Abstract
Lactose permease (LacY), a paradigm for the largest family of membrane transport proteins, catalyzes the coupled translocation of a galactoside and an H+ across the Escherichia coli membrane (galactoside/H+ symport). Initial X-ray structures reveal N- and C-terminal domains, each with six largely irregular transmembrane helices surrounding an aqueous cavity open to the cytoplasm. Recently, a structure with a narrow periplasmic opening and an occluded galactoside was obtained, confirming many observations and indicating that sugar binding involves induced fit. LacY catalyzes symport by an alternating access mechanism. Experimental findings garnered over 45 y indicate the following: (i) The limiting step for lactose/H+ symport in the absence of the H+ electrochemical gradient (∆µ̃H+) is deprotonation, whereas in the presence of ∆µ̃H+, the limiting step is opening of apo LacY on the other side of the membrane; (ii) LacY must be protonated to bind galactoside (the pK for binding is ∼10.5); (iii) galactoside binding and dissociation, not ∆µ̃H+, are the driving forces for alternating access; (iv) galactoside binding involves induced fit, causing transition to an occluded intermediate that undergoes alternating access; (v) galactoside dissociates, releasing the energy of binding; and (vi) Arg302 comes into proximity with protonated Glu325, causing deprotonation. Accumulation of galactoside against a concentration gradient does not involve a change in Kd for sugar on either side of the membrane, but the pKa (the affinity for H+) decreases markedly. Thus, transport is driven chemiosmotically but, contrary to expectation, ∆µ̃H+ acts kinetically to control the rate of the process.
Keywords: X-ray crystal structure, membrane proteins, transport, conformational change, MFS
The lactose permease of Escherichia coli (LacY) specifically binds and catalyzes symport of d-Gal and d-galactopyranosides with an H+ (galactoside/H+ symport), but it does not recognize the analogous glucopyranosides, which differ only in the orientation of the C4-OH of the pyranosyl ring (reviewed in refs. 1, 2). Typical of many major facilitator superfamily (MFS) members, LacY couples the free energy released from downhill translocation of H+ in response to an H+ electrochemical gradient (∆µ̃H+) to drive accumulation of galactopyranosides against a concentration gradient. Because coupling between sugar and H+ translocation is obligatory, in the absence of ∆µ̃H+, LacY can also transduce the energy released from the downhill transport of sugar to drive uphill H+ transport with the generation of ∆µ̃H+, the polarity of which depends upon the direction of the sugar gradient. However, the mechanism by which this so-called “chemiosmotic” process occurs remains obscure. This contribution aims at clarifying the specific steps underpinning the mechanism of galactoside/H+ symport.
Structural Evidence for an Occluded Intermediate
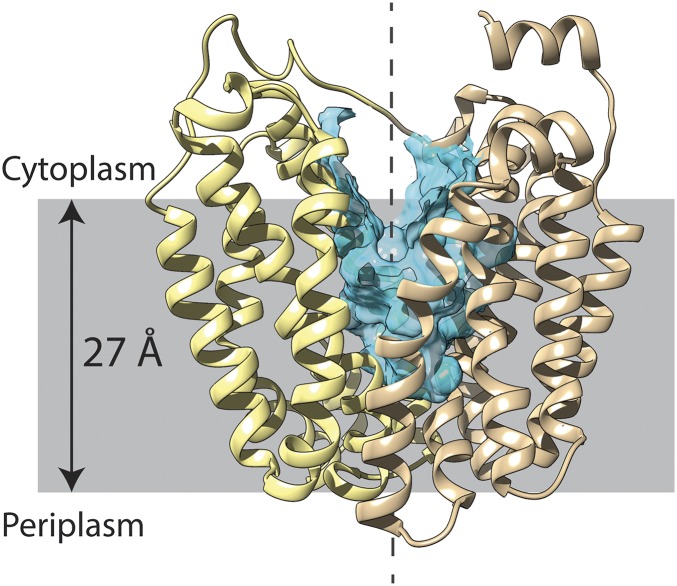
Initial X-ray structures of LacY were obtained with a conformationally restricted mutant C154G (3, 4) and WT LacY (5), and they are in an indistinguishable inward-facing conformation (Fig. 1). At the same time, a similar structure was determined for the glycerol-3-phosphate permease (6), which catalyzes phosphate/glycerol-3-phosphate exchange. The structures consist of two 6-helix bundles related by a quasi-twofold symmetry axis perpendicular to the membrane plane, linked by a long cytoplasmic loop between helices VI and VII. Furthermore, in each six-helix bundle, there are two 3-helix bundles with inverted symmetry. The two 6-helix bundles surround a deep hydrophilic cavity tightly sealed on the periplasmic face and open to the cytoplasmic side only (an inward-open conformation). The initial structures led to the “rocker-switch” model for transport in which the two 6-helix bundles rotate against each other around the middle of the protein, thereby exposing the substrate-binding site alternatively to either side of the membrane (also known as the alternating access model).
Fig. 1.
LacY ribbon presentation in an inward-open conformation with a twofold axis of symmetry (broken line). (Left) N-terminal helix bundle (light yellow). (Right) C-terminal helix bundle (tan). The cytoplasmic side is shown at the top. The blue region represents the hydrophilic cavity, and the gray-shaded area represents the membrane.
Although LacY contains 65–70% unequivocally hydrophobic side chains and crystal structures reflect only a single lowest energy conformation, the entire backbone appears to be accessible to water (7–9). In addition, an abundance of biochemical and spectroscopic data demonstrates that galactoside binding causes the molecule to open reciprocally on either side of the membrane, thereby providing almost unequivocal evidence for an alternating access model (discussed below). The first structure of LacY was obtained with a density at the apex of the central cavity, but because of limited resolution, the identity of the bound sugar and/or side-chain interactions was difficult to specify with certainty. However, biochemical and spectroscopic studies show that LacY contains a single galactoside-binding site and that the residues involved in sugar binding are located at or near the apex of the central, aqueous cavity in the approximate middle of the molecule.
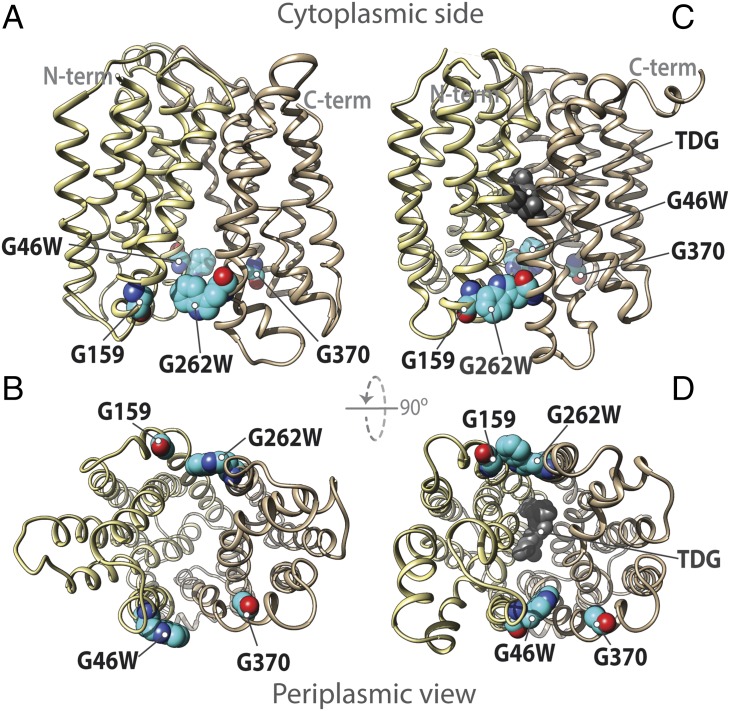
Among the conserved residues in LacY and many other MFS members are two Gly-Gly pairs between the N- and C-terminal six-helix bundles on the periplasmic side of LacY at the ends of helices II and XI (Gly46 and Gly370, respectively) and helices V and VIII (Gly159 and Gly262, respectively) (10). When Gly46 (helix II) and Gly262 (helix VIII) are replaced with bulky Trp residues (Fig. 2), transport activity is abrogated with little or no effect on galactoside affinity, but markedly increased accessibility of galactoside to the binding site is observed, indicating that the G46W/G262W mutant is open on the periplasmic side (11). Moreover, site-directed alkylation and stopped-flow binding kinetics indicate that the G46W/G262W mutant is physically open on the periplasmic side (an outward-open conformation).
Fig. 2.
Trp replacements in two pairs of Gly-Gly residues that connect the N- and C-terminal six-helix domains on the periplasmic side of LacY. The 12 transmembrane helices that make up LacY are colored light yellow [N-terminal (N-term) bundle] and tan [C-terminal (C-term) bundle]. Gly residues G159 and G370 in helices V and XI, respectively, and Trp replacements G46W (helix II) and G262W (helix VIII) are indicated. The putative outward-open structure is viewed from the side (A) or from the periplasm (B). The crystal structure of the almost occluded, narrow outward-open conformer of LacY with Gly→Trp replacements at positions 46 and 262 and bound galactoside (dark gray) is viewed from the side (C) or the periplasm (D), respectively.
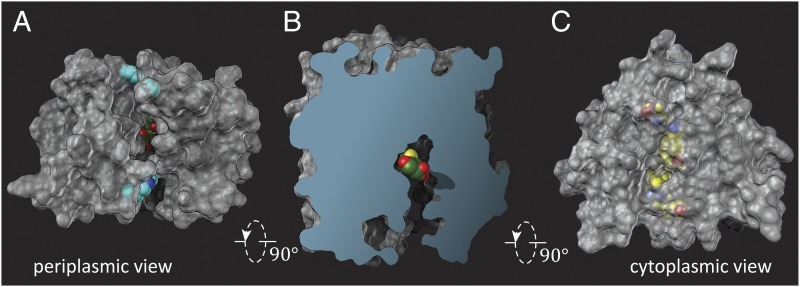
An X-ray structure of LacY mutant G46W/G262W cocrystallized in the presence of the relatively high-affinity, symmetrical lactose analog β-d-galactopyranosyl-1-thio-β-d-galactopyranoside (TDG) was determined at a resolution of 3.5 Å, and, importantly, crystals were not obtained in the absence of a galactoside (12). Two molecules in the asymmetrical unit are adjacent to one another but in opposite-facing orientations. Surprisingly, both molecules are in an almost occluded conformation with a narrow periplasmic opening and a single molecule of TDG in the central sugar-binding site. A space-filling view of the molecule from the periplasmic side (Fig. 3A) reveals the bound TDG through an opening that is too narrow to allow entrance or exit of the sugar (13) (∼3 Å at the narrowest point; Fig. 3B). In contrast, the cytoplasmic side of the molecule is tightly sealed (Fig. 3C). The double-Trp mutant is sufficiently open to bind galactoside rapidly (11), but when binding occurs and the mutant attempts to transition into an occluded state, it cannot do so completely because the bulky Trp residues block complete closure. Thus, the mutant binds galactoside, which initiates transition into an intermediate occluded state that it cannot complete, and this finding probably accounts for why the mutant is completely unable to catalyze transport of any type across the membrane. It is also apparent that the transport cycle includes an occluded intermediate conformer.
Fig. 3.
Surface renditions of LacY G46W/G262W molecule A. (A) View from the periplasmic side showing TDG (green and red spheres) just visible within the molecule. Trp residues are shown in blue. (B) Slab view. (C) View from the cytoplasmic side with the residues that form a zipper-like motif to seal that side.
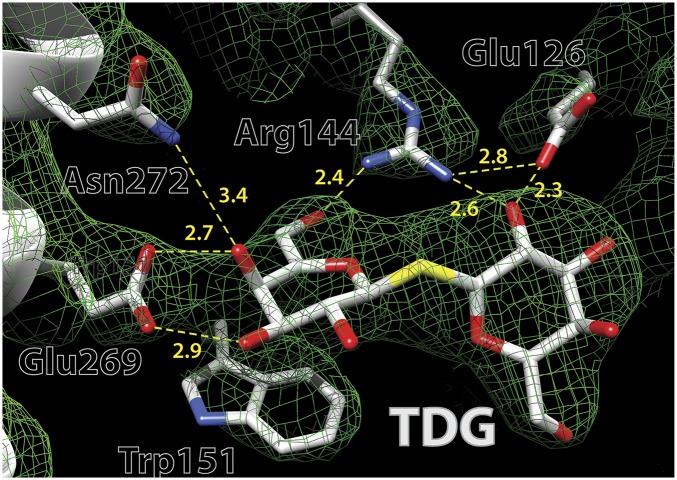
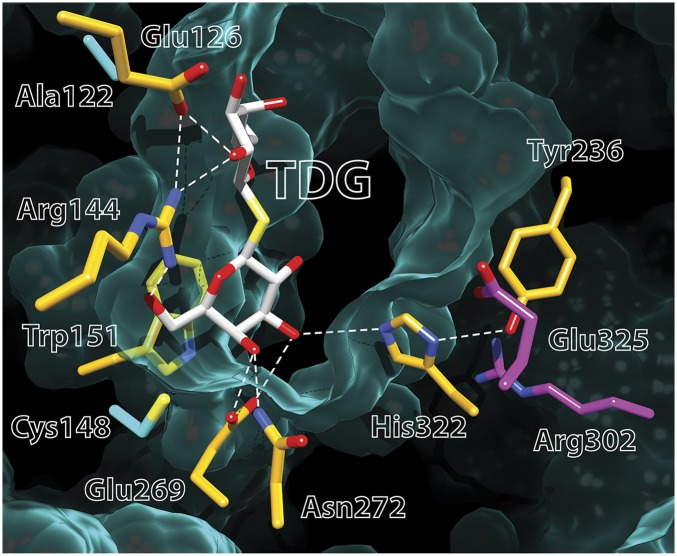
A TDG molecule is clearly defined in the almost occluded central cavity (Fig. 4) that allows assignment of likely H-bond interactions with the protein, although interatomic distances are only estimates at a resolution of 3.5 Å. Specificity is directed toward the galactopyranoside ring, and α-galactosides bind with higher affinity than the β-anomers (14–17). One galactopyranosyl ring of TDG stacks hydrophobically with Trp151 (helix V), confirming biochemical (18) and spectroscopic (19) findings. Glu269 (helix VIII) is the acceptor of H bonds from the C4-OH and C3-OH groups of the galactopyranosyl ring, indicating that it is probably the primary determinant for specificity. Even conservative replacement with an Asp abolishes binding and inactivates lactose transport (20–22). The η1 NH2 of Arg144 (helix V) donates an H bond to O5 in the ring and is also within H-bond distance of the C6-OH. The η2 NH2 group of Arg144 donates H bonds to the C2′-OH of TDG and to Glu126 Oε2. Conservative replacement of Arg144 with Lys, as well as neutral replacements, virtually destroys binding and transport (22, 23). Glu126 (helix IV) acts as an H-bond acceptor from the C2′-OH of TDG and is an H-bond acceptor from the η2 NH2 of Arg144. Replacement with Asp causes markedly diminished binding affinity, and removal of the carboxyl group abolishes binding and transport (21–23).
Fig. 4.
Electron density map contoured at 1σ (green mesh) of the sugar-binding site of LacY G46W/G262W. The density is superimposed on the structure, which is shown as sticks, with carbon atoms in gold, oxygen atoms in red, and nitrogen atoms in blue. Broken lines represent putative H bonds.
Remarkably, His322 (helix X), long thought to be involved in H+ transport by implication, likely acts as an H-bond donor/acceptor between the εNH of the imidazole ring and the C3-OH of TDG, and it is stabilized by an H-bond donor/acceptor between the δΝΗ of the imidazole and the OH of Tyr236, which was also thought to be involved in H+ transport (Fig. 5). All replacements for His322 exhibit little or no binding and no transport activity (22, 24, 25). Finally, Asn272 (helix VIII) donates an H bond to the C4-OH of TDG; Gln is the only replacement tolerated by LacY with respect to binding and transport (26).
Fig. 5.
Cytoplasmic view of the active site in double-Trp LacY. TDG is shown as green sticks, and side chains forming H bonds with TDG are shown in yellow. Broken lines represent likely H bonds. Ala122 and Cys148, which are close to TDG but do not make direct contact, are shown in cyan. Glu325 and Arg302 are shown in purple. The green felt-like area represents the Van der Waals lining of the cavity. Note the narrow opening on the periplasmic side.
In addition to the residues involved in galactoside binding, Cys148 (helix V), well known with respect to substrate protection against alkylation (reviewed in ref. 27), is close to bound TDG, but not sufficiently close to interact directly (Fig. 5). Similarly, replacement of Ala122 (helix IV) with bulky side chains or alkylation of A122C with bulky thiol reagents causes LacY to become specific for the monosaccharide Gal, and disaccharide binding and transport are blocked (28). However, Ala122 does not make direct contact with TDG either. Asp240 (helix VII) and Lys319 (helix X) interact relatively weakly, and mutants with double-neutral replacements (Cys or Ala) exhibit low but significant ability to catalyze lactose accumulation (29–31).
Although Glu325 (helix X) and Arg302 (helix IX) do not make direct contact with bound galactoside, both are critically involved in coupled H+ translocation. Neutral replacement of either residue yields mutants that are defective in all transport reactions that involve net H+ transport but catalyze equilibrium exchange and/or counterflow as well or better than WT (1, 2).
Sugar Binding Involves Induced Fit
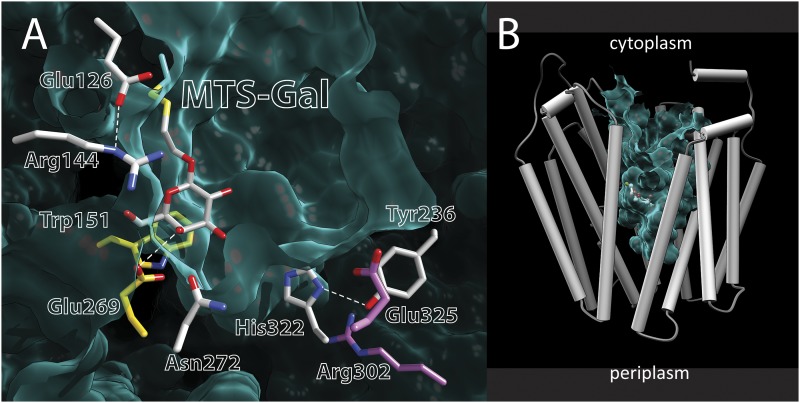
In the structure of single-Cys122 LacY with covalently bound methanethiosulfonyl (MTS)-Gal, a suicide inactivator for this mutant (32), the galactosyl moiety occupies the same position in the protein as in the double-Trp mutant (33). In addition, two important ligands, Trp151 and Glu269, interact with the galactopyranosyl ring (Fig. 6A). However, as opposed to the almost occluded, open-outward conformation of the double-Trp mutant, LacY with covalently bound MTS-Gal in the binding site exhibits an inward-open conformation (Fig. 6B), indicating that the galactoside must be fully liganded in order for LacY to transition into the occluded state. In view of this consideration and observations indicating that the alternating access mechanism of LacY is driven by galactoside binding and dissociation and not by ∆µ̃H+ (1, 2, 34–36), it seems highly likely that sugar binding involves induced fit. By this means, the N- and C-terminal bundles converge as given side chains from both the N- and C-terminal helix bundles ligate the galactoside. The energetic cost of binding and the resultant conformational change are regained upon sugar dissociation and provide the energy for a further structural change that allows deprotonation. With respect to induced fit, it is also notable that mutation of any single binding-site residue causes a marked decrease or complete loss of affinity (22). In brief, the mechanism of LacY resembles the mechanism of an enzyme, with the difference being that the protein, rather than the substrate, forms the transition state.
Fig. 6.
Crystal structure of single-Cys122 LacY with covalently bound MTS-Gal. (A) Side chains are shown as sticks. The yellow side chains (Glu269 and Trp151) make direct contact with the galactopyranosyl ring of MTS-Gal covalently bound to a Cys at position 122. The gray side chains are not sufficiently close to make contact with the galactopyranosyl ring. Glu325 and Arg302 (in purple) are involved in H+ transport. The green felt-like area represents the Van der Waals lining of the cavity. Note that the periplasmic side is closed. (B) Structure of single-Cys122 LacY with covalently bound MTS-Gal viewed from the side. Helices are depicted as rods, and MTS-Gal is shown as spheres colored by atom type with carbon in green. The aqueous central cavity open to the cytoplasmic side is colored light green.
Seven Independent Lines of Support for the Alternating Access Model
As postulated, alternating access involves reciprocal access of galactoside- and H+-binding sites to either side of the membrane. Over the past few years, almost incontrovertible evidence for this structural mechanism has accrued with LacY (reviewed in refs. 37, 38):
-
i)
Because thiol cross-linking yields the closest distance between Cys residues, it was suggested that galactoside binding induces closure of the cytoplasmic cavity (3).
-
ii)
Site-directed alkylation of Cys replacements at every position in LacY shows that Cys replacements on the periplasmic side exhibit increased reactivity upon galactoside binding, whereas Cys replacements on the cytoplasmic side show decreased reactivity (39–44).
-
iii)
Single-molecule FRET studies indicate that the periplasmic side opens and the cytoplasmic cavity closes upon sugar binding (45).
-
iv)
Double electron-electron resonance (DEER) reveals that LacY exists in at least four conformations even in the absence of galactoside and that galactoside binding induces a shift in the population toward longer distances on the periplasmic side and shorter distances on the cytoplasmic side (46, 47).
-
v)
Site-directed thiol cross-linking shows that the periplasmic cavity must open and close for transport to occur. Furthermore, the periplasmic side opens upon galactoside binding to approximately the same extent as observed with DEER (48).
-
vi)
Trp151→p-nitrophenyl-α-d-galactopyranoside (NPG) FRET exhibits practically identical kinetics of galactoside binding and displacement with LacY in inward- and outward-facing conformations (49).
-
vii)
Utilization of Trp→bimane or His→Trp FRET to determine opening/closing of periplasmic or cytoplasmic cavities combined with Trp151→NPG FRET to measure galactoside binding, both in real time, shows that opening and closing are reciprocal and that opening of the periplasmic cavity controls closing of the cytoplasmic cavity (12, 50–52).
Mechanism of Lactose/H+ Symport
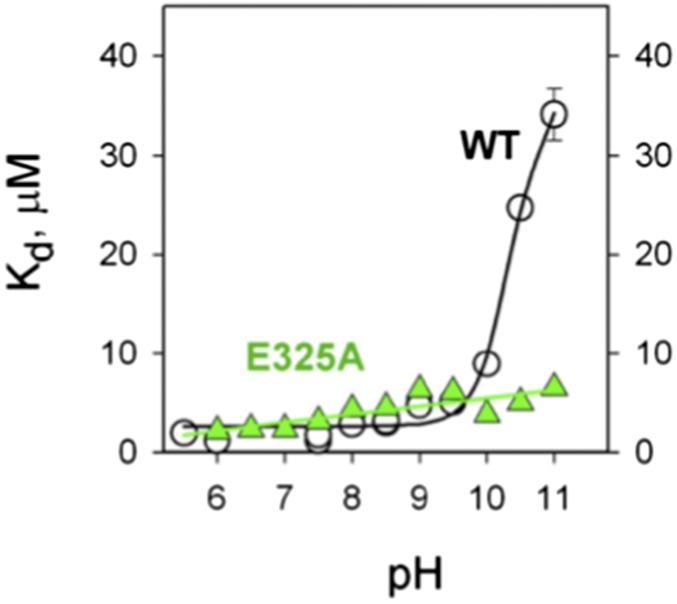
The affinity of WT LacY for galactosides (Kd) varies with pH to yield a pK of ∼10.5 (22, 50, 53). In addition, sugar binding to purified LacY in detergent does not induce a change in ambient pH under conditions where binding or release of 1 H+/LacY can be determined (53). Therefore, LacY is protonated over the physiological range of pH (Fig. 7). These observations and many others (1, 2) provide evidence for a symmetrical ordered kinetic mechanism in which protonation precedes galactoside binding on one side of the membrane and follows sugar dissociation on the other side (Fig. 8). Recent observations (54) also suggest that a similar ordered mechanism may be common to other members of the MFS as well. Importantly, as mentioned above, mutants with neutral replacements for Glu325 catalyze equilibrium exchange and counterflow (the shaded reactions in Fig. 8) but do not catalyze any reaction involving net H+ transport (55, 56). Dramatically, the titration observed in Fig. 7 is abolished in mutant E325A and mutants with other neutral replacements for Glu325, which bind with high affinity up to pH 11, when LacY begins to denature. This behavior is highly unusual and suggests that Glu325 may be the sole residue directly involved in H+ binding and transport [all 417 residues in LacY have been mutated and tested for transport activity (21)]. Thus, LacY cannot sustain a negative charge on Glu325 and bind galactoside simultaneously, and Glu325 must be protonated to bind sugar.
Fig. 7.
Effect of pH on the apparent Kd (Kdapp) for TDG binding to WT LacY (black) and the E325A mutant (green).
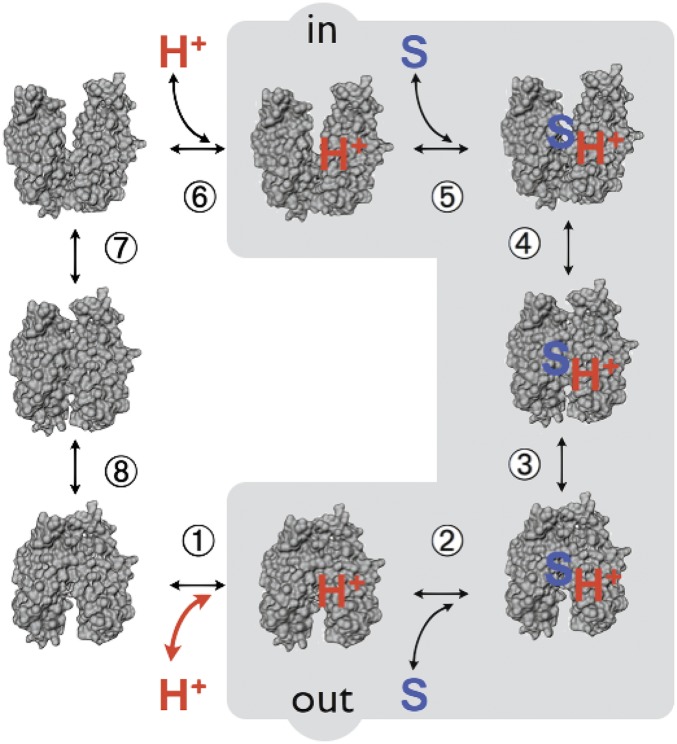
Fig. 8.
Kinetic scheme for galactoside/H+ symport, exchange, and counterflow. Symport starts with protonation of LacY (step 1 or 6 for influx or effux, respectively), which is required for high-affinity binding of lactose. Sugar (S) binding to protonated LacY (step 2 or 5) causes a conformational change to an occluded state (step 3 or 4), which can relax to either side where sugar dissociates first (step 2 or 5), followed by deprotonation (step 1 or 6) and return of unloaded LacY via an apo occluded intermediate (steps 7 and 8). Exchange or counterflow involves only steps 2–5 (gray shaded area). Because LacY catalyzes symport in both directions, when symport is in the influx direction (step 1, protonation), the pK is very alkaline (∼10.5), and step 6 (deprotonation) must have a much lower pK for deprotonation to occur (i.e., Arg302 approximates protonated Glu325). However, in the efflux direction, the pKs of these steps are reversed.
However, deprotonation is also critical for turnover, and with an apparent pK of 10.5, how does deprotonation occur? One possibility is that the pKa of Glu325, which is in a hydrophobic pocket, may decrease by becoming more accessible to water. However, evidence has been presented indicating that Arg302 is important in this capacity (57, 58). Like neutral replacements for Glu325, mutants R302A and R302S are also specifically defective in translocation reactions that involve H+ translocation—accumulation of lactose against a concentration gradient, as well as efflux—but they bind ligand and catalyze equilibrium exchange. Perhaps the positively charged guanidium group at position 302 facilitates deprotonation of Glu325. Although Tyr236 lies between Arg302 and Glu325 in the current structure (Fig. 5), double-mutant R302C/E325C exhibits excimer fluorescence when labeled with pyrene maleimide (59) and double-mutant R302H/E325H binds Mn(II) with micromolar affinity (60). Therefore, Arg302 and Glu325 may assume closer proximity in another conformation of LacY. Interestingly, a similar mechanism has been suggested for H+ transport through the Fo portion of F1/Fo-ATPase, where an Arg residue in subunit a is postulated to facilitate deprotonation of an Asp residue in the c subunit (reviewed in refs. 61, 62).
Because equilibrium exchange and counterflow are unaffected by imposition of ∆µ̃H+, it is apparent that the conformational change resulting in alternating accessibility of galactoside- and H+-binding sites to either side of the membrane is the result of sugar binding and dissociation, and not ∆µ̃H+ (reviewed in refs. 1, 2). It is also apparent that fully loaded LacY is not charged. Moreover, lactose/H+ symport from a high- to low-lactose concentration in the absence of ∆µ̃H+ exhibits a primary deuterium isotope effect that is not observed for ∆µ̃H+-driven lactose/H+ symport, equilibrium exchange, or counterflow (63, 64). Thus, it is likely that the rate-limiting step for lactose/H+ symport in the absence of ∆µ̃H+ is deprotonation (65, 66), whereas in the presence of ∆µ̃H+, opening of apo LacY on the other side of the membrane is rate-limiting. In other words, by changing the rate-limiting step, ∆µ̃H+ causes more rapid cycling.
Mechanism for Chemiosmotic Lactose/H+ Symport
Taken as a whole, the observations suggest the following considerations regarding the mechanism of chemiosmotic coupling in LacY:
-
i)
Symport in the absence or presence of ∆µ̃H+ is the same overall reaction. The limiting step for lactose/H+ symport in the absence of ∆µ̃H+ is deprotonation (a kinetic isotope effect is observed with D2O). The limiting step in the presence of a ∆µ̃H+ is likely the conformational change associated with opening of the cavity on the other side of the membrane.
-
ii)
LacY must be protonated (possibly Glu325 specifically) to bind sugar (the pK for binding is ∼10.5 and is abolished in mutants with neutral replacements for Glu325).
-
iii)
Sugar binding and dissociation, rather than ∆µ̃H+, are the driving force for alternating access.
-
iv)
Sugar binding involves induced fit, causing a transition to an occluded intermediate that undergoes alternating access.
-
v)
Sugar dissociates, releasing the energy of binding.
-
vi)
A conformational change allows Arg302 to approximate protonated Glu325, resulting in deprotonation.
-
vii)
Apo LacY opens on the other side of the membrane, and the cycle is reinitiated.
Strikingly, accumulation of galactoside against a concentration gradient does not involve a change in Kd for sugar on either side of the membrane, but the pK (the affinity for H+) decreases markedly. Moreover, it is apparent that ∆µ̃H+ does not have a direct effect on the global structural change that corresponds to alternating access. Thus, transport is driven chemiosmotically, and ∆µ̃H+ acts kinetically to control the rate of the process. Finally, it should be relatively simple and straightforward to test the generality of this basic notion by determining whether or not an imposed ∆µ̃H+ alters the rate of counterflow or equilibrium exchange with other members of the MFS.
Acknowledgments
This article is dedicated to the memory of my close friend and colleague, Wilhelmus Nicolaas Konings, who died on July 5, 2014. I am deeply indebted to the members of my research group and my collaborators over the past 40 years who contributed their minds, hearts, and hands to this work. At one time or another, the studies were supported financially by the National Heart (now Heart and Lung) Institute; the Roche Institute of Molecular Biology; the Howard Hughes Medical Institute; National Institutes of Health Grants DK51131, DK069463, and GM073210; and National Science Foundation Grant MCB-1129551.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madej MG, Kaback HR. 2014. The life and times of Lac permease: Crystals ain’t enough, but they certainly do help. Membrane Transporter Function: To Structure and Beyond, Springer Series in Biophysics: Transporters, eds Ziegler C, Kraemer R (Springer, Heidelberg) Vol 17, pp 121–158.
- 3.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301(5633):610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 4.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H+ symport in LacY. EMBO J. 2006;25(6):1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci USA. 2007;104(39):15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 7.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc Natl Acad Sci USA. 1998;95(11):6114–6117. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patzlaff JS, Moeller JA, Barry BA, Brooker RJ. Fourier transform infrared analysis of purified lactose permease: A monodisperse lactose permease preparation is stably folded, alpha-helical, and highly accessible to deuterium exchange. Biochemistry. 1998;37(44):15363–15375. doi: 10.1021/bi981142x. [DOI] [PubMed] [Google Scholar]
- 9.Sayeed WM, Baenziger JE. Structural characterization of the osmosensor ProP. Biochim Biophys Acta. 2009;1788(5):1108–1115. doi: 10.1016/j.bbamem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Kasho VN, Smirnova IN, Kaback HR. Sequence alignment and homology threading reveals prokaryotic and eukaryotic proteins similar to lactose permease. J Mol Biol. 2006;358(4):1060–1070. doi: 10.1016/j.jmb.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smirnova I, Kasho V, Sugihara J, Kaback HR. Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation. Proc Natl Acad Sci USA. 2013;110(22):8876–8881. doi: 10.1073/pnas.1306849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar H, et al. Structure of sugar-bound LacY. Proc Natl Acad Sci USA. 2014;111(5):1784–1788. doi: 10.1073/pnas.1324141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrini-Calace M, Maiwald T, Thornton JM. PoreWalker: A novel tool for the identification and characterization of channels in transmembrane proteins from their three-dimensional structure. PLOS Comput Biol. 2009;5(7):e1000440. doi: 10.1371/journal.pcbi.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandermann H., Jr beta-D-Galactoside transport in Escherichia coli: Substrate recognition. Eur J Biochem. 1977;80(2):507–515. doi: 10.1111/j.1432-1033.1977.tb11906.x. [DOI] [PubMed] [Google Scholar]
- 15.Sahin-Tóth M, Lawrence MC, Nishio T, Kaback HR. The C-4 hydroxyl group of galactopyranosides is the major determinant for ligand recognition by the lactose permease of Escherichia coli. Biochemistry. 2001;40(43):13015–13019. doi: 10.1021/bi011233l. [DOI] [PubMed] [Google Scholar]
- 16.Sahin-Tóth M, Akhoon KM, Runner J, Kaback HR. Ligand recognition by the lactose permease of Escherichia coli: Specificity and affinity are defined by distinct structural elements of galactopyranosides. Biochemistry. 2000;39(17):5097–5103. doi: 10.1021/bi0000263. [DOI] [PubMed] [Google Scholar]
- 17.Sahin-Tóth M, Gunawan P, Lawrence MC, Toyokuni T, Kaback HR. Binding of hydrophobic D-galactopyranosides to the lactose permease of Escherichia coli. Biochemistry. 2002;41(43):13039–13045. doi: 10.1021/bi0203076. [DOI] [PubMed] [Google Scholar]
- 18.Guan L, Hu Y, Kaback HR. Aromatic stacking in the sugar binding site of the lactose permease. Biochemistry. 2003;42(6):1377–1382. doi: 10.1021/bi027152m. [DOI] [PubMed] [Google Scholar]
- 19.Vázquez-Ibar JL, Guan L, Svrakic M, Kaback HR. Exploiting luminescence spectroscopy to elucidate the interaction between sugar and a tryptophan residue in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 2003;100(22):12706–12711. doi: 10.1073/pnas.1835645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ujwal ML, Sahin-Tóth M, Persson B, Kaback HR. Role of glutamate-269 in the lactose permease of Escherichia coli. Mol Membr Biol. 1994;11(1):9–16. doi: 10.3109/09687689409161024. [DOI] [PubMed] [Google Scholar]
- 21.Frillingos S, Sahin-Tóth M, Wu J, Kaback HR. Cys-scanning mutagenesis: A novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 1998;12(13):1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- 22.Smirnova I, Kasho V, Sugihara J, Choe JY, Kaback HR. Residues in the H+ translocation site define the pKa for sugar binding to LacY. Biochemistry. 2009;48(37):8852–8860. doi: 10.1021/bi9011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frillingos S, Gonzalez A, Kaback HR. Cysteine-scanning mutagenesis of helix IV and the adjoining loops in the lactose permease of Escherichia coli: Glu126 and Arg144 are essential. off. Biochemistry. 1997;36(47):14284–14290. doi: 10.1021/bi972314d. [DOI] [PubMed] [Google Scholar]
- 24.Padan E, Sarkar HK, Viitanen PV, Poonian MS, Kaback HR. Site-specific mutagenesis of histidine residues in the lac permease of Escherichia coli. Proc Natl Acad Sci USA. 1985;82(20):6765–6768. doi: 10.1073/pnas.82.20.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Püttner IB, Sarkar HK, Poonian MS, Kaback HR. lac permease of Escherichia coli: Histidine-205 and histidine-322 play different roles in lactose/H+ symport. Biochemistry. 1986;25(16):4483–4485. doi: 10.1021/bi00364a003. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Villafuerte MK, Andersson M, White SH, Kaback HR. Galactoside-binding site in LacY. Biochemistry. 2014;53(9):1536–1543. doi: 10.1021/bi401716z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaback HR, Sahin-Tóth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2(8):610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 28.Guan L, Sahin-Toth M, Kaback HR. Changing the lactose permease of Escherichia coli into a galactose-specific symporter. Proc Natl Acad Sci USA. 2002;99(10):6613–6618. doi: 10.1073/pnas.102178299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King SC, Hansen CL, Wilson TH. The interaction between aspartic acid 237 and lysine 358 in the lactose carrier of Escherichia coli. Biochim Biophys Acta. 1991;1062(2):177–186. doi: 10.1016/0005-2736(91)90390-t. [DOI] [PubMed] [Google Scholar]
- 30.Sahin-Tóth M, Dunten RL, Gonzalez A, Kaback HR. Functional interactions between putative intramembrane charged residues in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 1992;89(21):10547–10551. doi: 10.1073/pnas.89.21.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin-Tóth M, Kaback HR. Properties of interacting aspartic acid and lysine residues in the lactose permease of Escherichia coli. Biochemistry. 1993;32(38):10027–10035. doi: 10.1021/bi00089a019. [DOI] [PubMed] [Google Scholar]
- 32.Guan L, Sahin-Tóth M, Kálai T, Hideg K, Kaback HR. Probing the mechanism of a membrane transport protein with affinity inactivators. J Biol Chem. 2003;278(12):10641–10648. doi: 10.1074/jbc.M211355200. [DOI] [PubMed] [Google Scholar]
- 33.Chaptal V, et al. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc Natl Acad Sci USA. 2011;108(23):9361–9366. doi: 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efflux, exchange, and counterflow. Biochemistry. 1979;18(17):3691–3697. doi: 10.1021/bi00584a009. [DOI] [PubMed] [Google Scholar]
- 35.Kaczorowski GJ, Robertson DE, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2.Effect of imposed delata psi, delta pH, and Delta mu H+ Biochemistry. 1979;18(17):3697–3704. doi: 10.1021/bi00584a010. [DOI] [PubMed] [Google Scholar]
- 36.Garcia ML, Viitanen P, Foster DL, Kaback HR. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983;22(10):2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- 37.Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y. The alternating access transport mechanism in LacY. J Membr Biol. 2011;239(1-2):85–93. doi: 10.1007/s00232-010-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smirnova I, Kasho V, Kaback HR. Lactose permease and the alternating access mechanism. Biochemistry. 2011;50(45):9684–9693. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104(2):491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang X, Nie Y, Kaback HR. Site-directed alkylation studies with LacY provide evidence for the alternating access model of transport. Biochemistry. 2011;50(10):1634–1640. doi: 10.1021/bi101988s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X, et al. Evidence for an intermediate conformational state of LacY. Proc Natl Acad Sci USA. 2012;109(12):E698–E704. doi: 10.1073/pnas.1201107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang X, Driessen AJ, Feringa BL, Kaback HR. The periplasmic cavity of LacY mutant Cys154→Gly: How open is open? Biochemistry. 2013;52(37):6568–6574. doi: 10.1021/bi401026d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie Y, Ermolova N, Kaback HR. Site-directed alkylation of LacY: Effect of the proton electrochemical gradient. J Mol Biol. 2007;374(2):356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie Y, Kaback HR. Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc Natl Acad Sci USA. 2010;107(21):9903–9908. doi: 10.1073/pnas.1004515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majumdar DS, et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci USA. 2007;104(31):12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104(42):16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madej MG, Soro SN, Kaback HR. Apo-intermediate in the transport cycle of lactose permease (LacY) Proc Natl Acad Sci USA. 2012;109(44):E2970–E2978. doi: 10.1073/pnas.1211183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci USA. 2008;105(10):3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smirnova IN, Kasho VN, Kaback HR. Direct sugar binding to LacY measured by resonance energy transfer. Biochemistry. 2006;45(51):15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smirnova IN, Kasho V, Kaback HR. Protonation and sugar binding to LacY. Proc Natl Acad Sci USA. 2008;105(26):8896–8901. doi: 10.1073/pnas.0803577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smirnova I, Kasho V, Sugihara J, Kaback HR. Probing of the rates of alternating access in LacY with Trp fluorescence. Proc Natl Acad Sci USA. 2009;106(51):21561–21566. doi: 10.1073/pnas.0911434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smirnova I, Kasho V, Sugihara J, Kaback HR. Opening the periplasmic cavity in lactose permease is the limiting step for sugar binding. Proc Natl Acad Sci USA. 2011;108(37):15147–15151. doi: 10.1073/pnas.1112157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smirnova I, Kasho V, Sugihara J, Vázquez-Ibar JL, Kaback HR. Role of protons in sugar binding to LacY. Proc Natl Acad Sci USA. 2012;109(42):16835–16840. doi: 10.1073/pnas.1214890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madej MG, Kaback HR. Evolutionary mix-and-match with MFS transporters II. Proc Natl Acad Sci USA. 2013;110(50):E4831–E4838. doi: 10.1073/pnas.1319754110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrasco N, Antes LM, Poonian MS, Kaback HR. lac permease of Escherichia coli: Histidine-322 and glutamic acid-325 may be components of a charge-relay system. Biochemistry. 1986;25(16):4486–4488. doi: 10.1021/bi00364a004. [DOI] [PubMed] [Google Scholar]
- 56.Carrasco N, et al. Characterization of site-directed mutants in the lac permease of Escherichia coli. 2. Glutamate-325 replacements. Biochemistry. 1989;28(6):2533–2539. doi: 10.1021/bi00432a028. [DOI] [PubMed] [Google Scholar]
- 57.Sahin-Toth M, Kaback HR. Arg-302 facilitates deprotonation of Glu-325 in the transport mechanism of the lactose permease from Escherichia coli. Proc Natl Acad Sci USA. 2001;98(11):6068–6073. doi: 10.1073/pnas.111139698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson M, et al. Proton-coupled dynamics in lactose permease. Structure. 2012;20(11):1893–1904. doi: 10.1016/j.str.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung K, Jung H, Wu J, Privé GG, Kaback HR. Use of site-directed fluorescence labeling to study proximity relationships in the lactose permease of Escherichia coli. Biochemistry. 1993;32(46):12273–12278. doi: 10.1021/bi00097a001. [DOI] [PubMed] [Google Scholar]
- 60.He MM, Voss J, Hubbell WL, Kaback HR. Use of designed metal-binding sites to study helix proximity in the lactose permease of Escherichia coli. 2. Proximity of helix IX (Arg302) with helix X (His322 and Glu325) Biochemistry. 1995;34(48):15667–15670. doi: 10.1021/bi00048a010. [DOI] [PubMed] [Google Scholar]
- 61.Fillingame RH, Angevine CM, Dmitriev OY. Coupling proton movements to c-ring rotation in F(1)F(o) ATP synthase: Aqueous access channels and helix rotations at the a-c interface. Biochim Biophys Acta. 2002;1555(1-3):29–36. doi: 10.1016/s0005-2728(02)00250-5. [DOI] [PubMed] [Google Scholar]
- 62.Fillingame RH, Steed PR. Half channels mediating H(+) transport and the mechanism of gating in the Fo sector of Escherichia coli F1Fo ATP synthase. Biochim Biophys Acta. 2014;1837(7):1063–1068. doi: 10.1016/j.bbabio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Viitanen P, Garcia ML, Foster DL, Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983;22(10):2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]
- 64.Gaiko O, Bazzone A, Fendler K, Kaback HR. Electrophysiological characterization of uncoupled mutants of LacY. Biochemistry. 2013;52(46):8261–8266. doi: 10.1021/bi4013269. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Celma JJ, Smirnova IN, Kaback HR, Fendler K. Electrophysiological characterization of LacY. Proc Natl Acad Sci USA. 2009;106(18):7373–7378. doi: 10.1073/pnas.0902471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Celma JJ, Ploch J, Smirnova I, Kaback HR, Fendler K. Delineating electrogenic reactions during lactose/H+ symport. Biochemistry. 2010;49(29):6115–6121. doi: 10.1021/bi100492p. [DOI] [PMC free article] [PubMed] [Google Scholar]