Significance
Pertussis has reemerged as a major public health concern in many countries where vaccine uptake remains high and pertussis has been considered well controlled until recently. In our paper, we address the important scientific and practical problem of developing optimal booster vaccination schedules by using a genetic algorithm. Our results argue that booster vaccination schedules developed based on misdiagnosis of the problem are likely to be epidemiologically ineffective and economically costly.
Keywords: pertussis, booster schedule, immunization, genetic algorithm
Abstract
Pertussis has reemerged as a major public health concern in many countries where it was once considered well controlled. Although the mechanisms responsible for continued pertussis circulation and resurgence remain elusive and contentious, many countries have nevertheless recommended booster vaccinations, the timing and number of which vary widely. Here, using a stochastic, age-stratified transmission model, we searched for cost-effective booster vaccination strategies using a genetic algorithm. We did so assuming four hypothesized mechanisms underpinning contemporary pertussis epidemiology: (I) insufficient coverage, (II) frequent primary vaccine failure, (III) waning of vaccine-derived protection, and (IV) vaccine “leakiness.” For scenarios I–IV, successful booster strategies were identified and varied considerably by mechanism. Especially notable is the inability of booster schedules to alleviate resurgence when vaccines are leaky. Critically, our findings argue that the ultimate effectiveness of vaccine booster schedules will likely depend on correctly pinpointing the causes of resurgence, with misdiagnosis of the problem epidemiologically ineffective and economically costly.
Reconciling the historical successes of routine infant pertussis vaccination programs in reducing incidence (1–3) with the recent resurgence in a number of highly vaccinated countries (4, 5) has proved challenging, especially in the context of the global heterogeneity in contemporary pertussis epidemiology (6). A variety of explanations for pertussis resurgence have been proposed, ranging from improvements in surveillance and diagnostics (7) to reduced protection afforded by vaccination, whether due to the waning of vaccine-derived immunity (8), the evolution of the etiological agent (the bacterium Bordetella pertussis) (9), the switch from whole-cell to acellular vaccines (10), or the invasion and spread of Bordetella congeners (11). It has also been demonstrated that even in the absence of changes in the nature of transmission, vaccine, or reporting, pertussis resurgence might be expected in some countries as an inevitable consequence of insufficient historical vaccination (12).
Disentangling the many pathways to pertussis resurgence is particularly difficult because pertussis immunity and, in particular, vaccine-derived immunity are not well understood (13). With no known reliable serological marker of protection (14), the properties of infection- and vaccine-derived immunity against pertussis must be inferred indirectly (15). However, the models of pertussis immunity that best reconcile individual-level clinical data (16) and population-level incidence data (17), respectively, are strikingly different. Data from serological studies (16, 18) and animal models (19) paint a picture of a vaccine that protects against disease for a limited duration and may afford little protection against transmission. In contrast, large-scale pertussis incidence data from countries such as Denmark (20), England and Wales (21), Thailand (17), and Sweden (22, 23) are consistent with long-lasting vaccine-derived immunity and sufficiently little transmission among vaccinated and previously infected individuals as to generate herd immunity.
Despite the discussion surrounding the efficacy and epidemiological effectiveness of pertussis vaccines, several countries experiencing increased pertussis incidence have supplemented their existing routine infant immunization schedule with additional booster doses (24). Importantly, the timing and number of recommended booster doses vary considerably among countries. To examine the conundrum of how best to mitigate pertussis, we used a validated age-stratified transmission model integrated within a genetic algorithm (GA) framework. Our aim is to identify, assuming different causes of pertussis circulation, booster schedules that afford the greatest reduction in disease burden for the least economic cost.
Situational Analysis
Given the absence of consensus regarding the causes of ongoing pertussis transmission and resurgence, and potential heterogeneity underlying recent rises in incidence across different populations, we focused our attention on identifying successful booster schedules, assuming a subset of resurgence mechanisms described above. Because previous work on pertussis in the United States has indicated that resurgence substantially predated both the introduction of modern molecular diagnostics methods and the switch from whole-cell to acellular vaccines (5), we excluded these factors but discuss their potential impacts later. Instead, we focused on other candidate explanations, two that lead to persistent pertussis circulation and two that generate a clear rebound in incidence following a honeymoon period.
Scenario I: Insufficient Vaccine Coverage.
Perhaps the simplest reason behind the persistence of an infectious disease despite infant vaccination is inadequate uptake. This scenario would describe settings where logistic and economic factors may present significant barriers to large-scale immunization (25). According to the WHO, as of October 2013, in 10% of countries for which vaccination data are available, 80%, at most, of infants receive the recommended three doses of pertussis vaccine (26). Inadequate coverage may also result from the reluctance of a sizable fraction of the population to comply with recommended routine vaccination (27, 28), as evidenced by the strong spatial correlation between pertussis outbreaks and the concentration of unvaccinated or undervaccinated children in some US populations (28, 29). In our simulations for this scenario, we assumed an efficacious vaccine with 70% uptake (Fig. 1C).
Fig. 1.
Simulations generating the preboosting era initial conditions for GA runs. Preboosting incidence (solid red line) and infant vaccine uptake (dashed blue line) with 70% coverage (A), 70% efficacy (B), a 15-y mean duration of immunity (C), and leaky immunity preventing infection in 85.5% of risky contacts (D). In each case, we simulated 100 y prevaccine (plotted starting at year 50). In year 100, vaccination began at 60% of its eventual coverage level and increased linearly over 20 y. The various values for uptake, efficacy, waning, and leakiness were chosen to generate roughly similar DALYs lost before the introduction of boosters.
Scenario II: Low Efficacy.
Primary vaccine failure, where a vaccine sometimes fails to elicit protection when administered, is another mechanism by which a disease might persist despite infant vaccination efforts. For example, the outbreaks of pertussis in Canada beginning in the late 1980s have largely been attributed to the poor efficacy of the Connought Laboratories adsorbed vaccine that was used between 1985 and 1998 (30, 31). To explore this possibility, our simulations assumed 100% eventual vaccine uptake, with a 30% probability of failure in “take” (Fig. 1D).
Scenario III: Waning Vaccine Immunity.
Even if vaccination is widespread and initially efficacious, infant immunization programs alone may fail to achieve elimination if vaccine-derived immunity is temporary. Some waning of vaccine-derived immunity to pertussis is supported by data at both the population and individual scales, but whereas population-level patterns of incidence generally suggest long-lasting immunity for both whole-cell and acellular vaccines (17, 21, 22, 32), individual-level data on reinfections are better explained by a much shorter duration of protection (18). In this scenario, we assumed 100% uptake with a fully efficacious vaccine, with a mean duration of vaccine-derived immunity of 15 y (Fig. 1E). We also explored the impact of different durations of immunity, as discussed below.
Scenario IV: Leaky Immunity.
Finally, we examined vaccine “leakiness,” whereby, with some probability, a previously vaccinated individual may become infected upon exposure (33, 34). One potential mechanism for imperfect vaccine protection is strain polymorphism, such that the cocirculation of multiple strains of B. pertussis for which the vaccine induces limited cross-protection may permit a transmissible, symptomatic infection after contact with an infectious individual (35, 36). Thus, leaky vaccine-derived immunity is a further mechanism with the potential to facilitate the persistence of pertussis despite infant vaccination. Here, a vaccinated individual has a 14.5% probability of infection upon exposure (Fig. 1F).
In reality, it is likely that more than one of these mechanisms may be at play simultaneously in a given population. However, to disentangle the control impacts of each factor systematically, we make the pragmatic assumption that in each scenario, only the focal mechanism is responsible for ongoing chains of pertussis transmission. Given this simplifying assumption, it would be straightforward to evaluate the merits of introducing a single booster at a predefined age (22, 37–39). However, identifying successful, cost-effective, comprehensive booster schedules remains a nontrivial task because it requires the identification of appropriate timing, frequency, and uptake of boosting, stratified by age. Hence, this high dimensionality of the space of possible schedules, combined with the dynamical complexity of pertussis epidemiology (subject as it is to nonlinearity, stochasticity, and seasonal forcing), makes many traditional optimization tools ill-suited for the problem.
Here, we instead adopt a GA (40) to evolve cost-effective booster schedules by mimicking the operation of natural selection on a “population” of diverse schedules for booster vaccination (Methods and SI Appendix, section S2). Each strategy in the population is represented by a genotype that encodes its prescribed age-specific schedule of boosters (SI Appendix, Fig. S1A). Strategies receive reproductive opportunities based on their fitness (SI Appendix, Fig. S1B), which is calculated as a mixture of the disease burden and economic cost, both quantified in disability-adjusted life years (DALYs) lost (41). To evaluate the fitness of a strategy, we simulate transmission dynamics following the introduction of the booster schedule using our epidemiological model (Methods and SI Appendix, section S3). Strategies reproduce sexually, which allows for the generation of novel strategies via recombination (SI Appendix, Fig. S1C), and the resulting offspring have a chance to be further altered by point mutations (SI Appendix, Fig. S1D).
Because the past history of pertussis incidence and infant vaccination are known to have long-lasting effects on ongoing transmission dynamics (12), we simulated prebooster conditions with an identical history of vaccination and chose parameters of coverage, efficacy, duration, and leakiness to generate similar overall disease burden (measured in DALYs lost) in the two decades preceding the rollout of the candidate booster campaign (Fig. 1 C–F). Specifically, we assumed no vaccination for the first 100 y, after which routine immunization was introduced. In scenario I, uptake was assumed to be 40% in year 100, increasing to 70% over a 20-y period. In scenarios II–IV, uptake was 60% in year 100, eventually reaching complete coverage by year 120.
Visualizing the evolving population of booster schedules is challenging. Although the average strategy can provide insights into a GA’s overall behavior, we are often more interested in the “typical good strategy,” particularly if the algorithm has discovered multiple, comparably fit strategies. Continuing the analogy of booster schedules and genotypes, we are interested in the average high-fitness genotype that has been selected after multiple generations of selective pressure. To this end, we constructed a nearest-neighbor network of the most successful schedules found by the GA, with distance measured using the distance (sometimes called the taxi cab distance) between chromosomes. To find clusters in this network with high modularity (i.e., many connections within a cluster and few between clusters), we applied a multiway spectral community detection algorithm (42), which looks for a division based on the leading eigenvectors of the modularity matrix of the network. This method allows us to visualize the distribution of alleles within each cluster of schedules in a more intuitive way, such as looking at the interquartile range of booster coverage in each age group, without losing as much information about the correlation between traits as we would by looking at the same summary statistics over the whole population.
Fittest Strategy Families
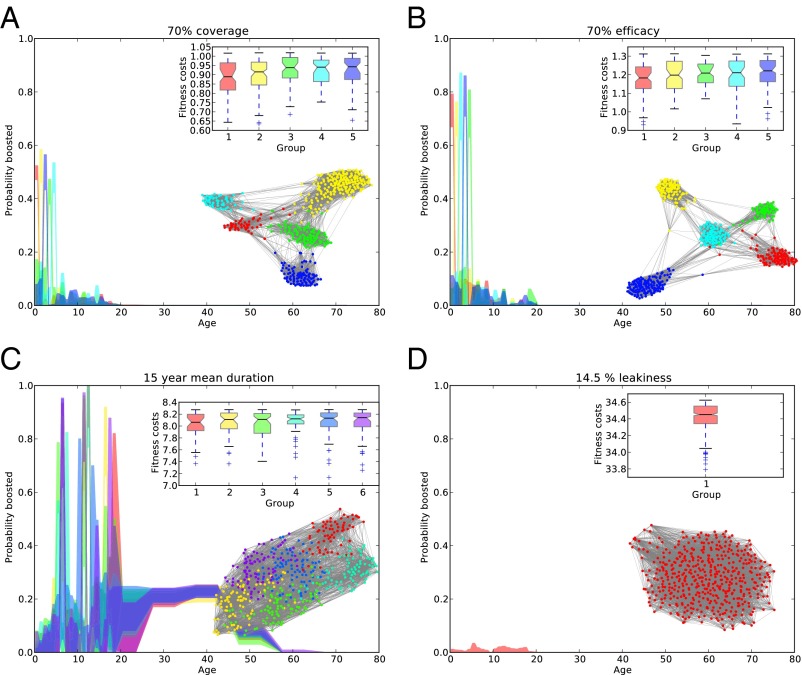
In all four scenarios, the GA rapidly converged on a population of booster schedules with lower combined vaccination and disease costs than the initial, randomly generated population of strategies (SI Appendix, Fig. S2). When the pertussis burden was primarily due to low coverage in the routine schedule (scenario I) or low vaccine efficacy (scenario II), the successful booster schedules found by our GA had, on average, very little vaccination in older age groups, focusing most of their effort on targeting young children (Fig. 2 A and B); this strategy achieved greater disease reduction at lower vaccination effort than the initial random population of strategies (SI Appendix, Fig. S3A). Specifically, for scenario I, we found five distinct clusters of strategies, each corresponding to the introduction of a single booster dose at the age of 1, 2, 3, 4, or 5 y (Fig. 2A). Our results for a low-efficacy vaccine were very similar (Fig. 2B and SI Appendix, Fig. S3B), with the exception that a greater vaccination effort was necessary to achieve the same effective coverage from the booster vaccine. In both cases, the most effective strategies came in the form of a single preschool booster, the precise timing of which had little effect on fitness as long as it came before children entered primary school, with the attendant high contact rates and strong age-assortative mixing (SI Appendix, Fig. S4 A and B). The results for scenarios I and II are explained by examining the right eigenvector of the next-generation matrix (see Fig. 5B), which quantifies the age-specific contribution to transmission. It indicates a steep increase in transmission after the transition from the toddler to primary school ages.
Fig. 2.
Families of successful booster strategies. Best booster schedules found for 70% infant coverage (A), 70% efficacy (B), a 15-y mean duration of immunity (C), and leaky immunity preventing 85.5% of infections (D) are shown. The best 500 booster schedules found in the last algorithm generation of each scenario are arranged into a network, where each schedule is connected to the nearest 25 (under distance between genomes) strategies. The strategies are clustered using a spectral partitioning algorithm (cluster indicated in the figure by color). The large plot shows the interquartile radius within each cluster of coverage at each age group (colors in plot correspond to colors in network). (Insets) Plot shows the fitness costs (in DALYs lost) of strategies in each family, ordered from most fit (red, Left) to the least fit (purple, Right).
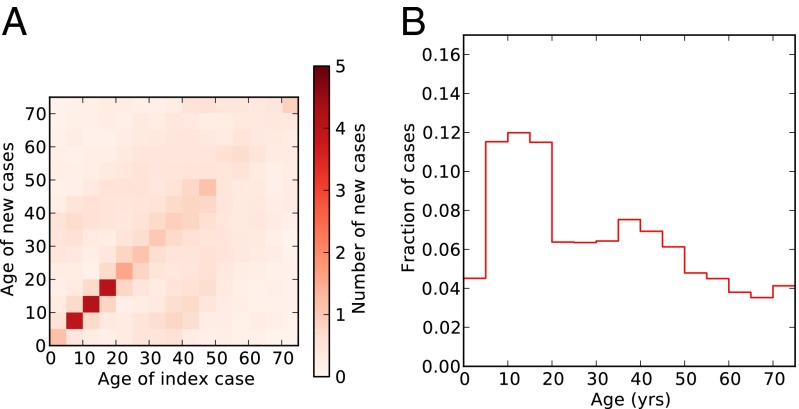
Fig. 5.
Contact structure. (A) Heat map shows the next-generation matrix: the number of cases in each age group expected to result from an index case in a given age group, with the column indicating the age group of the index case, the row indicating the age group of the resulting new cases, and shading indicating the number of cases (darker red for more cases). (B) Leading eigenvector (corresponding to eigenvalue 7.40) of the contact matrix (i.e., the age distribution of cases expected to replicate most quickly while retaining the same age distribution).
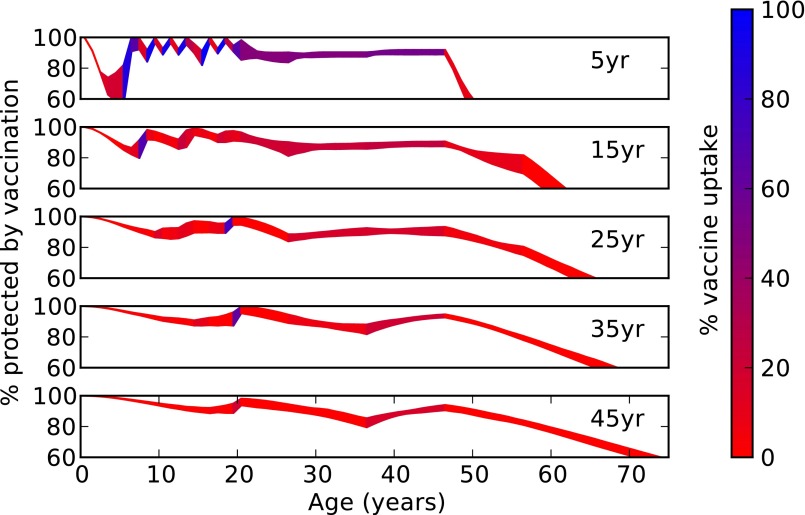
Booster schedules for waning vaccine-derived immunity (scenario III) differ greatly from those booster schedules evolved to deal with low coverage or efficacy (Fig. 2C). In this case, assuming a 15-y average duration of immunity in vaccinees, the most successful schedules call for a series of three boosters in the 6- to 19-y-old age groups approximately every 5 y, followed by additional boosters between 25 and 45 y of age. To dissect the evolution of strategies aimed at combatting waning vaccine immunity, we explored the population immunity profile by age as the mean duration of immunity varied (Fig. 3). Successful strategies aim to maintain herd immunity by ensuring the proportion of the population protected typically remains above ∼80%. Exceptions to this pattern occur when waning leads to an increased susceptible population in age groups with low transmission potential (Fig. 3), such as toddlers or the elderly (see Fig. 5).
Fig. 3.
Booster strategies to combat waning immunity. The most fit booster schedules under 5-y, 15-y, 25-y, 35-y, and 45-y mean durations of immunity, respectively, are plotted in the five panels. In each case, the 500 best strategies found during the past 25 generations of the GA are used. (In the case of 5-y immunity and 15-y immunity, the GA ran for 100 and 50 generations, respectively. All other cases ran for 25 generations.) The percentage of individuals protected by vaccination (in the absence of pertussis) is plotted for each age, with the interquartile range among the 500 strategies shaded in. The color of the shading indicates the median vaccine coverage among the strategies for that age, with blue indicating high levels of vaccination.
Finally, leaky immunity (scenario IV) presents a significantly different picture (Fig. 2D). The GA found no booster schedules that effectively reduce disease burden (SI Appendix, Fig. S3J). The nearest-neighbor network of the most successful strategies reveals no discernible community structure, and the best strategies eschew booster vaccination altogether (Fig. 2D). Again, the explanation lies in the patterns of susceptibility in the population. With infant vaccination already granting leaky protection to everyone, any remaining transmission already occurs among vaccinated individuals and cannot be disrupted by additional boosters with the same leaky vaccine (SI Appendix, Fig. S4D). We also examined the possibility that booster doses serve to reduce leakiness. Here, the best strategies resemble those strategies for a low level of primary vaccine failure, targeting the youngest age groups (SI Appendix, Fig. S5).
To quantify the penalties incurred by misdiagnosing the underlying reason for resurgence, we systematically implemented the best strategy for each scenario against all scenarios (Fig. 4A). As mentioned above, if pertussis resurgence is caused by pathogen evolution or strain polymorphism that renders the vaccine leaky (scenario IV), no booster strategy with a vaccine of the same composition can alleviate the problem. For scenario III, it is evident that correct identification of the cause of pertussis burden (waning vaccine immunity) and its associated parameter (duration of immunity) is paramount because ill-suited boosting strategies lead to high disease incidence (Fig. 4B). Our analyses indicate that even when the best booster schedule is adopted, the greatest overall costs arise when vaccine-derived immunity is transient, in large part, due to the expense associated with administering frequent boosters (Fig. 4C). Perhaps not surprisingly, there is only a small epidemiological cost to pay when mistaking insufficient uptake (scenario I) for primary vaccine failure (scenario II) and vice versa, given the broad similarity of their best boosters. In all cases, we found the overwhelming penalty is the failure of the incorrect strategy in suppressing disease (Fig. 4B) rather than economic costs via inefficient vaccine use (Fig. 4C). Similarly, we also examined the consequences of incorrectly estimating the duration of vaccine-derived immunity (SI Appendix, Fig. S6). Not surprisingly, there is a substantial epidemiological cost that is paid for overestimating the duration of immunity, with boosters administered too infrequently to break chains of transmission (SI Appendix, Fig. S6B). In contrast, there is a lesser economic burden incurred when immunity is underestimated (SI Appendix, Fig. S6C). Superficially, this result might appear to argue in favor of an overeager boosting strategy when vaccine-derived immunity is known to be temporary; however, this conclusion would need to be tempered by considering the concomitant evolutionary consequences of such a strategy (43, 44).
Fig. 4.
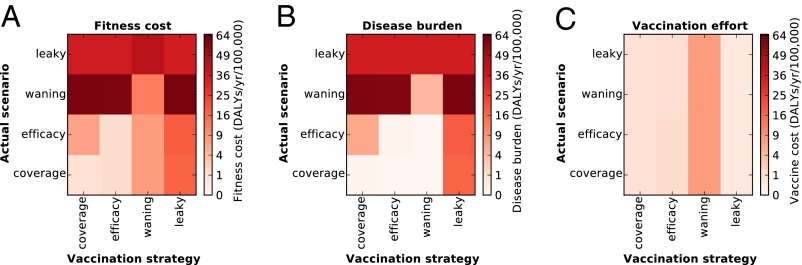
Costs of misapplication of strategies. (A) Total fitness costs (combined vaccination cost and disease burden) of applying a boosting schedule evolved for each scenario in each other scenario. The representative boosting schedules are constructed by taking the mean coverage at each age in the most fit strategy family (as shown in Fig. 2). These representative schedules (strategy used) are applied to each scenario (actual scenario), and the mean fitness cost among 120 replicant runs is plotted. The disease burden (B) and cost of vaccination effort (C) of these same strategies are plotted (so that A shows the sum of B and C).
Discussion
Complex applied problems are ubiquitous in epidemiology, and making predictions or policy recommendations frequently involves grappling with high-dimensional and hard-to-predict systems. In recent years, public health policy makers have made use of models to conduct a cost-benefit analysis of interventions for a number of high-profile infectious diseases, including rubella (45), the human papilloma virus (46), and influenza (47). Here, focusing on a comprehensive age-stratified schedule of pertussis vaccine boosters, we have adopted GAs as a useful tool for exploring complex optimization.
Our model is relatively simple and leaves out many putative mechanisms that may affect pertussis transmission and control, including the spatial or social aggregation of unvaccinated individuals (28), asymptomatic infections (48), and household structure (48). Other potential factors that could drive changes in the effectiveness of a booster vaccination strategy over the course of its use, such as vaccine-driven pathogen evolution, changing attitudes to vaccination, and changing public awareness of pertussis, are also excluded from our model. Perhaps equally importantly, our model does not attempt to mimic the real vaccination history and demographics of any particular location, instead exploring the relatively simple and defensible case of a linear increase in routine vaccine uptake after its introduction, followed by sustained high levels of infant vaccination. We stress that the precise structure of successful schedules we have obtained may be sensitive to the network of assumed contacts, which was derived from diary-based surveys of European populations (49).
Although the booster schedules discovered by the GA evolved in the absence of much of the complexity and uncertainty of the real world and are not intended to predict the quantitative impact of any particular vaccine schedule in any particular population, we submit that out results nevertheless have substantial implications for vaccine policy. Importantly, we have found that optimal booster schedules for controlling pertussis differ markedly depending on the mechanism responsible for the failure of routine immunization.
The four scenarios we considered are by no means an exhaustive exploration of the possible mechanisms by which an infant vaccine might fail to curtail the transmission of an infection. For example, we did not consider any scenarios incorporating diagnostic or surveillance changes that might generate the appearance of resurgence. The reason for this omission is that, in our GA, selection acts on the actual number and age distribution of infections, with a disproportionate contribution to DALYs lost by infant cases. Hence, the strategies that evolve in any scenario would not be affected by changes in reporting, including improved surveillance leading to an apparent resurgence.
Similarly, we did not investigate the possibility that vaccination may protect against disease but not transmission (19). In such a scenario, regardless of the strategy adopted, the ongoing dynamics of pertussis transmission would remain identical to the dynamics of pertussis transmission of the prevaccine era, with the incidence of disease in each age group simply reduced by the fraction of individuals who are protected by vaccination. Thus, in the case where this nonsterilizing immunity is lifelong, we expect the optimal strategy would be to administer a single dose of vaccine as early as possible so as to protect as much of the population as possible from disease. However, if this protection from disease wanes over time, we would expect a strategy with boosters continuing throughout life, timed to balance the cost of vaccination with the probability that immunity has waned since the last dose.
Another potential mechanism behind resurgence is natural immune boosting, which has previously been shown to have complex, often counterintuitive, interactions with infant vaccination (50, 51). We did not include this possibility on the basis of the epidemiological data. Some studies have found no empirical evidence in support of the immune boosting hypothesis (17, 21), whereas others have found that its epidemiological impact is small, even if statistically significant (52).
Our results reinforce the importance of ongoing efforts to understand vaccine-derived pertussis immunity better because it is central to developing cost-effective control strategies. If the cause of the resurgence is vaccine leakiness, then no worthwhile booster strategies are able to combat this problem (53), pointing toward the need for new vaccines (54). Our findings also emphasize the need for trouble-shooting pertussis resurgence; misdiagnosis of the problem will lead to implementing economically costly control measures with little or no epidemiological gains.
Methods
Transmission Dynamics.
We use a standard compartmental transmission model (55, 56) of pertussis that incorporates empirical age-specific rates of contact from the POLYMOD study (49). This model was previously parameterized using data from the vaccine-free period in Sweden and was validated by predicting age-specific pertussis incidence following the transition to infant immunization (22). Individuals are born susceptible, with routine infant vaccination occurring at 5 mo of age in order to mimic the protective effects afforded following the receipt of two doses of pertussis vaccine. Susceptible individuals become exposed through contact with infectious individuals and, after a latent period, become infectious. After recovering, individuals are immune to further infection. We assume infection-derived immunity to be lifelong, based on previous epidemiological studies of pertussis in Sweden (22), England and Wales (21), and Thailand (17), which found that repeat infections contributed very little to transmission (57). The number of individuals of each age in each state (susceptible, exposed, infectious, recovered, and vaccinated) is updated stochastically (56). The model equations and associated parameter values are presented in detail in SI Appendix, section S3.
To set the context for our results, in Fig. 5A, we present the next generation matrix, K, whose entries represent the expected number of new cases in age group i resulting from a single index case of age j in a naive population. School-aged children have the highest total contact rates, followed by 35- to 45 y-olds. However, the contacts of 35- to 45-y-olds are spread among a wide range of age groups, whereas mixing among school-aged children is very strongly age-assortative (49). Thus, although infections in school-aged children and 35- to 45-y-olds both generate a relatively large number of new infections, school-aged children tend to infect children their own age, who also have high contact rates, whereas the infectious contacts of 35- to 45-y-olds will include a wide range of age groups with varying frequency of contacts. This difference in transmission impact can be seen by repeated multiplication of the next-generation matrix, which, in the long term, will pick out the structure of the leading right eigenvector of the contact matrix (Fig. 5B). By analogy, if the contact matrix is thought of as a weighted network, with nodes corresponding to age groups and the weights of edges corresponding to the contact rates between them, then the entries of this eigenvector define the eigenvector centrality of each age group in the contact network. Fig. 5B clearly emphasizes the epidemiological significance of the school-aged groups, followed by those individuals aged 35–45 y.
GA.
We encoded booster schedules on a single chromosome containing the probability of vaccination for each age cohort, to be carried out at the start of the each school term. This encoding, which contains the “blueprint” for the schedule, can be considered the genotype of the strategy. Similarly, the simulated transmission dynamics under a specific booster schedule can be considered the phenotype of that strategy, with the combined costs of cumulative infections and vaccinations incurred during the simulation determining the strategy’s fitness. The burden of infections was quantified using the expected DALYs lost (41). For simplicity, we assumed that the economic costs of vaccination scale linearly with the number of doses administered, rather than considering logistic aspects of vaccination effort, which may be nonlinear. Further details on the calculation of strategy fitness can be found in SI Appendix, section S1.
After each GA generation, an offspring population of booster schedules was generated. To do so, parent schedules were chosen using “tournament selection” (58), in which random pairs of booster schedules are selected (with replacement) to compete for the opportunity to reproduce. In each tournament, the booster schedule with higher fitness has a higher probability (fixed at 90%) of winning. Carrying out N such tournaments, where N is the population size (set to 2,000 individuals), yields a pool of N parents. Note that although more fit strategies are likely to appear more often as parents than less fit strategies, a strategy’s reproductive opportunities are only determined by its rank. Tournament selection has the benefit of reduced sensitivity to the particular fitness function used compared with a selection method relying on the actual fitness values of strategies.
Once N parents have been selected, these parents are divided into pairs and each (not necessarily unique) couple produces a pair of offspring. The parent chromosomes undergo cross-over at a randomly chosen point (SI Appendix, Fig. S1C). In each child chromosome, point mutations occur (SI Appendix, Fig. S1D) at a fixed rate, μ (set to 0.05 per site in our experiments, which yields an average of 1.25 mutations per chromosome). This process leaves us with a new generation of booster strategies, ready to be evaluated through simulation. It is worthwhile to note that the choice of how to encode strategies can have important effects on the ease with which this reproductive process generates and preserves improved strategies, affecting both linkage and epistasis between “genes” as well as the topographical features of the fitness space itself (59, 60).
Supplementary Material
Acknowledgments
We thank Aaron King, Micaela Martinez-Bakker, Matthieu Domenech de Celles, and Felicia Magpantay for stimulating discussion. We also thank Alison Galvani, Meagan Fitzpatrick, and an anonymous reviewer for their insightful comments on this manuscript. This work was supported by the Research and Policy in Infectious Disease Dynamics Program of the Science and Technology Directorate, Department of Homeland Security, the Fogarty International Center, NIH; by a research grant from the NIH (Grant R01AI101155); and by the Models of Infectious Disease Agent Study, National Institute of General Medical Sciences (Grant U54-GM111274).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415573112/-/DCSupplemental.
References
- 1.Stewart GT. In: International Symposium on Pertussis. Manclark CR, Hill JC, editors. US Department of Health; Bethesda, MD: 1978. pp. 262–282. [Google Scholar]
- 2.Rohani P, Earn DJ, Grenfell BT. Opposite patterns of synchrony in sympatric disease metapopulations. Science. 1999;286(5441):968–971. doi: 10.1126/science.286.5441.968. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, et al. Effect of vaccination on Bordetella pertussis strains, China. Emerg Infect Dis. 2010;16(11):1695–1701. doi: 10.3201/eid1611.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE. EUVAC-NET Group Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24(9):761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 5.Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics. 2011;3(3-4):183–188. doi: 10.1016/j.epidem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Jackson D, Rohani P. The increasing application of multiplex nuclei detection tests to the diagnosis of syndromic infections. Epidemiol Infect. 2013;142(1):1–11. doi: 10.1017/S0950268813002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherry JD. The science and fiction of the “resurgence” of pertussis. Pediatrics. 2003;112(2):405–406. doi: 10.1542/peds.112.2.405. [DOI] [PubMed] [Google Scholar]
- 8.Tartof SY, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics. 2013;131(4):e1047–e1052. doi: 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 9.Mooi FR, van Der Maas NA, De Melker HE. Pertussis resurgence: Waning immunity and pathogen adaptation—Two sides of the coin. Epidemiol Infect. 2013;142(4):685–694. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro ED. Acellular vaccines and resurgence of pertussis. JAMA. 2012;308(20):2149–2150. doi: 10.1001/jama.2012.65031. [DOI] [PubMed] [Google Scholar]
- 11.Pittet LF, Emonet S, Schrenzel J, Siegrist CA, Posfay-Barbe KM. Bordetella holmesii: An under-recognised Bordetella species. Lancet Infect Dis. 2014;14(6):510–519. doi: 10.1016/S1473-3099(14)70021-0. [DOI] [PubMed] [Google Scholar]
- 12.Riolo MA, King AA, Rohani P. Can vaccine legacy explain the British pertussis resurgence? Vaccine. 2013;31(49):5903–5908. doi: 10.1016/j.vaccine.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills KH. Immunity to Bordetella pertussis. Microbes Infect. 2001;3(8):655–677. doi: 10.1016/s1286-4579(01)01421-6. [DOI] [PubMed] [Google Scholar]
- 14.Farizo KM, Burns DL, Finn TM, Gruber MF, Pratt RD. Clinical evaluation of pertussis vaccines: US Food and Drug Administration regulatory considerations. J Infect Dis. 2014;209(Suppl 1):S28–S31. doi: 10.1093/infdis/jit532. [DOI] [PubMed] [Google Scholar]
- 15.Lavine JS, Rohani P. Resolving pertussis immunity and vaccine effectiveness using incidence time series. Expert Rev Vaccines. 2012;11(11):1319–1329. doi: 10.1586/erv.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kretzschmar M, Teunis PFM, Pebody RG. Incidence and reproduction numbers of pertussis: Estimates from serological and social contact data in five European countries. PLoS Med. 2010;7(6):e1000291. doi: 10.1371/journal.pmed.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackwood JC, Cummings DA, Broutin H, Iamsirithaworn S, Rohani P. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc Natl Acad Sci USA. 2013;110(23):9595–9600. doi: 10.1073/pnas.1220908110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkinson D. Duration of effectiveness of pertussis vaccine: Evidence from a 10 year community study. Br Med J (Clin Res Ed) 1988;296(6622):612–614. doi: 10.1136/bmj.296.6622.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014;111(2):787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen A, Larsen SO. Epidemiology of pertussis in Denmark: The impact of herd immunity. Int J Epidemiol. 1994;23(6):1300–1308. doi: 10.1093/ije/23.6.1300. [DOI] [PubMed] [Google Scholar]
- 21.Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 2009;5(10):e1000647. doi: 10.1371/journal.ppat.1000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science. 2010;330(6006):982–985. doi: 10.1126/science.1194134. [DOI] [PubMed] [Google Scholar]
- 23.Domenech de Celles M, Riolo MA, Magpantay FMG, Rohani P, King AA. Epidemiological evidence for herd immunity induced by acellular pertussis vaccines. Proc Natl Acad Sci USA. 2014;111(7):E716–E717. doi: 10.1073/pnas.1323795111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zepp F, et al. Rationale for pertussis booster vaccination throughout life in Europe. Lancet Infect Dis. 2011;11(7):557–570. doi: 10.1016/S1473-3099(11)70007-X. [DOI] [PubMed] [Google Scholar]
- 25.Duclos P, Okwo-Bele J-M, Gacic-Dobo M, Cherian T. Global immunization: Status, progress, challenges and future. BMC Int Health Hum Rights. 2009;9(Suppl 1):S2. doi: 10.1186/1472-698X-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization Department of Immunization, Vaccines, and Biologicals (2013) World Health Organization Vaccine-Preventable Diseases: Monitoring System 2013 Global Summary. Available at apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragebcg.htm. Accessed May 19, 2014.
- 27.Gangarosa EJ, et al. Impact of anti-vaccine movements on pertussis control: The untold story. Lancet. 1998;351(9099):356–361. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- 28.Omer SB, et al. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–1396. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 29.Imdad A, et al. Religious exemptions for immunization and risk of pertussis in New York State, 2000-2011. Pediatrics. 2013;132(1):37–43. doi: 10.1542/peds.2012-3449. [DOI] [PubMed] [Google Scholar]
- 30.Halperin SA, Bortolussi R, MacLean D, Chisholm N. Persistence of pertussis in an immunized population: Results of the Nova Scotia Enhanced Pertussis Surveillance Program. J Pediatr. 1989;115(5 Pt 1):686–693. doi: 10.1016/s0022-3476(89)80643-2. [DOI] [PubMed] [Google Scholar]
- 31.Ntezayabo B, De Serres G, Duval B. Pertussis resurgence in Canada largely caused by a cohort effect. Pediatr Infect Dis J. 2003;22(1):22–27. doi: 10.1097/00006454-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Rohani P, Earn DJ, Grenfell BT. Impact of immunisation on pertussis transmission in England and Wales. Lancet. 2000;355(9200):285–286. doi: 10.1016/S0140-6736(99)04482-7. [DOI] [PubMed] [Google Scholar]
- 33.Halloran ME, Haber M, Longini IM., Jr Interpretation and estimation of vaccine efficacy under heterogeneity. Am J Epidemiol. 1992;136(3):328–343. doi: 10.1093/oxfordjournals.aje.a116498. [DOI] [PubMed] [Google Scholar]
- 34.Halloran ME, Longini IM, Struchiner CJ. Design and Analysis of Vaccine Studies. Springer; Berlin: 2010. [Google Scholar]
- 35.Preston NW. EFFECTIVENESS OF PERTUSSIS VACCINES. BMJ. 1965;2(5452):11–13. doi: 10.1136/bmj.2.5452.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kallonen T, He Q. Bordetella pertussis strain variation and evolution postvaccination. Expert Rev Vaccines. 2009;8(7):863–875. doi: 10.1586/erv.09.46. [DOI] [PubMed] [Google Scholar]
- 37.Hethcote HW. Simulations of pertussis epidemiology in the United States: Effects of adult booster vaccinations. Math Biosci. 1999;158(1):47–73. doi: 10.1016/s0025-5564(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 38.Edmunds WJ, Brisson M, Melegaro A, Gay NJ. The potential cost-effectiveness of acellular pertussis booster vaccination in England and Wales. Vaccine. 2002;20(9-10):1316–1330. doi: 10.1016/s0264-410x(01)00473-x. [DOI] [PubMed] [Google Scholar]
- 39.de Greeff SC, Mooi FR, Schellekens JFP, de Melker HE. Impact of acellular pertussis preschool booster vaccination on disease burden of pertussis in The Netherlands. Pediatr Infect Dis J. 2008;27(3):218–223. doi: 10.1097/INF.0b013e318161a2b9. [DOI] [PubMed] [Google Scholar]
- 40.Holland JH. Genetic algorithms. Sci Am. 1992;267:66–72. [Google Scholar]
- 41. World Health Organization Health Statistics and Information Systems (2014) Metrics: Disability-Adjusted Life Year (DALY). Available at www.who.int/healthinfo/global_burden_disease/metrics_daly/en/. Accessed July 23, 2014.
- 42.Riolo MA, Newman MEJ. 2014. First-principles multiway spectral partitioning of graphs. Journal of Complex Networks 2:121–140.
- 43.McLean AR. Vaccination, evolution and changes in the efficacy of vaccines: A theoretical framework. Proc Biol Sci. 1995;261(1362):389–393. doi: 10.1098/rspb.1995.0164. [DOI] [PubMed] [Google Scholar]
- 44.Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414(6865):751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- 45.Knox EG. Strategy for rubella vaccination. Int J Epidemiol. 1980;9(1):13–23. doi: 10.1093/ije/9.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769–a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science. 2009;325(5948):1705–1708. doi: 10.1126/science.1175570. [DOI] [PubMed] [Google Scholar]
- 48.de Greeff SC, et al. Pertussis disease burden in the household: How to protect young infants. Clin Infect Dis. 2010;50(10):1339–1345. doi: 10.1086/652281. [DOI] [PubMed] [Google Scholar]
- 49.Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Águas R, Gonçalves G, Gomes MGM. Pertussis: Increasing disease as a consequence of reducing transmission. Lancet Infect Dis. 2006;6(2):112–117. doi: 10.1016/S1473-3099(06)70384-X. [DOI] [PubMed] [Google Scholar]
- 51.Lavine JS, King AA, Bjørnstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proc Natl Acad Sci USA. 2011;108(17):7259–7264. doi: 10.1073/pnas.1014394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavine JS, King AA, Andreasen V, Bjørnstad ON. Immune boosting explains regime-shifts in prevaccine-era pertussis dynamics. PLoS ONE. 2013;8(8):e72086. doi: 10.1371/journal.pone.0072086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomes MGM, White LJ, Medley GF. The reinfection threshold. J Theor Biol. 2005;236(1):111–113. doi: 10.1016/j.jtbi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Clark TA, Messonnier NE, Hadler SC. Pertussis control: Time for something new? Trends Microbiol. 2012;20(5):211–213. doi: 10.1016/j.tim.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Anderson RM, May RM. Infectious Diseases of Humans. Oxford Univ Press; Oxford: 1991. [Google Scholar]
- 56.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton Univ Press; Princeton: 2008. [Google Scholar]
- 57.Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: A comparative study in 64 countries. Proc R Soc Lond B Biol Sci. 2010;282(1800):1–7. doi: 10.1098/rspb.2010.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg DE, Deb K. A comparative analysis of selection schemes used in genetic algorithms. Urbana. 1991;51:69–92. [Google Scholar]
- 59.Davidor Y. Epitasis variance: Suitability of a representation to genetic algorithms. Complex Systems. 1990;4:369–383. [Google Scholar]
- 60.Jones T, Forrest S. Fitness distance correlation as a measure of problem difficulty for genetic algorithms. In: Eshelman LJ, editor. Proceedings of the Sixth International Conference on Genetic Algorithms. Morgan Kaufmann; San Francisco: 1995. pp. 184–192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.