evaluation of the hGH-CDR3H-Coil-Herceptin Fusion.
The in vivo activity of the hGH–antibody fusion was assessed in a standard hypophysectomized rat model. Animals matched by initial weights and pretreatment growth rates were sorted and randomized to receive s.c. injections. Rats were given daily injections of either vehicle or Genotropin (0.1 mg/kg = 5 nmol/kg), or they were given a biweekly administration of vehicle, Herceptin (0.8 mg/kg = 5 nmol/kg), or increasing doses of hGH-CDR3H-coil-Herceptin fusion protein. Four different doses of hGH-CDR3H-coil-Herceptin were chosen: 1 mg/kg = 5 nmol/kg (which equals the daily dose of Genotropin in molarity); 3.5 mg/kg = 17.5 nmol/kg; 7 mg/kg = 35 nmol/kg; and 10 mg/kg = 50 nmol/kg. Body weight was monitored daily and the results are shown in Fig. 2D. No effect was observed for both vehicle-treated groups or the Herceptin control. A daily Genotropin injection gives a 15% increase in body weight. A dose-dependent response was observed for the hGH-CDR3H-coil-Herceptin fusion with 8%, 11%, 13%, and 15% increases in body weight observed for the four dose groups, respectively. A biweekly injection of 10 mg/kg hGH-CDR3H-coil-Herceptin fusion in rats gives an equal in vivo potency as daily administration of 0.1 mg/kg Genotropin, suggesting a potential for hGH-CDR3H-coil-Herceptin as a replacement therapy for daily hGH injections. In addition, at the end of the treatment, the thickness of tibial epiphysis from the knee was measured using histological analysis (Materials and Methods) from selected groups. As shown in SI Appendix, Fig. S9, the increase in growth plate thickness is consistent with body weight gain among treatment groups. These data indicate an increased and long-term in vivo efficacy achieved by the hGH–antibody fusion strategy that may result in weekly or bimonthly administration in humans.
One potential issue for the clinical use of the human antibody-CDR fusion proteins arises from possible immunogenicity of the coiled-coil linker sequences and the splice junctions. Computational analysis of the linker sequences for T-cell epitopes predicted low probability for immunogenicity (36, 37). To further assess the potential immunogenicity of the fusion proteins, a fusion construct with a rodent antibody scaffold and rodent growth hormone has been generated. If nontransient neutralizing antibodies are observed, it is likely that the sequences of the stalks can be optimized to reduce immunogenicity and maintain stability and biological activity.
Generation of an hLeptin–Herceptin CDR Fusion Protein.
To test the generality of the antibody-CDR fusion approach, we next attempted to fuse human leptin, also a member of the four-helix–bundle hormone family, into the Herceptin CDR3 regions of the heavy and light chains. Previous studies have indicated that residues at the N terminus of helix D of hLeptin are essential for binding to and activating hLeptin receptor (38, 39). These residues are on the opposite face of hLeptin relative to the free N and C termini. Moreover, like hGH, the N and C termini of hLeptin are in close proximity to each other (40) (SI Appendix, Fig. S10A). Thus, we envisioned that fusion of hLeptin into the CDR3 region of Herceptin with its termini connected to the Ig domain through a rigid coiled-coil stalk would allow generation of a functional antibody chimera with correct folding and biological activity similar to the native hormone. The hLeptin–Herceptin fusions were generated by fusing the N and C termini of hLeptin to either CDR3H or CDR3L of Herceptin using the coiled-coil stalk in a similar manner to the hGH antibody fusions (SI Appendix, Fig. S10B). Again, the hIgG1 constant regions of the chimeric antibodies bear seven mutations (E233P, L234V, L235A, ΔG236, A327G, A330S, and P331S) to reduce complement-dependent and antibody-dependent cell-mediated cytotoxicities.
The fusion proteins were transiently expressed in 293F cells and purified as described above with final yields of ∼6 mg/kg for hLeptin-CDR3H-coil-Herceptin and 10 mg/kg for hLeptin-CDR3L-coil-Herceptin (SI Appendix, Figs. S11 and S12). SDS/PAGE analysis indicated that the masses of the heavy and light chains of the purified fusion proteins match the calculated molecular weights. To determine the in vitro activities of the leptin fusion proteins, a leptin-dependent cell proliferation assay was carried out by using a Ba/F3 cell line with stable expression of human leptin receptor (hObR) on the cell surface, similar to Ba/F3-hGHR. Treatments of Ba/F3-hObR cells with rhLeptin, hLeptin-CDR3H-coil-Herceptin, and hLeptin-CDR3L-coil-Herceptin resulted in dose-dependent cell growth, with EC50 values of 25.0 ± 7.7, 26.7 ± 6.6, and 22.4 ± 7.6 pM, respectively (Fig. 3A), again demonstrating that the antibody CDR fusions fully retain the biological activity of the native hormone. Furthermore, the almost identical proliferative activities for the CDR3H and CDR3L fusion proteins provide additional support to the notion that multiple CDRs can be used to generate potent antibody chimeras. Additionally, flow cytometry analysis showed that fusion of hLeptin into CDR3H of Herceptin completely eliminates its binding to HER2 receptor (SI Appendix, Fig. S13), whereas CDR3L fusion leads to a significant reduction in antigen binding on HER2 highly overexpressing cells (SI Appendix, Fig. S14).
Fig. 3.
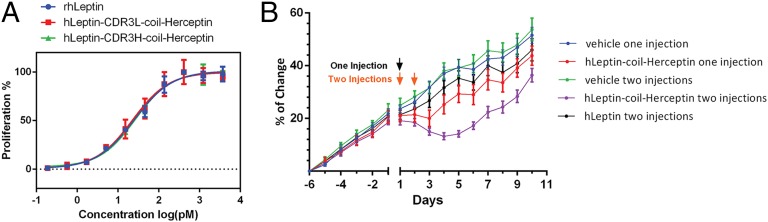
(A) hLeptin-coil-Herceptin fusion proteins stimulate proliferation of hLeptin-dependent Ba/F3-hObR cells in a dose-dependent manner. Cells were treated with various concentrations of hLeptin, hLeptin-CDR3H-coil-Herceptin, and hLeptin-CDR3L-coil-Herceptin, and cell viability was quantified using PrestoBlue reagent; assays were performed in triplicate. (B) Body weight changes of ob/ob mice treated with hLeptin or hLeptin-coil-Herceptin fusion. Groups of mice (n = 8) were injected s.c. with vehicle, hLeptin (0.2 mg/kg or 10 nmol/kg), or hLeptin-coil-Herceptin (2 mg/kg or 10 nmol/kg) with either a single injection on day 1 or one injection per d on 2 consecutive days (day 1 and day 2) as indicated on the figure. Their body weights were monitored daily for 10 d. Arrows indicate the time points at which vehicle or proteins were injected. The body weight change compared with the initial body weight (6 d before injection) was calculated for each group. Error bars represent the standard deviation.
Pharmacokinetic and Pharmacodynamic Properties of the hLeptin-CDR3H-Coil-Herceptin Fusion.
Next, we carried out a PK study in mice to determine the serum half-life of the leptin–antibody fusion protein. Single doses (70 μg) of hLeptin and hLeptin-CDR3H-coil-Herceptin were administered by i.v. injection into CD1 mice (three per group) in PBS (pH 7.4). Plasma samples were collected from day 0 to day 3 and the concentrations of hLeptin and hLeptin-CDR3H-coil-Herceptin were analyzed using the proliferative activity assay with Ba/F3-hObR cells (the endogenous mouse leptin activity was too low to be detected in this assay). As shown in SI Appendix, Fig. S15, the estimated terminal half-lives in mice are 12 h for hLeptin-CDR3H-coil-Herceptin and less than 30 min for hLeptin. In addition, a PK study by s.c. injection was performed under the same conditions (SI Appendix, Fig. S16). Compared with hLeptin (t1/2 = 0.6 h), the hLeptin–Herceptin fusion protein has an extended t1/2 of 11.8 h (SI Appendix, Table S2). The Fc–hLeptin fusion and PEGylated hLeptin were shown to have half-lives of 10 h in mice and 48 h in rat, respectively (41–43). Compared with the hGH-CDR3H-coil-Herceptin fusion, hLeptin-CDR3H-coil-Herceptin displays a reduced half-life, which is attributable in part to the different species used in the two PK studies, and may also be due to proteolytic degradation of the fusion protein in vivo (44).
We next examined the in vivo efficacy of the hLeptin-CDR3H-coil-Herceptin fusion protein in leptin-deficient ob/ob mice. ob/ob mice with similar body weights and body weight growth curves were sorted out and treated with various doses of PBS (pH 7.4) vehicle, hLeptin, and hLeptin-coil-Herceptin fusion protein for a 10-d period. Compared with mice treated with PBS vehicle which showed a >12% increase in body weight at day 10, daily injections of mice with the hLeptin-CDR3H-coil-Herceptin fusion protein (1 mg/kg = 5 nmol/kg) led to full suppression of body weight gain (SI Appendix, Fig. S17), whereas mice treated daily with hLeptin (5 mg/kg = 250 nmol/kg) showed a 5% increase in body weight at day 10. These results indicate that both hLeptin and the hLeptin–Herceptin fusion protein function in ob/ob mice, and that the extended serum half-life of the fusion protein enhances its efficacy. To further examine in vivo efficacy, ob/ob mice were treated with a single injection of the hLeptin-CDR3H-coil-Herceptin fusion protein (2 mg/kg = 10 nmol/kg) on day 1, and body weight changes were monitored for 10 d (Fig. 3B). These mice exhibited a 3% loss of body weight at day 3 before their body weight started to rebound, whereas the vehicle treatment group had a body weight increase of ∼3% per d. However, one injection per d on 2 consecutive days (day 1 and day 2) with 2 mg/kg (10 nmol/kg) of the hLeptin-CDR3H-coil-Herceptin fusion (and monitoring body weight changes for 10 d) led to an extended duration of action, resulting in 7% loss of body weight at day 5; mice body weight did not rebound until day 6. In comparison, injections of PBS vehicle and hLeptin (0.2 mg/kg = 10 nmol/kg) on both day 1 and day 2 led to 12% and 8% increases in body weight at day 5, respectively. The suppression of ob/ob mice body weight growth is consistent with the potent in vitro activity and increased serum half-life of the hLeptin-CDR3H-coil-Herceptin fusion relative to hLeptin, which requires more frequent dosing and/or higher doses to effectively control body weight. Thus, insertion of hLeptin into Herceptin CDRs provides an efficient and straightforward approach for generating long-acting hLeptin variants that in combination with incretin/glucagon coagonists may provide a useful strategy for treatment of obesity and diabetes, e.g., fusion of incretins into other CDR loops of the hLeptin-CDR3H-coil-Herceptin fusion to create bifunctional antibody chimeras.
It is known that leptin functions by activating leptin receptors located in the central nervous system and peripheral tissues (45, 46). We therefore analyzed whether hLeptin-CDR3H-coil-Herceptin up-regulated gene expression in liver and hypothalamus at multiple time points by quantitative PCR in mice treated with the fusion protein (Table 2). Three representative organ-specific leptin-regulated genes were selected, which include Glucokinase (GCK) (47), NADH dehydrogenase (ubiquinone) 1 α subcomplex (Ndufa8) (48), and cytokine signaling 3 (SOCS3) (49). GCK is a ubiquitously expressed, key metabolic gene with strong responsiveness to hLeptin. Upon treatment for 2 h with hLeptin-CDR3H-coil-Herceptin, GCK expression is significantly increased in liver and hypothalamus and gradually returns to baseline levels after 6 h. SOCS3 is a key regulator of diet-induced leptin in hypothalamic neurons that shows strong hypothalamic localization (49). Upon hLeptin–antibody fusion treatment, expression of SOCS3 is significantly up-regulated in the hypothalamus. Ndufa8 is a marker gene for the leptin-regulated mitochondrial oxidative phosphorylation (OXPHOS) pathway, which is significantly overrepresented in brain (48). We found that Ndufa8 was up-regulated in hypothalamus upon hLeptin–Herceptin treatment, whereas no significant difference was observed in liver. These results are consistent with the in vivo activity observed for hLeptin-CDR3H-coil-Herceptin.
Table 2.
Quantitative PCR analysis of gene expression regulated by leptin in mice treated with the hLeptin-coil-Herceptin fusion protein
| Liver | Hypothalamus | |||||
| Time, h | 2 | 4 | 6 | 2 | 4 | 6 |
| GCK | 2.16 ± 0.52* | 1.52 ± 0.65 | 0.80 ± 0.32 | 2.60 ± 1.05 | 0.80 ± 0.34 | 1.15 ± 0.41 |
| Ndufa8 | 1.02 ± 0.22 | 1.18 ± 0.22 | 1.10 ± 0.29 | 1.99 ± 0.14** | 0.93 ± 0.21 | 1.17 ± 0.26 |
| SOCS3 | 7.59 ± 1.15* | 4.69 ± 1.16* | 1.14 ± 0.30 | 5.58 ± 1.97* | 3.07 ± 0.91* | 2.22 ± 0.77 |
Data are presented as mean ± SEM of the normalized expression levels. *P < 0.05 vs. PBS treatment group; **P < 0.01 vs. PBS treatment group; n = 3.
Conclusion
We have successfully developed a strategy to fuse the human endocrine hormones hGH and hLeptin as functional domains into the CDRs of the humanized therapeutic antibody Herceptin. The resulting fusion proteins express in mammalian cells in good yields and maintain the biological activity of the native hormone in vitro. Pharmacokinetic analysis revealed extended half-lives of both fusion proteins, which result in significantly enhanced in vivo efficacy in rodent models. This work together with previous studies (16–18) suggests a general and versatile strategy for the efficient generation of antibody agonists that can be developed as long-acting protein therapeutics. For example, this approach should be applicable to more complex two-chain proteins such as insulin and relaxin, bifunctional antibody agonists such as an Exendin 4-hLeptin coagonist, and bispecific antibodies for cancer immunotherapy.
Materials and Methods
Construction of Hormone–Antibody Fusion Expression Vectors.
The genes encoding human GH and human leptin were synthesized by GenScript and amplified by PCR using PfuUltra II DNA polymerase (Agilent Technologies). The cloning of hGH–BLV1H12 was performed similarly as described (16, 17). The genes encoding Herceptin heavy chain and light chain were synthesized by IDT and amplified by PCR. The fusion genes were assembled through overlapping PCR and digested using restriction enzymes EcoRI-HF and NheI-HF (New England Biolabs). The final mammalian expression vectors of the Herceptin-derived antibody fusions were constructed by in-frame ligation of the assembled genes into the pFuse backbone vector (InvivoGen) using T4 DNA ligase (New England Biolabs). The resulting mammalian expression vectors were confirmed by DNA sequencing.
Expression and Purification of Fusion Proteins.
Fusion proteins were expressed through transient transfection of FreeStyle 293F cells (Life Technologies) with the constructed pFuse expression vectors for secretion into culture media. FreeStyle 293F suspension cells were cultured at 37 °C with 5% (vol/vol) CO2 in FreeStyle 293 expression medium (Life Technologies) in shaker flasks (125 rpm). At a density of 106 cells per mL, 293F cells were transfected with heavy- and light-chain plasmids in the presence of 293fectin (Life Technologies) at a ratio of 2:1: 6, as suggested by the manufacturer. Expression medium containing secreted proteins was harvested every 48 h twice after transfection. Fusion proteins were purified by protein G chromatography (Thermo Fisher Scientific) according to the manufacturer’s instruction and analyzed by SDS/PAGE.
NB2 Proliferation Assay.
The rat Nb2-11 cell line from Sigma was maintained in RPMI with 10% (vol/vol) FBS, 10% (vol/vol) horse serum (Life Technologies), and 55 µM 2-mercaptoethanol (Life Technologies). Proliferation assays were performed in a 96-well culture plate with 50,000 cells in 200 µL assay medium (10% horse serum in RPMI with 55 µM 2-mercaptoethanol) per well. Cells were incubated with varied concentrations of antibody fusions for 72 h. At the end of the treatment, 20 µL PrestoBlue (Life Technologies) was added to each well, and fluorescence signal was recorded on a SpectraMax fluorescence plate reader at 590 nm with 550-nm excitation.
hGHR-Ba/F3 Proliferation Assay.
Murine Ba/F3 cells were stably transduced with hGHR. Clonal selected hGHR-Ba/F3 cells were maintained in 10% FBS in RPMI1640 with 50 ng/mL hGH. Proliferation assays were performed in 96-well culture plates with 20,000 cells in 200 µL assay medium (10% FBS in RPMI1640) per well. Varied concentrations of fusion antibodies were incubated with cells for 72 h. At the end of incubation, 20 µL PrestoBlue was added to each well, and fluorescence signal was recorded on a SpectraMax fluorescence plate reader at 590 nm with 550-nm excitation.
STAT5 Phosphorylation Assay.
Human IM9 cells from ATCC were maintained in 10% FBS in RPMI1640. Cells (2 × 105) were seeded in V-bottom 96-well plates in 200 µL assay medium (1% charcoal-stripped FBS in RPMI) and starved overnight. Starved cells were stimulated with hGH and antibody fusion proteins at varied concentrations for 10 min at 37 °C. After stimulation, cells were fixed by 4% formaldehyde at 37 °C for 10 min and permeabilized with 90% methanol. Cells were then blocked with 5% (g/mL) BSA at room temperature for 10 min and stained with Alexa Fluor 488-conjugated anti-pSTAT5 (Tyr694) (C71E5) rabbit mAb (Cell Signaling Technology) following the manufacturer-suggested protocol. Cells were then washed with PBS and analyzed by LSR II flow cytometer (Becton Dickinson).
Flow Cytometry Analysis of HER2 Binding.
Cells were blocked with blocking buffer (PBS supplemented with 3% BSA) at 4 °C for 10 min and then incubated with 1.5 µg/mL antibody fusion proteins in blocking buffer for 1 h. Cells were then washed with PBS and incubated with Alexa Fluor 647-conjugated goat anti-human IgG (Life Technologies) in blocking buffer following the manufacturer-suggested protocol. After incubation, cells were washed and analyzed by LSR II flow cytometer (Becton Dickinson).
Ba/F3-hObR Proliferation Assay.
Murine Ba/F3 cells were stably transduced with hLeptin. Clonal selected Ba/F3-hObR cells were maintained in 10% FBS in RPMI1640 with 20 nM hLeptin (R&D Systems). Proliferation assays were performed in 96-well culture plates with 20,000 cells in 200 µL assay medium (10% FBS in RPMI1640) per well. Increasing concentrations of antibody fusions were incubated with cells for 72 h. At the end of the incubation period, 20 µL PrestoBlue was added to each well and fluorescence signal was recorded on a SpectraMax fluorescence plate reader at 590 nm with 550-nm excitation.
All animals and pharmacology studies are detailed in SI Appendix, Materials and Methods. All animal experiments were approved by the Institutional Animal Care and Use Committee at the California Institute for Biomedical Research.
Supplementary Material
Acknowledgments
This work was supported by funding from the California Institute for Biomedical Research to The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423668112/-/DCSupplemental.
References
- 1.Leader B, Baca QJ, Golan DE. Protein therapeutics: A summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 2.Nussey S, Whitehead S. Endocrinology: An Integrated Approach. BIOS Scientific; Oxford: 2001. [PubMed] [Google Scholar]
- 3.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99(6):2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143–154. doi: 10.4158/EP.14.2.143. [DOI] [PubMed] [Google Scholar]
- 5.Cawley P, Wilkinson I, Ross RJ. Developing long-acting growth hormone formulations. Clin Endocrinol (Oxf) 2013;79(3):305–309. doi: 10.1111/cen.12240. [DOI] [PubMed] [Google Scholar]
- 6.Laursen T, et al. Long-term effects of continuous subcutaneous infusion versus daily subcutaneous injections of growth hormone (GH) on the insulin-like growth factor system, insulin sensitivity, body composition, and bone and lipoprotein metabolism in GH-deficient adults. J Clin Endocrinol Metab. 2001;86(3):1222–1228. doi: 10.1210/jcem.86.3.7323. [DOI] [PubMed] [Google Scholar]
- 7.Bendele A, Seely J, Richey C, Sennello G, Shopp G. Short communication: Renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol Sci. 1998;42(2):152–157. doi: 10.1006/toxs.1997.2396. [DOI] [PubMed] [Google Scholar]
- 8.Gaberc-Porekar V, Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Devel. 2008;11(2):242–250. [PubMed] [Google Scholar]
- 9.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22(6):868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Chou K, Perry CM. Metreleptin: First global approval. Drugs. 2013;73(9):989–997. doi: 10.1007/s40265-013-0074-7. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: The effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93(1):26–31. doi: 10.1210/jc.2007-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppari R, Bjørbæk C. Leptin revisited: Its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11(9):692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safar Zadeh E, et al. The liver diseases of lipodystrophy: The long-term effect of leptin treatment. J Hepatol. 2013;59(1):131–137. doi: 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, et al. Reshaping antibody diversity. Cell. 2013;153(6):1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Functional antibody CDR3 fusion proteins with enhanced pharmacological properties. Angew Chem Int Ed Engl. 2013;52(32):8295–8298. doi: 10.1002/anie.201303656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. An antibody CDR3-erythropoietin fusion protein. ACS Chem Biol. 2013;8(10):2117–2121. doi: 10.1021/cb4004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, et al. Rational design of CXCR4 specific antibodies with elongated CDRs. J Am Chem Soc. 2014;136(30):10557–10560. doi: 10.1021/ja5042447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: Crystal structure of the complex. Science. 1992;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 20.Walsh ST, Sylvester JE, Kossiakoff AA. The high- and low-affinity receptor binding sites of growth hormone are allosterically coupled. Proc Natl Acad Sci USA. 2004;101(49):17078–17083. doi: 10.1073/pnas.0403336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popp MW, Dougan SK, Chuang TY, Spooner E, Ploegh HL. Sortase-catalyzed transformations that improve the properties of cytokines. Proc Natl Acad Sci USA. 2011;108(8):3169–3174. doi: 10.1073/pnas.1016863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozzola M, et al. Evaluation of growth hormone bioactivity using the Nb2 cell bioassay in children with growth disorders. J Endocrinol Invest. 1998;21(11):765–770. doi: 10.1007/BF03348043. [DOI] [PubMed] [Google Scholar]
- 23.Friesen HG. Receptor assays for growth hormone. Acta Paediatr Scand Suppl. 1990;370:87–91, discussion 92. doi: 10.1111/j.1651-2227.1990.tb11680.x. [DOI] [PubMed] [Google Scholar]
- 24.Cho H, et al. Optimized clinical performance of growth hormone with an expanded genetic code. Proc Natl Acad Sci USA. 2011;108(22):9060–9065. doi: 10.1073/pnas.1100387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleland JL, et al. A novel long-acting human growth hormone fusion protein (VRS-317): Enhanced in vivo potency and half-life. J Pharm Sci. 2012;101(8):2744–2754. doi: 10.1002/jps.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson IR, et al. A ligand-receptor fusion of growth hormone forms a dimer and is a potent long-acting agonist. Nat Med. 2007;13(9):1108–1113. doi: 10.1038/nm1610. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. An antibody with a variable-region coiled-coil “knob” domain. Angew Chem Int Ed Engl. 2014;53(1):132–135. doi: 10.1002/anie.201307939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baselga J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26(4 Suppl 12):78–83. [PubMed] [Google Scholar]
- 29.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21(2):309–318. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 30.Shak S. Herceptin Multinational Investigator Study Group Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Semin Oncol. 1999;26(4 Suppl 12):71–77. [PubMed] [Google Scholar]
- 31.Cho HS, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki N, Fujii N. Optimization of the loop length for folding of a helix-loop-helix peptide. Tetrahedron Lett. 1999;40(33):6013–6017. [Google Scholar]
- 33.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29(8):2613–2624. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 34.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276(9):6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 35.Osborn BL, et al. Albutropin: A growth hormone-albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur J Pharmacol. 2002;456(1–3):149–158. doi: 10.1016/s0014-2999(02)02644-4. [DOI] [PubMed] [Google Scholar]
- 36.Lundegaard C, Lund O, Nielsen M. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics. 2008;24(11):1397–1398. doi: 10.1093/bioinformatics/btn128. [DOI] [PubMed] [Google Scholar]
- 37.Calis JJ, et al. Properties of MHC class I presented peptides that enhance immunogenicity. PLOS Comput Biol. 2013;9(10):e1003266. doi: 10.1371/journal.pcbi.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peelman F, et al. Mapping of binding site III in the leptin receptor and modeling of a hexameric leptin⋅leptin receptor complex. J Biol Chem. 2006;281(22):15496–15504. doi: 10.1074/jbc.M512622200. [DOI] [PubMed] [Google Scholar]
- 39.Peelman F, et al. Mapping of the leptin binding sites and design of a leptin antagonist. J Biol Chem. 2004;279(39):41038–41046. doi: 10.1074/jbc.M404962200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387(6629):206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 41.Müller TD, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J Pept Sci. 2012;18(6):383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- 42.Kahler A, et al. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol. 1998;275(1 Pt 2):R180–R185. doi: 10.1152/ajpregu.1998.275.1.R180. [DOI] [PubMed] [Google Scholar]
- 43.Lo KM, et al. Engineering a pharmacologically superior form of leptin for the treatment of obesity. Protein Eng Des Sel. 2005;18(1):1–10. doi: 10.1093/protein/gzh102. [DOI] [PubMed] [Google Scholar]
- 44.Hill RA, Margetic S, Pegg GG, Gazzola C. Leptin: Its pharmacokinetics and tissue distribution. Int J Obes Relat Metab Disord. 1998;22(8):765–770. doi: 10.1038/sj.ijo.0800656. [DOI] [PubMed] [Google Scholar]
- 45.Huo L, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9(6):537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berglund ED, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122(3):1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma A, et al. Hepatic gene expression profiling reveals key pathways involved in leptin-mediated weight loss in ob/ob mice. PLoS ONE. 2010;5(8):e12147. doi: 10.1371/journal.pone.0012147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung YC, et al. Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci. 2008;28(47):12419–12426. doi: 10.1523/JNEUROSCI.3412-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10(7):739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.