Significance
Viral infections still constitute a major health issue. Upon viral infection, interferon (IFN) elicits an antiviral response by activating multiple effector systems. The ubiquitin-like protein ISG15 is strongly induced by IFN and mediates its antiviral effect by being covalently conjugated to cellular and viral proteins. The protease USP18, which is also an important negative regulator of the IFN response, counteracts ISG15 conjugation. Within this study, we have generated knock-in mice expressing USP18, which selectively lacks protease activity. ISG15 modification was enhanced in these animals, but the IFN response was unaltered. This clearly shows that USP18 has enzymatic and nonenzymatic properties in vivo. Elevated ISGylation increased resistance to influenza B infections, qualifying USP18 protease inhibition as a potential antiviral strategy.
Keywords: ISG15, UBP43, interferon, ubiquitin isopeptidase, influenza
Abstract
Protein modification by the ubiquitin-like protein ISG15 is an interferon (IFN) effector system, which plays a major role in antiviral defense. ISG15 modification is counteracted by the isopeptidase USP18, a major negative regulator of IFN signaling, which was also shown to exert its regulatory function in an isopeptidase-independent manner. To dissect enzymatic and nonenzymatic functions of USP18 in vivo, we generated knock-in mice (USP18C61A/C61A) expressing enzymatically inactive USP18. USP18C61A/C61A mice displayed increased levels of ISG15 conjugates, validating that USP18 is a major ISG15 isopeptidase in vivo. Unlike USP18−/− mice, USP18C61A/C61A animals did not exhibit morphological abnormalities, fatal IFN hypersensitivity, or increased lethality, clearly showing that major USP18 functions are unrelated to its protease activity. Strikingly, elevated ISGylation in USP18C61A/C61A mice was accompanied by increased viral resistance against vaccinia virus and influenza B virus infections. Enhanced resistance upon influenza B infection in USP18C61A/C61A mice was completely reversed in USP18C61A/C61A mice, which additionally lack ISG15, providing evidence that the observed reduction in viral titers is ISG15 dependent. These results suggest that increasing ISGylation by specific inhibition of USP18 protease activity could constitute a promising antiviral strategy with only a minimal risk of severe adverse effects.
Protein modification by the ubiquitin-like (UBL) modifier interferon (IFN)-stimulated gene 15 (ISG15) is strongly induced by type I IFNs and represents one of the major antiviral IFN effector systems (1). ISG15 has weak sequence identity to ubiquitin and is structurally characterized by two ubiquitin-like domains connected by a short linker region (2). In analogy to ubiquitin, ISG15 is covalently conjugated to protein targets via the consecutive action of an E1-activating enzyme (Ube1L) (3), an E2-conjugating enzyme (UbcH8) (4), and a few E3 ligases. All enzymes involved in the conjugation process are themselves strongly inducible by type I IFNs. The major E3 ligases for human and murine ISG15 modification (ISGylation) are hHERC5 (5, 6) and mHERC6 (7, 8), respectively. hHERC5 is associated with polyribosomes and newly synthesized proteins are modified by ISG15 in a cotranslational manner (9). Thus, it was suggested that ISG15 modification of virus-derived proteins, which are the most prevalent translational products in a virus-infected cell, mediates the antiviral activity of the ISGylation system. In line with this suggestion, ISG15 modification of the human papilloma virus protein HPV16 L1 interfered with virus assembly and decreased the infectivity of HPV in a dominant manner (9). Moreover, ISGylation of the influenza virus protein NS1A prevents interaction with importin-α and thereby interferes with the capability of NS1A to counteract host defense (10). ISG15 is also conjugated to cellular proteins. For example, ISGylation increases the stability of the IFN regulatory factor 3 (IRF3), resulting in enhanced activation of IRF3 target genes (11, 12). Consistent with the antiviral function, mice lacking ISG15 are more susceptible to influenza A and B, Sindbis, and herpes virus infections (13). However, antiviral responses against vesicular stomatitis virus (VSV) and lymphocytic choriomeningitis virus (LCMV) are not altered (14). For both influenza B and Sindbis virus infection, the antiviral function of ISG15 is dependent on its ability to form conjugates because mice lacking the ISG15 E1 enzyme, UbE1L, are also susceptible to these infections (15, 16). Several viruses including influenza B virus, vaccinia virus (VACV), and Crimean Congo hemorrhagic fever virus (CCHFV) have evolved strategies to target ISG15 conjugation, further suggesting an important antiviral role of ISGylation (3, 17–19).
Conjugation of ISG15 to its substrates is counteracted by the activity of ubiquitin-specific protease 18 (USP18/UBP43) (20). In vitro studies also provided evidence for other USPs to be cross-reactive for ISG15, but the relevance of these isopeptidases for deISGylation in vivo is unclear (21). Beyond enhanced ISGylation, USP18 knockout (USP18−/−) mice develop severe brain malformations and have a shortened lifespan (22). In addition, these animals fail to properly terminate IFN-signaling, exhibit enhanced IFN target gene expression, and show lethal hypersensitivity to poly(I:C) injections (23). Furthermore, IFN desensitization, which assures long-term refractoriness of the IFN signaling pathway, is abrogated in USP18−/− mice (24), underlining the role of USP18 as a critical negative regulator of the IFN pathway. USP18−/− mice also exhibit increased resistance to intracranial (i.c.) infections with VSV and LCMV (25). The phenotypic alterations seen in USP18−/− mice were first exclusively ascribed to enhanced ISG15 modification. However, subsequent analysis of mice doubly deficient for either USP18 and ISG15 (26) or USP18 and Ube1L (27) surprisingly revealed that the lack of ISGylation cannot rescue the brain injuries and is not able to overcome the lethality induced by poly(I:C) in USP18−/− mice. Furthermore, irrespective of its protease activity, USP18 was shown to compete with JAK1 for binding to the IFNAR2, thereby negatively regulating the type I IFN response (28) in an isopeptidase-independent manner.
To differentiate between enzymatic and nonenzymatic physiological functions of USP18 and to study the consequences of selective inhibition of USP18 protease activity in vivo, we have now generated and characterized knock-in mice expressing mutant USP18 protein selectively lacking the isopeptidase activity.
Results
Generation of Mice Expressing Catalytically Inactive USP18 Protein.
In murine USP18 the cysteine at position 61 (Cys61) was reported to be essential for protease activity (20). To validate that mutation of Cys61 inactivates the isopeptidase activity, USP18 carrying an N-terminal S-peptide epitope tag (S-USP18) and mutants thereof were expressed in HEK293T cells. In particular the codons of USP18 for either Cys61 (C61A), Cys62 (C62A), or both (C61A/C62A) were replaced by those encoding for alanine. Subsequently, protein extracts of transfected cells were analyzed for the ISG15-specific reactivity of USP18 and its derivatives using ISG15-vinylsulfone (ISG15-VS). ISG15-VS acts as an active site-directed probe and irreversibly reacts with the catalytic core of ISG15 proteases. ISG15-VS was readily linked to WT USP18 and USP18C62A but neither USP18C61A nor USP18C61A/C62A did react with ISG15-VS (Fig. S1A), showing that Cys61 is essential for the protease activity of USP18. To generate mice that express catalytically inactive USP18C61A, a knock-in targeting strategy was applied (Fig. S1B). Via homologous recombination, the codon for the active site cysteine (C61) was replaced by a codon specific for alanine (C61A). Positive ES cell clones were identified and used to generate germ-line chimeras and heterozygous animals were mated to yield homozygous mice (USP18C61A/C61A). Successful mutation was validated by Southern blot analysis (Fig. S1C). cDNA sequencing proved that USP18C61A/C61A animals express a mutant USP18C61A transcript (Fig. S1D). To confirm that targeted mutagenesis of the USP18 gene locus did not alter regulatory properties, bone-marrow–derived macrophages (BMMs) were isolated and stimulated with IFN-β. Western blot analysis showed that the induction kinetics and protein levels of USP18C61A protein were similar to that observed in WT cells (Fig. S1E). USP18C61A/C61A animals were born according to Mendelian ratios and in contrast to USP18−/− mice, did not exhibit macroscopic alterations, reduced life expectancy, or brain abnormalities. Even no abnormalities were detected in USP18C61A/C61A mice backcrossed to C57BL/6, a genetic background, in which USP18 deficiency causes embryonic lethality accompanied by malformation and growth retardation around embryonic day 15.5 (E15.5) (Fig. S2). Thus, we established a mouse strain in which the endogenous USP18 gene was mutated to express a USP18 protein selectively lacking its isopeptidase activity.
USP18C61A/C61A Mice Demonstrate Increased ISGylation.
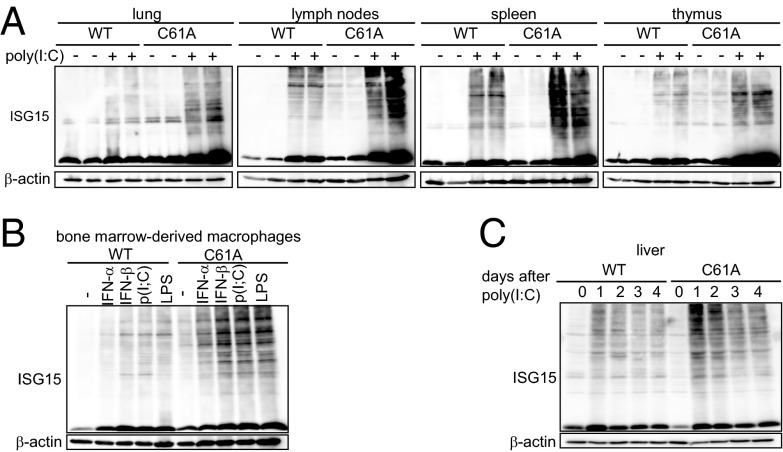
In addition to functioning as a deconjugating enzyme for ISG15, it has been shown in cells that USP18 can regulate IFN signaling in a protease-independent manner (28). Thus, the enhanced ISGylation seen in the USP18-deficient mice could have been due to either the enhanced IFN signaling or impaired deISGylation. In addition, previous in vitro studies have provided evidence for other USPs that exhibit cross-reactivity to ISG15 (21, 29). Therefore, we evaluated whether the loss of USP18 isopeptidase activity is sufficient to augment ISG15 modification in vivo. USP18C61A/C61A or WT mice were stimulated with poly(I:C), and ISG15-conjugated proteins were monitored in lysates from liver, lung, lymph nodes, spleen, and thymus (Fig. 1A and Fig. S3A). In addition, ISG15 modification was analyzed in BMMs treated with IFN-α, IFN-β, poly(I:C), or LPS (Fig. 1B and Fig. S3B). Inactivation of the USP18 isopeptidase activity significantly increased levels of ISGylated substrates upon IFN-β, poly(I:C), or LPS stimulation and in all assayed tissues and cell types, indicating that USP18 isopeptidase function is necessary to counteract ISGylation of multiple proteins. Remarkably, lungs from USP18C61A/C61A mice also displayed significantly enhanced levels of unconjugated ISG15. We also followed the kinetics of ISGylation over several days (Fig. 1C and Fig. S3C). Both in WT and in USP18C61A/C61A mice, the levels of ISG15-conjugated proteins reached a maximum in liver lysates 24 h after induction with poly(I:C). Levels of ISG15-modified substrates in USP18C61A/C61A mice compared with WT controls were significantly elevated on days 1, 2, 3, and 4 after induction. However, on days 3 and 4 after induction, levels of ISGylation decreased in both genotypes, suggesting that either other isopeptidases deconjugate ISG15 or that ISGylated substrates are removed by physiologic turnover. In contrast, overall ubiquitin modification did not differ between WT and USP18C61A/C61A murine embryonic fibroblasts (MEFs) (Fig. S4A). Together, these results clearly show that selective inactivation of the USP18 isopeptidase activity causes increased and prolonged ISGylation, which appears to be only partially compensated by other isopeptidases in vivo, if at all.
Fig. 1.
Inactivation of USP18 isopeptidase enhances ISGylation. (A) USP18C61A/C61A mice (C61A) showed enhanced ISGylation in different organs. Mice were injected with poly(I:C) and 24 h later protein lysates were analyzed by immunoblotting. (B) USP18C61A/C61A BMMs displayed enhanced ISGylation levels after treatment with 250 units/mL IFN-α, 250 units/mL IFN-β, 25 µg/mL poly(I:C) [p(I:C)] or 100 ng/mL LPS for 24 h. (C) Time course of ISGylation in liver lysates. WT and USP18C61A/C61A animals were treated i.p. once with 5 µg/g bodyweight poly(I:C) and ISGylation was analyzed 1–4 d after injection. Western blots are representative of three to five independent experiments (densitrometric analysis is depicted in Fig. S3).
USP18 Protease Activity Is Not Critical for IFN Induction, Signaling, or Desensitization.
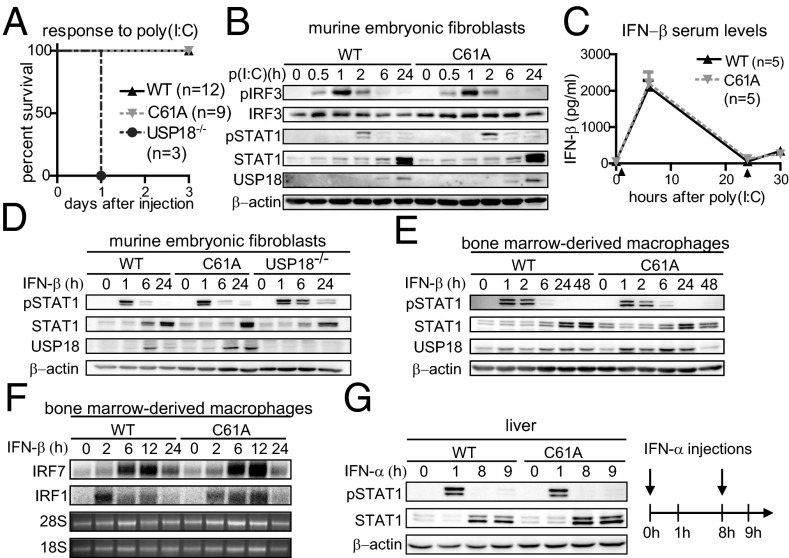
Several reports suggested a role of ISG15 and USP18 in the regulation of the type I IFN response. The importance of USP18-mediated regulation is most strikingly shown by the fact that the lack of USP18 results in lethal hypersensitivity upon poly(I:C) injection (23, 26). To evaluate the contribution of the USP18 protease function in poly(I:C)-induced hypersensitivity, USP18C61A/C61A mice were challenged with this IFN-inducing TLR3 agonist. In contrast to USP18−/− mice, in which 100% of the mice succumbed to poly(I:C)-induced lethality within 1 d, neither USP18C61A/C61A nor WT mice died (Fig. 2A), showing that the protease activity of USP18 is not essential to counteract the proinflammatory response upon poly(I:C) administration. TLR3 stimulation activates a signaling cascade, which culminates in the activation of IRF3 and induces expression of type I IFNs (30). Furthermore, ISG15 was reported to be conjugated to IRF3, thereby increasing its stability (11, 12). Thus, we evaluated stability and phosphorylation of IRF3 and type I IFN induction upon poly(I:C) stimulation in USP18C61A/C61A cells and mice. Neither the total contents of IRF3 nor the kinetics or the intensities of IRF3 phosphorylation differed between cells of both genotypes (Fig. 2B). Concordantly, IFN-β serum levels in USP18C61A/C61A mice treated with poly(I:C) were not affected (Fig. 2C). These results strongly indicate that the enzymatic activity of USP18 is dispensable for normal poly(I:C)-mediated IFN induction and that its loss does not affect IRF3 stability or activation. USP18-deficient fibroblasts also did not exhibit alterations in the kinetic of IRF3 phosphorylation (Fig. S4B). To analyze the potential role of USP18 isopeptidase activity in IFN signaling, STAT1 phosphorylation after IFN-β stimulation was monitored in MEFs and BMMs. As reported previously (23), STAT1 phosphorylation was prolonged in USP18-deficient MEFs (Fig. 2D). In contrast, kinetics and intensity of STAT1 phosphorylation did not differ between WT and USP18C61A/C61A MEFs (Fig. 2D). Similarly, no differences were detected between WT and USP18C61A/C61A BMMs (Fig. 2E). In addition, Northern blot analysis showed that expression of the type I IFN target genes IRF1 and IRF7 was not altered in USP18C61A/C61A cells (Fig. 2F). IFN-α stimulation can cause a state of refractoriness (desensitization) in which a second stimulus can no longer induce IFN signaling. Lack of USP18 was shown to abrogate this desensitizing effect of IFN-α in vivo (24), whereas USP18 expression was sufficient to establish this state of refractoriness (31). To monitor IFN-mediated desensitization in vivo, animals were first injected with IFN-α, which induces STAT1 phosphorylation after 1 h. Subsequently, mice received a second IFN-α injection 8 h later and P-STAT1 was analyzed 1 h after the second treatment. The initial injection of IFN-α induced P-STAT1 in liver protein lysates of WT and USP18C61A/C61A mutant mice, whereas the second injection failed to activate STAT1 in both genotypes (Fig. 2G). Thus, desensitization was observed in WT and USP18C61A/C61A animals, indicating that the enzymatic activity of USP18 is dispensable for this effect. Collectively, these data show that in vivo type I IFN induction and signaling is not influenced by the loss of USP18 isopeptidase activity, strongly suggesting that regulatory properties of USP18 within this context are exerted in a nonenzymatic manner.
Fig. 2.
Lack of USP18 protease activity does not alter type I IFN signaling, IFN-α desensitization, or sensitivity to poly(I:C) injections. (A) Survival curve after poly(I:C) injection. In contrast to USP18 knockout (USP18−/−) mice, USP18C61A/C61A (C61A) and WT mice are not lethally hypersensitive to poly(I:C) injection. (B) Lack of USP18 isopeptidase activity does not affect IRF3 phosphorylation (P-IRF3) or stability. Primary MEFs isolated from USP18C61A/C61A mice and WT controls were stimulated with 25 µg/mL poly(I:C) and analyzed by immunoblotting. (C) Unaltered IFN-β serum levels in USP18C61A/C61A mice upon stimulation with poly(I:C). Mice were injected with 5 µg/g bodyweight poly(I:C) at 0 and 24 h. Serum levels of IFN-β from poly(I:C)-stimulated mice (n = 5) were analyzed by ELISA. (D and E) MEFs and BMMs isolated from USP18C61A/C61A mice show normal STAT1Tyr701 phosphorylation (P-STAT1) upon IFN-β induction. MEFs and BMMs of the indicated genotypes were stimulated with 250 units/mL IFN-β and analyzed by immunoblotting. (F) Induction of IRF7 and IRF1 in IFN-β–stimulated BMMs was analyzed by Northern blot. Ribosomal 28S and 18S RNA served as a loading control. (G) Normal JAK-STAT signaling refractoriness after IFN-α injections. USP18C61A/C61A mice showed a normal desensitization reaction after reiterated IFN-α injection (8 h after the first administration).
Enhanced Resistance of USP18C61A/C61A MEFs to the Cytopathic Effect of VSV Infection.
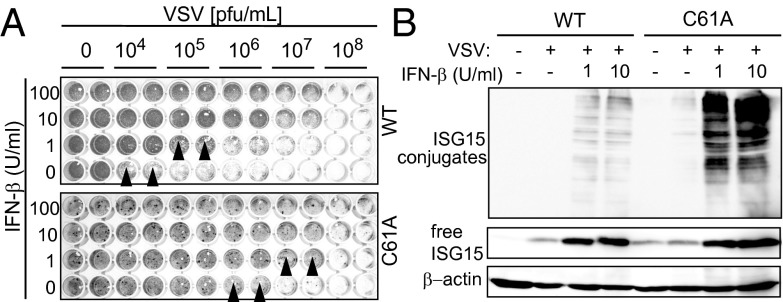
As ISGylation represents one of the major type I IFN effector systems, the role of USP18 enzymatic activity was assessed in viral infection models. USP18−/− mice were reported to be resistant to both i.c. VSV and LCMV infection (25). When WT and USP18C61A/C61A MEFs were challenged with VSV, we found that USP18C61A/C61A cells were about 100-fold more resistant against the cytopathic effect of VSV than WT cells. The same effect was seen upon prestimulation with IFN-β (Fig. 3A). This was accompanied by a dramatic increase in ISGylation in USP18C61A/C61A cells (Fig. 3B). However, the pronounced cytoprotective effect in cell culture was not mirrored by altered survival rates of USP18C61A/C61A mice upon intracranial (i.c.) (Fig. S5A) or i.v. infection with VSV (Fig. S5B). Also during infection with the noncytopathic LCMV, the survival kinetics did not differ between USP18C61A/C61A mice and WT controls upon i.c. infection (Fig. S5C). These results show a correlation between enhanced ISGylation and viral resistance upon VSV infection in cell culture, which does not translate into enhanced survival in vivo.
Fig. 3.
Lack of USP18 isopeptidase activity increases resistance to the cytopathic effects of VSV. (A) MEFs were prestimulated with indicated amounts of IFN-β for 24 h and subsequently infected with VSV. Twenty-four hours later, cells were stained with crystal violet and cell survival was monitored by microscopy. USP18C61A/C61A MEFs (C61A) are protected against VSV-mediated cell lysis at higher virus concentrations (indicated by black arrowheads). One representative assay of the three independent experiments is shown. (B) ISGylation levels in whole cell lysates of VSV-infected MEFs were analyzed by Western blot. USP18C61A/C61A cells showed enhanced amounts of ISG15 conjugates after IFN-β stimulation. One representative assay of the three independent experiments is shown.
Inactivation of USP18 Protease Activity Increases Resistance to VACV Infections.
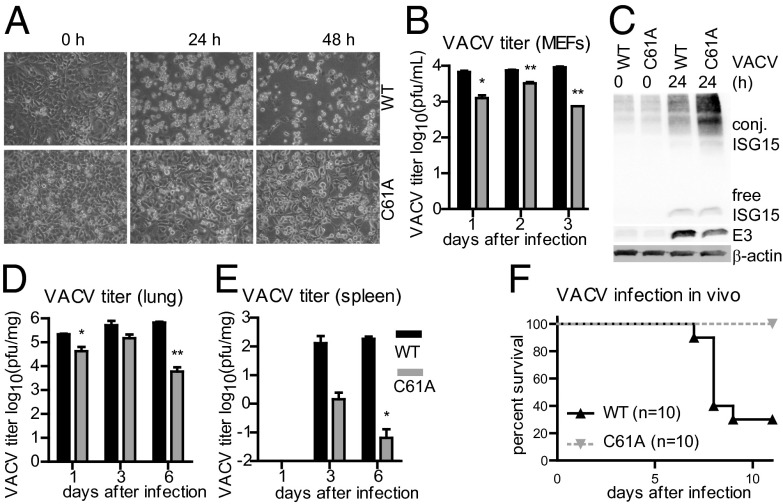
ISG15 plays an important role in the host response to VACV infection and the viral E3 protein functions as an immune evasion protein to inhibit ISG15 conjugation (17, 19). Thus, we characterized the role of USP18 isopeptidase activity in VACV infection. USP18C61A/C61A MEFs were protected against the cytopathic effects of VACV infection, whereas WT cells were highly susceptible (Fig. 4A). This effect was accompanied by significantly lower virus titers in cultures of USP18C61A/C61A cells in comparison with WT controls (Fig. 4B). IRF3 phosphorylation after infection was unaltered between WT and USP18C61A/C61A MEFs (Fig. S6). USP18C61A/C61A BMMs showed lower levels of VACV E3 protein, coinciding with enhanced ISGylation (Fig. 4C). As VACV E3 was previously shown to counteract the host defense, we asked whether E3 might be ISGylated in cells lacking USP18 isopeptidase activity. However, neither endogenous E3 nor E3 overexpressed in USP18C61A/C61A cells could be shown to be modified by ISG15 (Fig. S7).
Fig. 4.
Inactivation of USP18 protease activity enhances antiviral activity upon VACV infection. (A) WT MEFs or USP18C61A/C61A (C61A) MEFs were infected with VACV at 0.01 pfu per cell. Cytopathic effect in the cells was examined by phase-contrast microscopy. (B) VACV titers are diminished in USP18C61A/C61A cells. One-step virus growth of VACV-infected (0.001 pfu per cell) USP18C61A/C61A or WT MEFs. Cells were infected and virus yields were determined by plaque assays for VACV. (C) Enhanced ISGylation and reduced E3 protein levels in USP18C61A/C61A BMMs upon VACV infection. USP18C61A/C61A or WT BMMs were infected with 0.01 pfu per cell VACV and protein lysates subjected to immunoblotting using the indicated antibodies. (D and E) USP18C61A/C61A (C61A) animals showed decreased viral titers at days 1, 3, and 6 postinfection in lung or spleen, respectively. USP18C61A/C61A or WT mice were infected i.n. with VACV at 105 pfu per mouse. The extent of infectious particles from WT (black bars) and USP18C61A/C61A (gray bars) mice were evaluated by plaque assay in BSC-40 cells. Results represent mean values from samples of four animals per day and group with SE of mean (SEM). (F) USP18C61A/C61A mice displayed an improved survival after VACV infection in vivo. USP18C61A/C61A or WT mice were infected i.n. with VACV at 105 pfu per mouse [Western Reserve (WR) strain]. The survival of each group (n = 10) is indicated.
Upon infection of USP18C61A/C61A mice with VACV, significantly reduced viral titers were detected in the lung (Fig. 4D) and spleen (Fig. 4E). Most strikingly, after infection with 105 pfu per mouse, all USP18C61A/C61A mice survived the infection, whereas 7 of 10 WT animals died between days 7 and 9 (Fig. 4F). Altogether, inactivation of the USP18 isopeptidase activity increases resistance to VACV both in cell culture and in mice.
Inactivation of USP18 Protease Activity Increases Resistance to Influenza B Virus Infections.
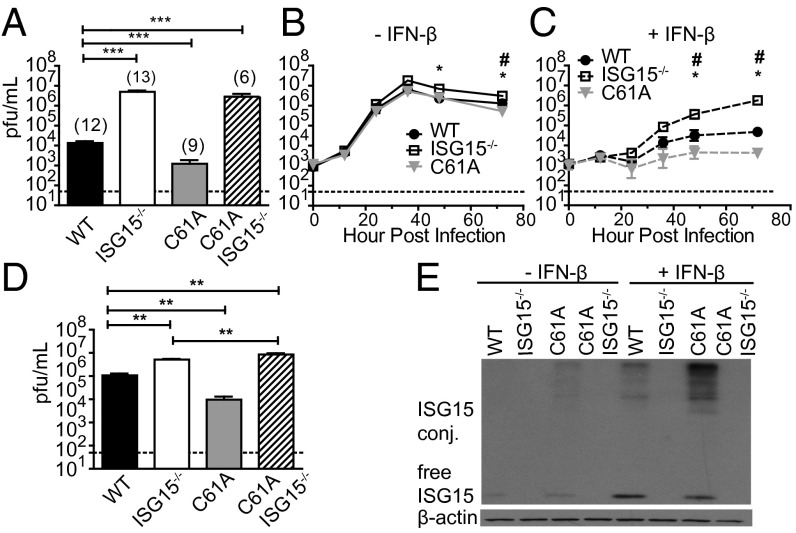
Previous studies have demonstrated a critical role for ISGylation in the control of influenza B virus infection (15). To evaluate, if increasing the levels of ISGylation by USP18 protease inactivation can boost antiviral activity, USP18C61A/C61A mice were infected with influenza B/Yamagata/88 virus. As WT mice do not develop severe disease symptoms following influenza B virus infection, we evaluated viral loads within the lungs of mice to assess resistance to infection. As previously reported (13) viral titers were increased nearly 1000-fold in ISG15−/− animals (Fig. 5A). Interestingly, in the infected USP18C61A/C61A mice, we observed a sevenfold decrease in viral loads at 3 d postinfection compared with WT mice (Fig. 5A). Because USP18 has been previously shown to regulate IFN signaling independent of its ISG15 deconjugating activity we wanted to determine if the resistance that we observed in the USP18C61A/C61A-infected mice was dependent on ISG15. ISG15−/− mice were bred onto the USP18C61A/C61A background and viral loads were analyzed in these ISG15−/−USP18C61A/C61A mice following influenza B virus infection. When ISG15 was eliminated from USP18C61A/C61A mice, viral titers were similar to those observed in ISG15−/− mice (Fig. 5A), providing strong evidence that the loss of ISG15 deconjugation causes increased resistance against influenza B virus infection. To determine whether ISGylation directly affects influenza B virus replication, we next evaluated viral replication in primary mouse tracheal epithelial cells (mTECs). mTECs derived from WT, ISG15−/−, USP18C61A/C61A, or ISG15−/−USP18C61A/C61A mice were either left untreated or were pretreated with IFN-β and then infected with influenza B virus. In mTECs not pretreated with IFN, we detected only minimal differences in viral growth between the three genotypes (Fig. 5B). Following pretreatment with IFN, we observed a significant decrease in viral growth in the USP18C61A/C61A cells with viral replication being reduced by about 7-fold after 72 h postinfection, whereas mTECs derived from ISG15−/− mice exhibited a 33-fold increase in viral titers compared with WT controls (Fig. 5 C and D). Once again the reduced replication observed in the USP18C61A/C61A mTECs was dependent on ISG15 because mTECs derived from ISG15−/−USP18C61A/C61A mice exhibited increased replication similar to that observed in the ISG15−/− mTECs (Fig. 5 C and D). ISG15 protein expression analysis from IFN-stimulated mTECs confirmed that in both WT and USP18C61A/C61A mTECs, ISG15 and ISG15 conjugates were induced, with an increase in the levels of ISG15 conjugates being observed in the USP18C61A/C61A cells (Fig. 5E and Fig. S8). Interestingly, whereas the amount of ISG15 conjugates was increased in the USP18C61A/C61A mTECs, we also observed an increase in the levels of total ISG15 protein in these cells compared with WT cells (Fig. 5E and Fig. S8). Together these results clearly show that the selective inactivation of the USP18 protease activity results in decreased influenza B viral replication both in cells and in vivo.
Fig. 5.
Inactivation of USP18 protease activity enhances resistance to influenza B infection in vivo and in vitro. (A) Mice were infected with 1.2 × 104 pfu influenza B/Yamagata/88 virus i.n. Three days later, viral loads in lung were determined. (B–D) mTECs from mice were either left unstimulated (B) or stimulated with 30 units IFN-β/mL for 24 h (C) before infection with 9.0 × 105 pfu of influenza B/Yamagata/88. Viral titers were determined by plaque assays. Growth curves were determined from two infections, three replicates per experiment. #, USP18C61A/C61A (C61A) vs. WT P < 0.05; *, ISG15−/− vs. WT P < 0.05. (D) Viral titers in IFN-β stimulated cultures at 48 h postinfection. **P < 0.005. (E) mTECs were stimulated with 30 units/mL IFN-β for 24 h. ISG15 and β-actin protein levels were assessed by Western blot analysis (densitrometric analysis in Fig. S5).
Discussion
The findings from our study are briefly summarized in a model depicted in Fig. S9 and answer several questions concerning the function of USP18 in vivo. First, the given results show that in general, the molecular function of USP18 as a negative regulator of IFN signaling in vivo is mediated in an isopeptidase-independent manner. In contrast to the complete knockout, USP18C61A/C61A mice did not develop brain injuries, displayed no prolongation of STAT1 phosphorylation, and were not hypersensitive to the administration of poly(I:C). Previous experiments provided evidence for a mechanism where the C terminus of USP18 binds to IFNAR2 and competes with JAK1 for binding (28). Interestingly, mice harboring an amino acid exchange of the conserved leucine 361 at the C terminus in USP18 (USP18Ity9) exhibit increased STAT1 expression upon IFN-β induction, suggesting that the C terminus of USP18 is indeed critical for the negative regulatory role in IFN signaling (32). USP18 together with USP12 is the smallest member of the USP family and does not comprise any other prominent domains besides the catalytic core (33). Therefore, it was surprising that USP18C61A/C61A mice develop a phenotype so different from the mice lacking USP18. Recently, also USPL1, a SUMO-deconjugating enzyme, was shown to exert the stabilization of Cajal bodies in an isopeptidase-independent manner (34), suggesting that nonenzymatic functions of USPs might be more common than generally assumed. Thus, loss-of-function experiments and RNAi-based screens inhibiting ubiquitin- and UBL-deconjugating enzymes (DUBs) need to be cautiously interpreted.
Second, enhanced ISGylation in USP18C61A/C61A mice showed that USP18, which so far is the only DUB reported to be specific for ISG15, is a major deconjugating enzyme for ISG15 in vivo. All components of the ISGylation system (ISG15, Ube1L, UbcH8, and HERC5/HERC6) are strongly induced by IFN. Therefore, enhanced activation of IFN signaling observed in USP18−/− mice would force ISGylation even if USP18 isopeptidase activity is directed against other proteins or if loss of the protein can be compensated. We now show increased and prolonged ISGylation in USP18C61A/C61A mice, where the IFN signaling pathway is not altered in general, clearly indicating that USP18 is a major ISG15 isopeptidase in vivo and that other cross-reactive USPs (21) cannot fully compensate for the loss of USP18 protease function. However, efficient ISGylation in USP18C61A/C61A mice clearly shows that processing of the ISG15 precursor protein is exerted in an USP18-independent manner.
Third, our results suggest that increasing the levels of ISG15 conjugation can be protective against viral infection. Studies using ISG15−/− or Ube1L−/− mice have clearly shown that ISGylation is essential for antiviral activity against some viruses (13, 15). Previous studies in the USP18−/− mice had shown resistance to viral infection (25). However, it was unclear if this was due to increased ISG15 conjugates or hypersensitive IFN signaling. Furthermore embryonic lethality of USP18−/− animals on a C57BL/6 background (Fig. S2) made it impossible to analyze the consequences of viral infection in this immunologically well-characterized background. The results from our study provide strong evidence that enhancing the levels of ISG15 conjugates can protect the host from at least some viral infections. It is interesting to note that both influenza B virus and VACV have developed immune evasion strategies to target ISG15 conjugation. In the case of influenza B virus, the NS1 protein can block the binding of ISG15 to UbE1L (3), and therefore block conjugate formation, whereas in the case of VACV, the E3 protein can inhibit ISG15 conjugation (17, 19). In both of these cases, inactivation of the USP18 protease activity protected the mice from infection. The increased resistance observed in USP18−/− mice infected i.c. with either VSV or LCMV is likely due to the enhanced IFN signaling and not due to the antiviral actions of ISG15. This is supported by the lack of a phenotype for either virus infection observed in the ISG15−/− mice and because no protection was noted when USP18C61A/C61A mice were challenged with VSV or LCMV. In a recent study, USP18 was reported to specifically suppress the IFN response in metallophilic macrophages (35). This suppression permits a limited amplification of VSV, which in turn secures replication of a critical amount of virus particles necessary to efficiently promote adaptive immunity. In agreement with this function, USP18-deficient mice exhibited reduced survival upon i.v. VSV infection where the adaptive immune response is critical to prevent lethality. In contrast, USP18−/− mice exhibit increased resistance to i.c. VSV infections where viral clearance is mainly IFN dependent (25). As USP18C61A/C61A and WT mice did not differ in survival upon i.c. or i.v. infection with VSV, it is unlikely that USP18 protease activity contributes to the reported suppression of the IFN response in metallophilic macrophages.
There are several potential mechanisms that could mediate the increased resistance observed in the USP18C61A/C61A mice. ISGylation can occur in a cotranslational manner and ISG15 modification of viral proteins has been shown to result in improper viral assembly (9) or interfere with the capability of distinct viral proteins like E3 from VACV or NS1A from influenza to counteract cellular defense mechanisms (10, 17). As we observed reduced levels of VACV E3 upon infection of BMMs, which correlated with increased levels of overall ISGylation, it was tempting to speculate that ISG15 modification of E3 in USP18C61A/C61A animals might account for the observed increase in resistance. However, we were unable to detect ISGylation of E3 (Fig. S7), even when overexpressed in USP18C61A/C61A MEFs followed by IFN stimulation, or in HEK293T cells together with expression constructs for ISG15 and E1-, E2-, and E3-conjugating enzymes (Fig. S7). Therefore, the reduced E3 protein levels most likely rather mirrors reduced viral load in USP18C61A/C61A cells than being the cause for the enhanced resistance of USP18C61A/C61A cells. Thus, the exact molecular mechanisms leading to enhanced viral resistance still require further investigation.
It is quite conceivable that the lack of USP18 isopeptidase activity increases the pool of ISG15-modified viral proteins thereby enhancing antiviral activity in USP18C61A/C61A mice.
Alternatively, antiviral activities mediated by ISG15-modified cellular proteins could be enhanced. Although previous studies have shown that ISGylation of IRF3 enhanced its stability, resulting in increased IFN and ISG production, neither poly(I:C) nor VACV infection substantially altered IRF3 phosphorylation or stability. Future studies will be needed to explore the potential targets of enhanced ISGylation in this system.
Altogether, our results suggest that small molecule inhibition of the USP18 protease activity might be an attractive strategy for the development of antiviral therapies. USP18C61A/C61A mice represent a well-suited model system to mimic the consequences of such a therapeutic strategy and represent a valuable tool to study the physiological consequences of USP18 protease inhibition and enhanced ISGylation in innate immunity and tumorigenesis.
Whereas the severe phenotype of USP18−/− mice raised concerns about negative consequences of USP18 protease inhibition, the present study puts former results in perspective. Lack of USP18 isopeptidase function increases ISGylation and viral resistance without inducing any obvious physical alterations or symptoms, restoring confidence that USP18 protease inhibition could be a well-tolerable antiviral therapeutic approach.
Experimental Procedures
Generation of USP18C61A/C61A mice is described in detail in Fig. S1.
Mice.
Mice were housed under specific pathogen free conditions and experiments were performed in accordance with good animal practice as defined by the relevant national, international, and/or local animal welfare bodies, and with the Royal Decree (RD 1201/2005). All animal work was approved by the Ethical Committee of Animal Experimentation (CEEA-CNB) of the Centro Nacional de Biotecnología (CNB-CSIC). Permit 10015. Studies performed at Washington University were approved by the Animals Studies Committee at Washington University protocol 20120181. USP18C61A/C61A mice on a 129/C57BL/6 background were used for LCMV, VACV, and VSV infections and poly(I:C) hypersensitivity experiments. For influenza B infections, mice were fully backcrossed to C57BL/6, confirmed by congenic SNP analysis.
Poly(I:C) and IFN-α Injections.
Mice were treated with 5 µg/g bodyweight poly(I:C) (Invivogen) or IFN-α (1,000 units/g bodyweight) (Calbiochem) intraperitoneally (i.p.).
ELISA.
Blood serum samples from mice (n = 5) were treated with poly(I:C) and analyzed with the IFN-β ELISA kit (PBL InterferonSource).
Isolation of Primary Cells and Cell Culture.
MEFs and BMMs were isolated, cultured (26), and stimulated with IFN-α (Calbiochem), IFN-β (PBL InterferonSource), LPS (Sigma), and poly(I:C) (high molecular weight) (Invivogen).
Northern, Southern, and Western Blots.
Northern and Southern blots were performed as described (14). For Western blotting, cells and organs were lysed in radioimmunoprecipitation assay buffer. Antibodies used are as follows: P-STAT1 (Tyr701), STAT1, IRF3, phospho-IRF3 (Cell Signaling), USP18 (rabbit antiserum), ISG15 (14), β-actin (I-19, Santa Cruz), and S-Tag (Novagen). Protein expression in mTECs was analyzed using anti-ISG15 antiserum (15) and anti–β-actin (Sigma AC-74).
ISG15-VS Probe Reaction.
cDNA of mUSP18 in pTriEx2 plasmid was mutated with QuikChange (Stratagene), transfected in HEK 293T cells (FuGENE HD), and lysed after 48 h in 50 mM Tris (pH 7.4), 5 mM MgCl2, 250 mM sucrose, 1 mM DTT (36). Lysates (20 µg) were incubated with 1 µg of HA-ISG15-VS probe (Boston Biochem) for 1 h at 37 °C.
Murine Tracheal Epithelial Cell Cultures.
Primary mTECs were generated from the tracheas of mice as previously described (37). Viral infections of mice and mTECs are described in Supporting Information.
Statistical Analysis.
Statistical differences were evaluated using the Student's t test (GraphPad Prism). Differences were considered to be significant when *P < 0.05, **P < 0.01, or ***P < 0.001.
Supplementary Material
Acknowledgments
We thank M. Ditter and K. Monte for excellent technical assistance and B. L. Jacobs for anti E3 VACV antiserum. This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grants KN590/1-2, KN590/3-1, and KN590/3-2 (to K.-P.K.); National Institutes of Health (NIH) Grant R01 AI080672; a Pew Scholar Award (to D.J.L.); and NIH Training Grant GM 007067 (to D.J.M.) and Spanish Ministry of Health FIS2011-00127 (to S.G.). Experimental support was provided by the Speed Congenics Facility of the Rheumatic Diseases Core Center (P30AR048335). M.P. is funded by DFG (FOR1336, SFB 942, PR 577/8-2) and the Bundesministerium für Bildung und Forschung (Krankheitsbezogenes Kompetenznetz Multiple Sklerose).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412881112/-/DCSupplemental.
References
- 1.Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narasimhan J, et al. Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J Biol Chem. 2005;280(29):27356–27365. doi: 10.1074/jbc.M502814200. [DOI] [PubMed] [Google Scholar]
- 3.Yuan W, Krug RM. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 2001;20(3):362–371. doi: 10.1093/emboj/20.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C, et al. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci USA. 2004;101(20):7578–7582. doi: 10.1073/pnas.0402528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dastur A, Beaudenon S, Kelley M, Krug RM, Huibregtse JM. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J Biol Chem. 2006;281(7):4334–4338. doi: 10.1074/jbc.M512830200. [DOI] [PubMed] [Google Scholar]
- 6.Wong JJY, Pung YF, Sze NSK, Chin KC. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc Natl Acad Sci USA. 2006;103(28):10735–10740. doi: 10.1073/pnas.0600397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketscher L, Basters A, Prinz M, Knobeloch KP. mHERC6 is the essential ISG15 E3 ligase in the murine system. Biochem Biophys Res Commun. 2012;417(1):135–140. doi: 10.1016/j.bbrc.2011.11.071. [DOI] [PubMed] [Google Scholar]
- 8.Oudshoorn D, et al. HERC6 is the main E3 ligase for global ISG15 conjugation in mouse cells. PLoS ONE. 2012;7(1):e29870. doi: 10.1371/journal.pone.0029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durfee LA, Lyon N, Seo K, Huibregtse JM. The ISG15 conjugation system broadly targets newly synthesized proteins: Implications for the antiviral function of ISG15. Mol Cell. 2010;38(5):722–732. doi: 10.1016/j.molcel.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C, Hsiang TY, Kuo RL, Krug RM. ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc Natl Acad Sci USA. 2010;107(5):2253–2258. doi: 10.1073/pnas.0909144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G, et al. ISG15 enhances the innate antiviral response by inhibition of IRF-3 degradation. Cell Mol Biol (Noisy-le-grand) 2006;52(1):29–41. [PubMed] [Google Scholar]
- 12.Shi HX, et al. Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification. Mol Cell Biol. 2010;30(10):2424–2436. doi: 10.1128/MCB.01466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenschow DJ, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci USA. 2007;104(4):1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osiak A, Utermöhlen O, Niendorf S, Horak I, Knobeloch KP. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol Cell Biol. 2005;25(15):6338–6345. doi: 10.1128/MCB.25.15.6338-6345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai C, et al. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J Virol. 2009;83(2):1147–1151. doi: 10.1128/JVI.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannakopoulos NV, et al. ISG15 Arg151 and the ISG15-conjugating enzyme UbE1L are important for innate immune control of Sindbis virus. J Virol. 2009;83(4):1602–1610. doi: 10.1128/JVI.01590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra S, Cáceres A, Knobeloch KP, Horak I, Esteban M. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathog. 2008;4(7):e1000096. doi: 10.1371/journal.ppat.1000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frias-Staheli N, et al. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe. 2007;2(6):404–416. doi: 10.1016/j.chom.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eduardo-Correia B, Martínez-Romero C, García-Sastre A, Guerra S. ISG15 is counteracted by vaccinia virus E3 protein and controls the proinflammatory response against viral infection. J Virol. 2014;88(4):2312–2318. doi: 10.1128/JVI.03293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem. 2002;277(12):9976–9981. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- 21.Catic A, et al. Screen for ISG15-crossreactive deubiquitinases. PLoS ONE. 2007;2(7):e679. doi: 10.1371/journal.pone.0000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie KJ, et al. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 2002;16(17):2207–2212. doi: 10.1101/gad.1010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malakhova OA, et al. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17(4):455–460. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarasin-Filipowicz M, et al. Alpha interferon induces long-lasting refractoriness of JAK-STAT signaling in the mouse liver through induction of USP18/UBP43. Mol Cell Biol. 2009;29(17):4841–4851. doi: 10.1128/MCB.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie KJ, et al. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat Med. 2004;10(12):1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 26.Knobeloch KP, Utermöhlen O, Kisser A, Prinz M, Horak I. Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol Cell Biol. 2005;25(24):11030–11034. doi: 10.1128/MCB.25.24.11030-11034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KI, et al. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol Cell Biol. 2006;26(2):472–479. doi: 10.1128/MCB.26.2.472-479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malakhova OA, et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25(11):2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye Y, et al. Polyubiquitin binding and cross-reactivity in the USP domain deubiquitinase USP21. EMBO Rep. 2011;12(4):350–357. doi: 10.1038/embor.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 31.François-Newton V, et al. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon α response. PLoS ONE. 2011;6(7):e22200. doi: 10.1371/journal.pone.0022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richer E, et al. N-ethyl-N-nitrosourea-induced mutation in ubiquitin-specific peptidase 18 causes hyperactivation of IFN-αß signaling and suppresses STAT4-induced IFN-γ production, resulting in increased susceptibility to Salmonella typhimurium. J Immunol. 2010;185(6):3593–3601. doi: 10.4049/jimmunol.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komander D, Clague MJ, Urbé S. Breaking the chains: Structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10(8):550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 34.Schulz S, et al. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep. 2012;13(10):930–938. doi: 10.1038/embor.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honke N, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol. 2012;13(1):51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- 36.Borodovsky A, et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9(10):1149–1159. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 37.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.