Significance
A single primary cilium extends from the surface of many mammalian cells—often into an aqueous lumen, such as a kidney duct. In kidney epithelial cells, primary cilia are believed to sense fluid flow. This mechanosensory function is critical for proper organ function. Fluid flow is assumed to deflect cilia, leading to activation of transmembrane ion channels. This study defines the mechanical contributions of both bending and pivoting at the base to ciliary deflection. In addition, we report that active intracellular forces drive ciliary pivoting. This cell-directed cilia movement may be important for tuning ciliary mechanosensitivity.
Keywords: primary cilium, mechanosensing, ciliary motility, flexural rigidity, active fluctuations
Abstract
Primary cilia are ubiquitous, microtubule-based organelles that play diverse roles in sensory transduction in many eukaryotic cells. They interrogate the cellular environment through chemosensing, osmosensing, and mechanosensing using receptors and ion channels in the ciliary membrane. Little is known about the mechanical and structural properties of the cilium and how these properties contribute to ciliary perception. We probed the mechanical responses of primary cilia from kidney epithelial cells [Madin–Darby canine kidney-II (MDCK-II)], which sense fluid flow in renal ducts. We found that, on manipulation with an optical trap, cilia deflect by bending along their length and pivoting around an effective hinge located below the basal body. The calculated bending rigidity indicates weak microtubule doublet coupling. Primary cilia of MDCK cells lack interdoublet dynein motors. Nevertheless, we found that the organelles display active motility. 3D tracking showed correlated fluctuations of the cilium and basal body. These angular movements seemed random but were dependent on ATP and cytoplasmic myosin-II in the cell cortex. We conclude that force generation by the actin cytoskeleton surrounding the basal body results in active ciliary movement. We speculate that actin-driven ciliary movement might tune and calibrate ciliary sensory functions.
A living cell is a dynamic, nonequilibrium system dependent on chemical and mechanical communication with its environment. Communication is mediated in many mammalian cells by the primary cilium, a specialized antenna that typically extends from a cell’s apical surface. The primary cilium, with its specialized and segregated membrane compartment, has emerged as a key signaling center that transduces mechanical and chemical extracellular cues (1–4). Flow detection in the kidney epithelium promotes cell homeostasis, whereas defects in sensing or signaling can lead to polycystic kidney disease (5). Forces generated by fluid flow are thought to lead to Ca2+ influx through a transient receptor potential (TRP) family Ca2+ channel in the ciliary membrane, polycystin-2 (PC2) (6–9) (Fig. 1A). It has also been shown that ciliary mechanosensation regulates kidney epithelial cell size through the mammalian target-of-rapamycin pathway independent of Ca2+ transients (10). How do the mechanical properties of the cilium facilitate these cellular responses? Both bending and pivoting could trigger membrane channels. Previous studies have used fluid flow to estimate primary cilium bending stiffness based on deformation profiles (11–14), but the precise mechanical properties of the cilium remain unknown. It is likely that mechanosensation needs subtle coordination and calibration of the intracellular machinery involving adaptation and feedback mechanisms reacting to external stimuli, such as is the case in mammalian inner-ear cells, where active mechanical processes are crucial for hearing acuity (15, 16). We here applied micromanipulation and imaging methods to measure the mechanical properties of primary cilia, their cellular anchoring, and their fluctuations.
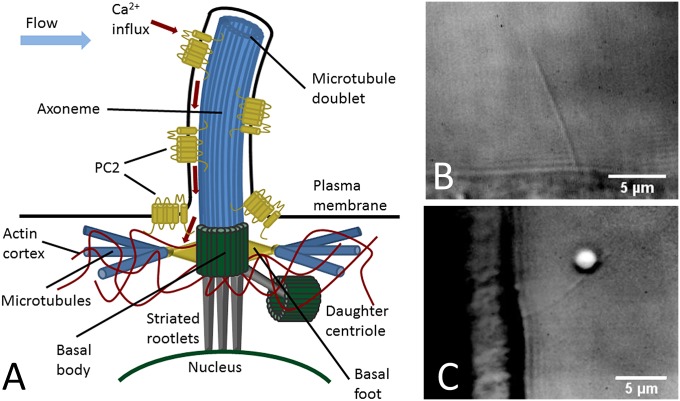
Fig. 1.
Mechanical structure and anchoring of the primary cilium. (A) Schematic sketch of a primary cilium with PC2 Ca2+ channels and its intracellular anchoring. (B) Differential interference contrast (DIC) micrograph of a primary cilium deflected in buffer flow of ∼4.8 µm/s. (C) DIC micrograph of a primary cilium with an attached bead deflected by an optical trap.
The core of the primary cilium is an axoneme made up of microtubules. Nine microtubule doublets extend from the cylindrical basal body formed by the cell’s mother centriole and provide the mechanical backbone of the cilium. Unlike beating cilia and flagella (9 + 2 cilia), primary cilia (9 + 0 cilia) lack a central microtubule doublet and cross-linking supports. As a consequence, the doublet microtubules can lose their ninefold symmetry as they extend away from the basal body. Electron micrographs have revealed so-called 9v arrangements in distal portions of cilia, in which the number of microtubule doublets varies from the usual nine down to a single doublet (17). These fundamental structural differences are expected to make the elastic properties of 9 + 0 cilia distinctly different from those of 9 + 2 cilia.
Most 9 + 0 primary cilia are presumed to be passive sensors because they lack the dynein motors that drive beating of active cilia (18), with one exception: motile 9 + 0 cilia in the organizer node of early embryos possess dynein arms and drive fluid flow (19). Cilia in the epithelium lining renal ducts are thought to passively respond to flow in the ducts. Surprisingly, we found active ciliary fluctuations. Even in the absence of dyneins, internal activity in the cell can cause cilia to move, just as a skyscraper can sway because of both wind and an earthquake. The mechanical properties of the cilium and its anchoring inside the cell determine the response of the assembly to both external and internal forces. The distinct nonthermal component of cilium motion that we document here challenges the view that the cilium is a strictly immotile sensor and raises the possibility that endogenous cilium fluctuations may have a functional role.
Results
Flexural Rigidity of Primary Cilia.
As an essential step toward understanding ciliary mechanosensation, we set out to characterize the elastic properties of both the cilium and its basal anchor in the cell. We used an optical trap to exert controlled forces on individual primary cilia of Madin–Darby canine kidney (MDCK) cells while imaging them with differential interference contrast microscopy (Fig. 1 B and C). Cilia develop in nondividing, polarized MDCK cells in culture after confluence is reached. Cells were grown to confluence on elastic polycarbonate membranes (PCMs) that were then folded and mounted in a microscope chamber, such that the cilia extended parallel to the specimen plane of the microscope (Materials and Methods). The orientation of cilia in MDCK cells is typically close to normal to the apical cell surface. This orientation is established and maintained by the anchoring of the basal body in the cell cortex and against the nucleus by distal appendages and striated rootlets (20). The microtubule doublets of the ciliary axoneme are likely to be the main contributors to the cilium’s flexural rigidity (Fig. 1A). To assess how the microtubule doublets are mechanically coupled in the cilium, we first measured its flexural rigidity. For a columnar bundle of rod-like filaments, the flexural rigidity depends strongly on the degree of lateral cross-linking and varies with the individual filament flexural rigidity as (21)
| [1] |
where E is the Young’s modulus of the (assumed to be) homogeneous material composing the filaments (here microtubule doublets), IB and If are geometrical factors, is the flexural rigidity of the bundle, is the flexural rigidity of an individual filament (microtubule doublet), is the number of filaments, and is an exponent that varies between one and three depending on the cross-linking within the bundle (assumed to be a hollow cylinder with a wall strength of one filament and a circular cross-section) (21). In the limit of weak cross-linking (), the bundle rigidity is the sum of the flexural rigidities of the individual filaments. In the strongly cross-linked regime (), the bundle rigidity corresponds to the flexural rigidity of a solid, thin shell of circular cross-section. Because n = 9, Eq. 1 predicts that the degree of interdoublet cross-linking in the cilium strongly affects its flexural rigidity. The conspicuous absence of interdoublet structures in 9 + 0 cilia suggests that they might be more flexible than 9 + 2 cilia (22). To directly measure bending stiffness, we used two different approaches (Table 1). First, in the bend and relax experiments (Movie S1), we laterally displaced the tips of cilia with an optical trap and watched the time course of the tip relaxation to its equilibrium position (Fig. 2A). At low Reynolds number (here ), the relaxation time constant depends on the balance between the elastic restoring force acting on the cilium and the viscous drag produced by the external medium, leading to a simple exponential relaxation curve (23, 24). For small bending angles, the time constant is related to the bending stiffness approximately as
| [2] |
where designates the tip position parallel to the cell monolayer at time , is the length of the cilium, is the drag on a cylinder with the cilium’s dimensions (10 µm in length and 200 nm in diameter), and is the coefficient associated with the lowest-order mode in the family of solutions to the equations of motion for a slender rod with one end clamped and one end free (24). Second, in the bead-attached bending experiments, we used the optical trap to deflect a cilium with the help of an attached silica bead of 1.5 µm in diameter and measured the force needed to bend the cilium by a given amount (SI Materials and Methods, Fig. S1, and Movie S2). The disadvantages of the latter method were (i) that the proximity of the refractile cell monolayer and PCM typically perturbed the force signal and (ii) that cell debris accumulated in the trap from the culture medium, which added to the force measurement error. The values that we measured from the two methods agreed within a factor of two, with the bead-attached bending experiments yielding EIB = 2.5 ± 1.5 × 10−23 N·m2 and the bend and relax experiments yielding EIB = 3.6 ± 0.8 × 10−23 N·m2.
Table 1.
Bending stiffness (EIB) of primary cilia (values from this work and referenced published work)
Fig. 2.
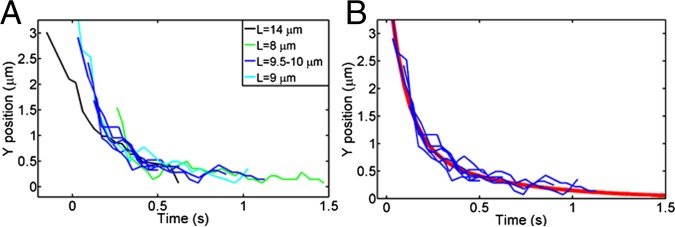
Biexponential relaxation curves of cilia deflection. (A) Relaxation curves of cilia of different lengths from the bend and relax experiments. Cilia of different lengths relax with different time constants, which was expected from Eq. 2. (B) Relaxation curves of cilia between 9 and 10 µm in length. The red line is a double-exponential fit to these curves (τ1 = 120 ms; τ2 = 1,500 ms).
By using optical traps, we avoided uncertainties in the fluid velocity field that can affect deformation measurements. Values measured here and those reported in the literature from flow experiments (Table 1) (11–13), with the exception those in the work by Downs et al. (12), consistently fall in the range of 1–5 × 10−23 N·m2. Reported bending stiffness values for individual microtubules are 0.1–3.4 × 10−23 N·m2 (23, 25–30) (1–10 times lower). Given the number of microtubules (n = 9; expression 1) and the reported bending stiffness of microtubules, we, therefore, conclude that . In other words, a weak-coupling model best explains the measured values. Although we do not account for it, the nine microtubule doublets might each be somewhat more rigid than a single microtubule. However, strong coupling would lead to an about 10-fold stiffer response, which should be easily distinguishable. Weak coupling is consistent with the lack of interdoublet structural proteins in the primary cilium as well as reports showing the gradual loss of columnar order in the primary cilium along its length (17). Assuming weak coupling ( in Eq. 1), we estimate the stiffness of a single microtubule doublet to be 0.3–0.4 ± 0.2 × 10−23 N·m2—approximately one-third of the only other estimate of doublet stiffness of which we are aware [1.4 × 10−23 N·m2 measured from the deformation of demembranated sperm flagella doublets under flow in the presence of 0.1 mM ATP (31)]. The stiffness values that we measured for the primary cilium are 100–1,000 times lower than those of sperm flagella, consistent with the highly cross-linked structure of the latter (31, 32).
Viscoelastic Anchoring of Primary Cilia.
When we closely examined the relaxation curves of cilia from the bend and relax experiments, we found systematic deviations from single-exponential time dependence. Tip relaxation curves could typically be better fit by a sum of two exponentials (Fig. 2B). Initial fast relaxation occurs on the order of hundreds of milliseconds followed by a slower reorientation of the cilium to its resting position normal to the cell surface (Movie S1). This evidence points to a viscoelastically hinged rather than a rigidly clamped boundary condition (BC) for ciliary deflection. [By clamped BC, we mean y(z = 0) = 0 and dy/dz|x = 0 = 0, where y measures displacement parallel to the cell surface and z measures position along the cilium, with z = 0 corresponding to the cilium attachment point at the cell. In the case of a limited hinge, the first condition still holds, but the second does not. Rather, deviations in slope away from zero (i.e., normal to the cell surface) are penalized by an elastic energy cost.] Two characteristic relaxation times can arise from the effective drag coefficients for ciliary hinging and bending and their respective elastic restoring forces. We can draw a rough conclusion about the ratio between internal and external drag coefficients from the fact that we observed two clearly distinct relaxation times. The slower overall reorientation dynamics mean that cell-internal viscous drag must dominate over external fluid drag on the cilium. This fact can be seen as follows. The amplitudes of the two deflections, cilium bending and hinge pivoting, were roughly equal. This observation implies that the effective elastic constants were roughly equal as well, with the bending elastic constant, of course, depending on cilium length. Although the bend relaxation is only determined by the cell-external drag, the pivoting relaxation feels both internal and external drag. If the internal drag was small and the relaxation was dominated by external drag, then the two relaxation times should be close to equal. Because they are not, the cell-internal drag must dominate over the pivoting relaxation.
Based on these results, we conclude that cilium deflection under external force involves both axoneme bending and cilium base pivoting. We extracted the bending stiffness values quoted in Table 1 by fitting EIB to the fast decay time constant, because it is evident from Movie S1 that the backbone curvature relaxes on the fast timescale. However, the existence of a hinge at the cilium base modifies the coefficient in Eq. 2 and leads to a systematic underestimate of the values given in Table 1 by an amount dependent on the ratio of the cilium bending stiffness to the compliance of the hinge at its base. From our estimates of this ratio, we conclude that the values for the bend and relax experiments in Table 1 represent an underestimate by 10% or less.
Spontaneous Angular Fluctuations of Primary Cilia.
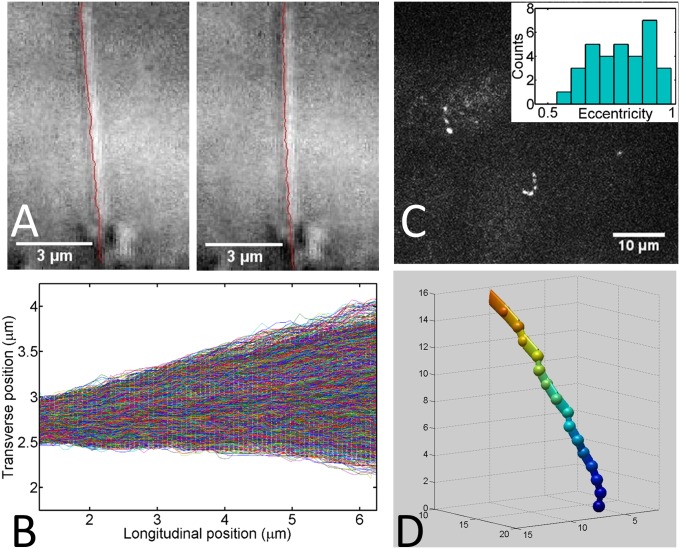
Even in the absence of external forcing by fluid flow or the optical trap, cilia exhibit angular fluctuations and, if long enough, detectable bending fluctuations. Given the micrometer-length scale of cilia, these fluctuations could be purely thermal. We recorded these spontaneous fluctuations of cilia with high temporal resolution. We then reconstructed the backbone of the cilium for each movie frame using a custom-written MATLAB algorithm (Fig. 3A). An overlay of 6,500 backbones from one such movie is shown in Fig. 3B. The cilia that we analyzed moved within a limited range of angles over time, sweeping out a circular sector and giving the appearance of a stiff rod rotating on a limited hinge. To determine whether a hinged rigid rod is an accurate description of the spontaneous cilium motion as opposed to a bending rod with a fixed attachment angle, we plotted the angle between each point on the cilium and a reference line normal to the apical cell membrane in each frame. For the case of a hinged stiff rod, this angle would be constant along the rod, whereas a flexing rod would exhibit a distribution of angles along its length because of the nonzero curvature. The angles convincingly collapsed onto a single value (SI Materials and Methods and Figs. S2–S4), which leads us to conclude that the main component of the spontaneous motion of short cilia (<10 µm) is a rigid rod pivoting around a hinge point inside the cell. The fact that internal flexing of the cilium was not detectable in the absence of strong external perturbations makes sense given the absence of dynein and the stiffness values reported above: purely thermal bending for a 10-µm-long cilium with a flexural rigidity value of 3 × 10−23 N·m2 and a clamped end at the cell surface would only result in average tip displacements on the order of 200 nm (, where d2 is the mean squared displacement) (33), much less than the observed overall tip displacements (∼2 µm in Fig. 3B).
Fig. 3.
Spontaneous fluctuations of primary cilia. (A) Differential interference contrast micrographs of a primary cilium viewed sideways with the tracked backbone superimposed. (B) The 6,500 superimposed backbones from one cilium tracking experiment recorded at 25 Hz. (C) Maximum intensity projection of an en face confocal stack containing several smoothened yellow fluorescent protein (YFP)-labeled cilia recorded at 2 Hz. To maximize temporal resolution, widely spaced z planes were imaged, corresponding to the bright dots in the projection (Movie S3). (Inset) Distribution of eccentricities for 34 cilia in the xy plane 4 µm above the cell surface. (D) 3D reconstruction of a cilium from the confocal stack in C at higher spatial resolution. The reconstruction consists of 17 planes 1 µm apart. Axes are marked in micrometers (Movie S4).
Actively Driven Nonthermal Fluctuations.
The evidence that cilia pivot around a hinge within the cell in the absence of an external force suggests that the orientation and the motion of primary cilia may be coupled to the mechanical activity of the cell cortex. To test for regular patterns in the motion of cilia, we established an additional assay that enabled us to track ciliary motion in 3D. We imaged confluent layers of MDCK cells with fluorescently labeled cilia grown on a coverslip with a spinning disk confocal microscope in an en face geometry. To maximize temporal resolution, we undersampled focal planes in the z dimension. As a result, each cilium looks like beads on a string in a maximum intensity projection of a single stack (Fig. 3C and Movie S3). We used the SpotTracker ImageJ plugin (34) to track time trajectories in each confocal plane, then reconstructed the cilium backbone along three dimensions with MATLAB, and examined position fluctuations over time (Fig. 3D and Movie S4). To compare the 3D data to the 2D data, we projected the movement of the cilium along one axis (x or y) and analyzed the variance along the length of the cilium. Plots of the transverse variance along the cilium length revealed no significant difference between the side-view experiments and the en face experiments (Fig. 4A) and confirmed the hinged pivoting motions. We performed confocal imaging experiments at room temperature as well as 37 °C and found similar behavior (Fig. 4A).
Fig. 4.
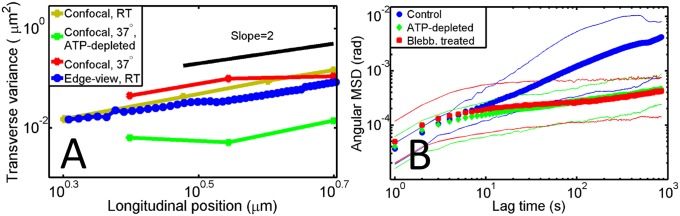
Analysis of ciliary fluctuations. (A) Variance of the cilium’s transverse position along its length for side-viewed cells imaged in differential interference contrast (blue circles, n = 14) and from confocal stacks of fluorescently labeled cells at room temperature (RT; 21 °C) and 37 °C and ATP-depleted cells at 37 °C (yellow, n = 10; red, n = 6; and green, n = 18, respectively). (B) Angular MSD vs. lag time for control (blue, n = 14), ATP-depleted (green, n = 9), and blebbistatin-treated (Blebb; 50 µM; red, n = 9) cells.
The en face experiments allowed us to look for anisotropy of the 9 + 0 ciliary motion, which could reflect constraining anchoring conditions or anisotropic active driving. It is known that 9 + 2 motile cilia perform strongly anisotropic motions, with their beating stroke occurring in a plane defined by their singular basal foot, which extends from the basal body (35). Basal feet in primary cilia vary in number from two to five (36) and have been proposed to stabilize the cilium (Fig. 1A). We conjectured that a particular arrangement of basal feet could lead to anisotropic motion in the primary cilium as well. To test for anisotropy, we analyzed thresholded maximum intensity projections from confocal movies in a plane 4 µm above the apical cell surface. We observed no obvious regular pattern, such as motion on a circle or arc. We next determined the eccentricity ε of the best-fit ellipse to the maximum intensity pattern in the intersecting plane and plotted a histogram of the eccentricity values (Fig. 3C, Inset). For perfectly isotropic motion, ε = 1, whereas for motion entirely along one dimension, ε = 0. We see a broad distribution of values with an average ε = 0.79 ± 0.12, indicating, at most, weak anisotropy in the cilium motion (eccentricity is a biased indicator, and any noise leads to values of ε < 1). The embedding of the cilium in the cell through its basal feet, thus, does not create a favored plane of motion, or there is enough variation in the number and arrangement of feet among cilia that no clear trend emerges.
Because cilia are microscopic in size, thermal Brownian motion will be at least part of their observed fluctuations. However, it is possible that cilium motion has an actively driven component, despite the fact that they contain no dynein motors. Cells are nonequilibrium systems with vigorous cytoskeletal dynamics. Aggregate random forces generated by stochastic motor activity have recently been documented to stir the cytoplasm (37) and enhance the transport of small proteins and organelles in the cytoplasm (38). The basal body is embedded in the actin cortex (Figs. 1A and 5), and recent work showed that focal adhesion proteins localize near the basal body and indicates a link between cilia and the myosin-containing actin meshwork in the cell cortex (39). Because we have established that pivoting around a cell-internal hinge is a key aspect of primary cilia movement, cortical actin–myosin fluctuations could exert forces at or above the effective hinge, which could then be translated into angular motion of the primary cilium.
Fig. 5.
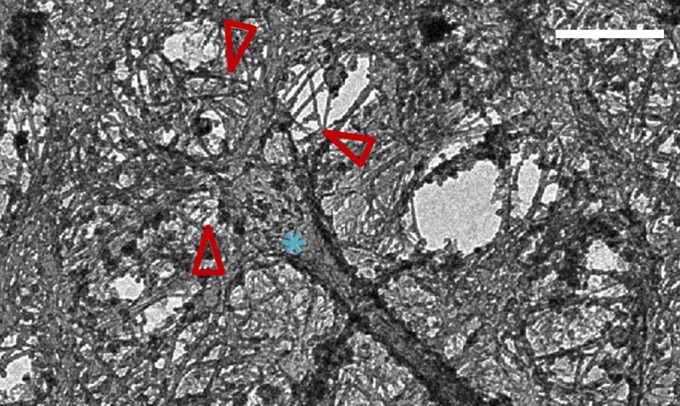
EM of a membrane-extracted apical domain of an MDCK cell. 3D stereo image shows the base of a single primary cilium (asterisk) embedded in a network of actin filaments (red arrows). (Scale bar: 500 nm.)
To test this possibility, we analyzed cilium motion in the presence of biochemical perturbations. We quantified cilia movement in the side-view geometry by calculating the angular mean-squared displacement (MSD). We found that, in unperturbed control cells, the angular MSD grew subdiffusively with time, with a power law exponent of (Fig. 4B). Subdiffusive exponents < 1 are expected generically in thermally driven, viscoelastically confined systems, where Brownian motion is influenced by the elasticity of the surrounding matrix (40). In the cell cytoskeleton, one would expect to see thermal motions only at short times of 100 ms or less, whereas at longer times, nonthermal, actively driven cytoskeletal fluctuations typically take over (41). Direct methods to test for active cytoskeletal driving are to either deplete the energy supply or disable specific motor proteins. Intriguingly, both depletion of ATP and treatment of ciliated MDCK cells with blebbistatin, a nonmuscle myosin II motor inhibitor (42), led to a significant drop in the overall angular MSD amplitude of ciliary motion. Furthermore, both treatments resulted in a much lower power law scaling exponent of (Fig. 4B). The large drop in fluctuation amplitudes is indicative of a strong active component in the motion of primary cilia. We found a comparable drop in ciliary fluctuations on ATP depletion in the confocal en face experiments at 37 °C (Fig. 4A), indicating that the activity is not dependent on temperature or geometry. Because ATP depletion and blebbistatin treatment led to identical suppression of spontaneous fluctuations, we surmise that actin–myosin activity—acting on the cilium base and the basal body, which is embedded in the actin cortex—translates cellular fluctuations into ciliary movements.
Cilium and Centrosome Fluctuations Are Positively Correlated.
The cilium grows from the mother centriole, which forms the basal body, and is, in turn, connected to the daughter centriole and the nucleus through bundles of microtubules, the striated rootlets, and the striated connector (Fig. 1A) (36). This whole complex is embedded in the actin cortex during ciliogenesis (Fig. 5) (20). To localize the pivot point in this complex, we tracked both cilium and centriole positions simultaneously in cells expressing emerald–melanin-concentrating hormone receptor-1 (a ciliary membrane protein) and monomeric red fluorescent protein fused to the pericentrin-AKAP450 centrosomal targeting domain (mRFP-PACT; a centrosome marker). If the hinge point precisely coincided with the basal body, we would expect no correlation between cilium and basal body fluctuations. However, if the hinge point was below or above the centrioles, the fluctuations would be correlated or anticorrelated, respectively. PACT labeling was visible on both the daughter centriole and the basal body; however, to maximize temporal resolution, we recorded only the focal plane containing the basal body. Fig. 6A shows a time series of fluctuations for a cilium–centrosome pair, and Fig. 6B shows the associated correlation plot. In the majority of cases, cilium and centrosome fluctuations were well-correlated (rx,avg = 0.60 ± 0.43 and ry,avg = 0.78 ± 0.09); their Pearson correlation coefficients are listed in Table 2. Because the correlations in all but one case are positive, we conclude that the hinge point of the ciliary motion must be located below the centrosome, somewhere closer to the nucleus, such as depicted in Fig. 6B, Inset.
Fig. 6.
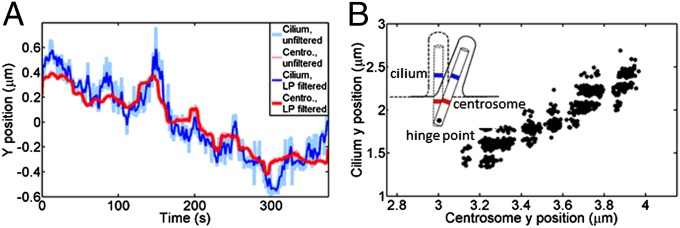
Correlations between cilium and centrosome fluctuations from confocal data. (A) Low pass-filtered (LP filtered; 0.33-Hz cutoff) cilium fluctuations (blue) in a plane ∼1 µm above the apical membrane and centrosome (Centro.) fluctuations (red) recorded at a 2-Hz frame rate. (B) Correlation plot of the cilium–centrosome fluctuations plotted in A. Cilium and centrosome fluctuations are correlated with a Pearson correlation coefficient value of r = 0.89. (Inset) Schematic of the proposed location of the hinge point and cilium–centrosome motion. Blue denotes the tracked point at the cilium base (1 µm above the membrane), and red denotes the centrosome label.
Table 2.
Pearson correlation coefficient (PCC) values for cilium–centrosome fluctuations in x and y directions
| Cilium | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| x PCC | 0.84 | 0.85 | 0.72 | 0.06 | 0.37 | −0.22 | 0.94 | 0.92 | 0.88 |
| y PCC | 0.89 | 0.86 | 0.75 | 0.72 | 0.68 | 0.68 | 0.71 | 0.80 | 0.92 |
Discussion
Deflection of the primary cilium in MDCK kidney epithelial cells occurs through a combination of bending and pivoting. We characterized the flexural rigidity of cilium bending and determined that the pivot point for the limited hinge is located below the basal body. In contrast to the current paradigm that primary cilia are immotile, our experiments revealed that primary cilia perform cell-directed active movements. Fluctuations in the actin–myosin network seem to act above the pivot point to cause correlated centrosome and cilia movements.
The primary cilium is soft compared with motile axonemes. The bending stiffness of 9 + 0 cilia is only on the order of 1–10 times that of an individual microtubule, whereas 9 + 2 axonemes are 100–1,000 times stiffer than primary cilia. The bending stiffness of the cilium and the mechanical nature of its attachment to the cell body will affect functional aspects of mechanosensing, such as cell membrane tension or shear strain in the whole organelle. These parameters, in turn, should affect the location and gating of mechanosensitive channels, about which there has been some debate (6, 7, 43, 44). Our results allow us to speculate further about plausible sensory mechanisms. Because the system has a hinged BC, it has an additional rotational degree of freedom compared with a system with a clamped BC, and therefore, tension in the ciliary membrane caused by externally imposed curvature should be lower at a given tip deflection than in the clamped case. The additional rotational degree of freedom allows the cilium to deflect farther in a given flow field before bending. Whereas mechanosensitive channels at the base could be triggered when the cilium pivots, greater flow forces would be required to trigger channels along the length of the cilium. Thus, we speculate that channels located at the base might be more responsive to weak flow than channels located along the cilium length. In addition, channels might be gated by cytoskeletal attachments through shear between the plasma membrane, the ECM, and the axoneme.
Transmission of force from the actin cortex to the cilium might occur through simple steric interactions or specific connections to the cytoskeleton through striated rootlets (20) or basal body-associated focal adhesion proteins (39, 45). The hinging degree of freedom could be important for other cellular processes, such as ciliary orientation in migrating fibroblasts, which have been found to align their cilia in their direction of motion (4). The previously unidentified actively driven cilium motion quantified here has an origin that is very different from the dynein motors driving beating in 9 + 2 cilia. The correlation between ciliary fluctuations and both myosin II activity and basal body movements shows that primary cilia motions report underlying cortical dynamics. One could compare the primary cilium with an antenna that has both receiving and transmitting functions. The question remains what the physiological role of such activity might be. A very basic function might be to establish and maintain the overall orientation of the cilium normal to the apical cell surface. The existence of cell-directed pivoting raises the intriguing possibility that active fluctuations could be integrated into feedback loops that have a functional role in tuning the response to extracellular forces, possibly similar to what is known to occur in inner-ear hair cells (15, 16). Speculatively, cell-directed ciliary pivoting could gate base-located ion channels, leading to a rise in ciliary calcium levels, which could bring signaling pathways closer to the threshold for activation, effectively calibrating and sensitizing the response to flow. Interestingly, previous reports have shown that myosin II motor inhibition with the Rho-kinase inhibitor Y27632 abolishes the flow-induced calcium response of murine epithelial kidney cells (46). Furthermore, there is evidence that the Lkb1 pathway in mammalian target-of-rapamycin signaling is locally activated at the cilia basal body through ciliary flow sensing, highlighting another possible physiological role for ciliary fluctuations (10).
The connection between the actin cytoskeleton and primary cilia is just beginning to be appreciated. A screen for proteins that effect ciliary length revealed that depletion of actin regulatory proteins significantly reduces cilium formation (47). In addition, that study showed that inhibiting actin polymerization with cytochalasin D promotes cilia formation (47). The challenge now is to understand the integrated function of the primary cilium and the dynamic actin–myosin cell cortex.
Materials and Methods
Cell Culture.
MDCK-II cells (a gift from Andreas Janshoff, Georg-August Universität, Göttingen, Germany) were cultured in MEM with 2 mM l-glutamine, 1% penicillin-streptomycin, and 10% (vol/vol) FBS. Cells for the edge-viewing experiments were imaged 1–2 d after full confluence (5–7 d after seeding) on poly-l-lysine–coated PCMs. For confocal experiments, cells were cultured on LabTek cover glass chambers in MEM with 3 mM l-glutamine and 960 μg/mL Geneticin (G-418) for selection of transfected cells.
Transfection.
Cells for the confocal experiments were stably transfected with smoothened YFP (48), Smoothened Tomato (49), or emerald–melanin-concentrating hormone receptor-1 (created by Michael Davidson, National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL), and those used in the centrosome-tracking experiments were additionally transfected with mRFP PACT (created by Alex Ritter, National Institutes of Health, Bethesda). Transfection was done using the FuGene transfection reagent.
Imaging Chambers for Side-View Geometry.
Imaging chambers were constructed out of a microscope slide and a coverslip sealed together on all sides with extrathin double-stick tape, creating a chamber ∼80 μm in height. Cells on PCMs were folded over for side viewing as described in ref. 50. PCMs were mounted in the chamber with ∼50 μL medium.
Blebbistatin Treatment.
Confluent cells were incubated in 50 μM active blebbistatin (−) enantiomer (100 μM racemic solution; 203389; CalBiochem) at 37 °C for 30 min and then mounted with blebbistatin-containing medium for video-tracking experiments.
ATP Depletion.
Cells were incubated in glucose-free DMEM solution for 3 h at 37 °C. Then, Antimycin A and 2-Deoxy-d-glucose were added to final concentrations of 10 μM and 10 mM, respectively. The cells were incubated 10–15 min at 37 °C and mounted in the glucose-free Antimycin A- and 2-Deoxy-d-glucose–containing solution for video-tracking experiments.
Microscopy.
Edge-viewing experiments were performed using the custom-built differential interference contrast/opticaltrapping setup described in ref. 51. Images were acquired with an MTI analog camera at a rate of 25 Hz and read out through a frame grabber card and a custom-written LabView VI. Confocal experiments were performed with a 63× objective on a Marianas Intelligent Imaging Innovations spinning disk confocal, and frames were acquired at ∼2 Hz.
EM.
MDCK-II cells were cultured on Nunc glass transwells (Thermo Scientific) 2 d after full confluence. Membranes were extracted, and samples were prepared for EM by rotary shadowing as previously described (52). Metal replicas were then separated from the glass by floating them on 10% bleach to remove organic materials and mounted on EM grids. Images were acquired at 80 kV.
Data Analysis.
Tracking of cilia in the confocal data was done using the ImageJ plugin SpotTracker (34). Reconstruction and analysis were done in MATLAB. Edge-viewing tracking and analysis were done using custom-written MATLAB routines.
Supplementary Material
Acknowledgments
We thank Hiroaki Ishikawa, Claus Heussinger, Giovanni Marelli, and Andrej Vilfan for helpful discussion and Andreas Janshoff for providing us with the Madin–Darby canine kidney-II cell line. We acknowledge discussions at the Woods Hole Marine Biology Laboratory. We also thank Monica Bettencourt-Dias, Tristan Ursell, and Kimberly Yasutis for help with early experiments monitoring cilia movement. Rafael Vilasmil from the National Eye Institute Flow Cytometry Core sorted cells for this study. This research was supported by the Intramural Program of the National Institutes of Health, National Institute of Child Health and Human Development; the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 803 Project A7 and 937 Project A2; the Center for Nanoscale Microscopy and Molecular Physiology of the Brain; and European Research Council Grant PF7 ERC-2013-AdG, Project 340528 (to C.F.S.). Fellowship funding was provided by the Göttingen Graduate School for Neurosciences, Biophysics, and Molecular Biosciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421845112/-/DCSupplemental.
References
- 1.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19(13):R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15(1):105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 3.Singla V, Reiter JF. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science. 2006;313(5787):629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 4.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123(Pt 4):499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12(11):R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 6.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504(7479):311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504(7479):315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184(1):71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 9.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol. 2003;191(3):193–200. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- 10.Boehlke C, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12(11):1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272(1 Pt 2):F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- 12.Downs ME, Nguyen AM, Herzog FA, Hoey DA, Jacobs CR. An experimental and computational analysis of primary cilia deflection under fluid flow. Comput Methods Biomech Biomed Engin. 2014;17(1):2–10. doi: 10.1080/10255842.2011.653784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young YN, Downs M, Jacobs CR. Dynamics of the primary cilium in shear flow. Biophys J. 2012;103(4):629–639. doi: 10.1016/j.bpj.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rydholm S, et al. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol. 2010;298(5):F1096–F1102. doi: 10.1152/ajprenal.00657.2009. [DOI] [PubMed] [Google Scholar]
- 15.Hudspeth AJ. How the ear’s works work. Nature. 1989;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 16.Martin P, Hudspeth AJ. Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. Proc Natl Acad Sci USA. 1999;96(25):14306–14311. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluenz E, et al. Beyond 9+0: Noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 2010;24(9):3117–3121. doi: 10.1096/fj.09-151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes BG. Ciliated secretory cells in the pars distalis of the mouse hypophysis. J Ultrastruct Res. 1961;5:453–467. doi: 10.1016/s0022-5320(61)80019-1. [DOI] [PubMed] [Google Scholar]
- 19.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133(21):4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 20.Marshall WF. Basal bodies: Platforms for building cilia. In: Yoder B, editor. Ciliary Function in Mammalian Development. Elsevier; Oxford: 2008. pp. 1–17. [DOI] [PubMed] [Google Scholar]
- 21.Heussinger C, Schüller F, Frey E. Statics and dynamics of the wormlike bundle model. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81(2 Pt 1):021904. doi: 10.1103/PhysRevE.81.021904. [DOI] [PubMed] [Google Scholar]
- 22.Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129(6):687–693. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felgner H, Frank R, Schliwa M. Flexural rigidity of microtubules measured with the use of optical tweezers. J Cell Sci. 1996;109(Pt 2):509–516. doi: 10.1242/jcs.109.2.509. [DOI] [PubMed] [Google Scholar]
- 24.Wiggins CH, Riveline D, Ott A, Goldstein RE. Trapping and wiggling: Elastohydrodynamics of driven microfilaments. Biophys J. 1998;74(2 Pt 1):1043–1060. doi: 10.1016/S0006-3495(98)74029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Heuvel MG, Bolhuis S, Dekker C. Persistence length measurements from stochastic single-microtubule trajectories. Nano Lett. 2007;7(10):3138–3144. doi: 10.1021/nl071696y. [DOI] [PubMed] [Google Scholar]
- 26.Janson ME, Dogterom M. A bending mode analysis for growing microtubules: Evidence for a velocity-dependent rigidity. Biophys J. 2004;87(4):2723–2736. doi: 10.1529/biophysj.103.038877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Mameren J, Vermeulen KC, Gittes F, Schmidt CF. Leveraging single protein polymers to measure flexural rigidity. J Phys Chem B. 2009;113(12):3837–3844. doi: 10.1021/jp808328a. [DOI] [PubMed] [Google Scholar]
- 28.Mickey B, Howard J. Rigidity of microtubules is increased by stabilizing agents. J Cell Biol. 1995;130(4):909–917. doi: 10.1083/jcb.130.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dogterom M, Yurke B. Measurement of the force-velocity relation for growing microtubules. Science. 1997;278(5339):856–860. doi: 10.1126/science.278.5339.856. [DOI] [PubMed] [Google Scholar]
- 30.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993;120(4):923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishijima S, Hiramoto Y. Flexural rigidity of echinoderm sperm flagella. Cell Struct Funct. 1994;19(6):349–362. doi: 10.1247/csf.19.349. [DOI] [PubMed] [Google Scholar]
- 32.Okuno M, Hiramoto Y. Mechanical stimulation of starfish sperm flagella. J Exp Biol. 1976;65(2):401–413. doi: 10.1242/jeb.65.2.401. [DOI] [PubMed] [Google Scholar]
- 33.Danuser G, Tran PT, Salmon ED. Tracking differential interference contrast diffraction line images with nanometre sensitivity. J Microsc. 2000;198(Pt 1):34–53. doi: 10.1046/j.1365-2818.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 34.Sage D, Neumann FR, Hediger F, Gasser SM, Unser M. Automatic tracking of individual fluorescence particles: Application to the study of chromosome dynamics. IEEE Trans Image Process. 2005;14(9):1372–1383. doi: 10.1109/tip.2005.852787. [DOI] [PubMed] [Google Scholar]
- 35.Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol Cell. 1991;72(1-2):3–14. doi: 10.1016/0248-4900(91)90072-u. [DOI] [PubMed] [Google Scholar]
- 36.Bornens M. Organelle positioning and cell polarity. Nat Rev Mol Cell Biol. 2008;9(11):874–886. doi: 10.1038/nrm2524. [DOI] [PubMed] [Google Scholar]
- 37.Fakhri N, et al. High-resolution mapping of intracellular fluctuations using carbon nanotubes. Science. 2014;344(6187):1031–1035. doi: 10.1126/science.1250170. [DOI] [PubMed] [Google Scholar]
- 38.Guo M, et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell. 2014;158(4):822–832. doi: 10.1016/j.cell.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antoniades I, Stylianou P, Skourides PA. Making the connection: Ciliary adhesion complexes anchor basal bodies to the actin cytoskeleton. Dev Cell. 2014;28(1):70–80. doi: 10.1016/j.devcel.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Gittes F, Schnurr B, Olmsted PD, MacKintosh FC, Schmidt CF. Microscopic viscoelasticity: Shear moduli of soft materials determined from thermal fluctuations. Phys Rev Lett. 1997;79(17):3286–3289. [Google Scholar]
- 41.Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA. Cytoplasmic diffusion: Molecular motors mix it up. J Cell Biol. 2008;183(4):583–587. doi: 10.1083/jcb.200806149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25(4-5):337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 43.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M-Z, et al. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci USA. 2004;101(8):2311–2316. doi: 10.1073/pnas.0400073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188(6):877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301(1):23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464(7291):1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 49.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: Pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA. 2009;106(9):3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth KE, Rieder CL, Bowser SS. Flexible-substratum technique for viewing cells from the side: Some in vivo properties of primary (9+0) cilia in cultured kidney epithelia. J Cell Sci. 1988;89(Pt 4):457–466. doi: 10.1242/jcs.89.4.457. [DOI] [PubMed] [Google Scholar]
- 51.Battle C, Lautscham L, Schmidt CF. Differential interference contrast microscopy using light-emitting diode illumination in conjunction with dual optical traps. Rev Sci Instrum. 2013;84(5):053703. doi: 10.1063/1.4804597. [DOI] [PubMed] [Google Scholar]
- 52.Burnette DT, et al. A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol. 2011;13(4):371–381. doi: 10.1038/ncb2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.