Significance
The human voice is a major source for auditorily communicated social signals. The voice in general, and emotional cues embedded in vocalizations in particular, receive enhanced decoding in sensory cortical areas of the auditory system. This enhanced cortical decoding is assumed to be remotely driven by the amygdala, which responds to socially and emotionally meaningful stimuli. Here, we tested for the first time, to our knowledge, how damage to either the left or right amygdala impairs the cortical processing of human voices and vocal affect. Amygdala damage generally leads to reduced cortical processing of human voices in the hemisphere corresponding to the side of the amygdala damage, whereas only left amygdala damage impaired the cortical processing of vocal affect.
Keywords: amygdala, lesion, voice, emotion, fMRI
Abstract
We tested whether human amygdala lesions impair vocal processing in intact cortical networks. In two functional MRI experiments, patients with unilateral amygdala resection either listened to voices and nonvocal sounds or heard binaural vocalizations with attention directed toward or away from emotional information on one side. In experiment 1, all patients showed reduced activation to voices in the ipsilesional auditory cortex. In experiment 2, emotional voices evoked increased activity in both the auditory cortex and the intact amygdala for right-damaged patients, whereas no such effects were found for left-damaged amygdala patients. Furthermore, the left inferior frontal cortex was functionally connected with the intact amygdala in right-damaged patients, but only with homologous right frontal areas and not with the amygdala in left-damaged patients. Thus, unilateral amygdala damage leads to globally reduced ipsilesional cortical voice processing, but only left amygdala lesions are sufficient to suppress the enhanced auditory cortical processing of vocal emotions.
Socially relevant and emotionally charged stimuli evoke increased activation in sensory cortices, both during the visual processing of emotional pictures or facial expressions (1, 2) and during the auditory processing of vocally expressed emotions (3–5). Such increases are assumed to be remotely driven by the amygdala, which is critically involved in decoding the emotional value of stimuli (6–8). Moreover, these effects seem to be predominantly (although not exclusively) mediated by ipsilateral anatomical (9) and functional connections between amygdala and sensory areas (10, 11).
In line with this view, recent studies conducted in patients with amygdala lesions reported impairments in the recognition of facial expressions (12), emotional words (13), or vocal emotions (14, 15). Furthermore, studies in both human patients (16, 17) and monkeys (18) showed significant changes in visual cortical activations to facial expressions following lesions of the amygdala. These changes in cortical processing are assumed to be remotely driven by the impaired emotional processing in the amygdala (10, 16). Distant effects of amygdala damage have also been observed for visual stimuli in cats (19) and for auditory stimuli in rats (8). However, other results have challenged this view, with some studies reporting no impairment in recognition (20–25) or changes in cortical processing for emotional stimuli in patients with amygdala lesions (26). Notably, Edmiston et al. (26) observed normal visual increases in response to emotional scenes for patients with unilateral amygdala resection, arguing against a direct role for the amygdala in modulating activity in sensory cortical areas. However, in that study (26), such increases could be related to attentional effects driven by greater interest or complexity of emotional scenes (27, 28).
Thus, evidence for impaired cortical responses to emotional stimuli after unilateral amygdala damage in humans remains inconsistent. In addition, unlike in rodents (8), to date, no study has investigated how the cortical processing of emotionally salient auditory stimuli might be affected by amygdala lesions in humans. Here, we tested for the first time, to our knowledge, whether unilateral amygdala damage in patients with left or right medial temporal lobe (MTL) lesions would modify auditory responses in intact cortical areas to voices and vocally expressed emotions. Previous studies consistently found differential activity in several subregions of auditory cortex in response to vocal emotions (29, 30), as well as in the amygdala (4, 5, 29, 31, 32), especially for angry voices (3–5). These auditory effects predominate in the superior temporal gyrus (STG) and superior temporal sulcus (STS), attributed to the processing of emotional valence in the amygdala (10) and presumably mediated by direct anatomical connections between the latter and auditory cortex (9, 33). Previous studies also consistently reported a response to emotional voices in the inferior frontal cortex, which may support higher level categorization processes (34) and thus constitutes an important component of the distributed network involved in detecting and decoding vocal emotions (29, 35).
We therefore hypothesized that cortical processing of human vocalizations in general, and of vocal emotions in particular, might be impaired in patients with lesions to the amygdala. This impairment is thought to result from a reduced emotional decoding of affective vocal cues in the amygdala, which is generally sensitive to emotional cues in voices (4, 5, 29, 31, 36) and usually is assumed to enhance cortical processing remotely (10, 16, 19). We also hypothesized that left and right amygdala lesions might have different effects. Whereas a right MTL lesion may strongly impair the processing of facial expressions due to well-known hemispheric asymmetries in face processing (12, 17, 37), the left amygdala seems to be more strongly involved in the decoding of emotional cues expressed in speech (13) or speech-like material (3, 4, 29). In two experiments, we tested brain responses to human vocalizations in general (experiment 1) and to emotional vocalizations embedded in pseudolanguage (experiment 2) (Fig. 1) while 10 patients with unilateral left amygdala lesions and 10 patients with unilateral right amygdala lesions (SI Results, Fig. S1A, and Table S1) underwent functional MRI (fMRI) scanning. In experiment 2, emotional voices were presented in either the attended or unattended ear during a dichotic listening task (38). We expected, first, that vocalizations, as socially salient stimuli, would generally produce weaker cortical processing in interconnected regions due to unilateral amygdala damage (experiment 1) (39). Second, in keeping with predominant left amygdala activity in healthy individuals during the processing of vocal emotions, we expected more severe impairment in cortical processing of emotional cues in patients with left amygdala lesions compared with right amygdala lesions (experiment 2) (29, 40).
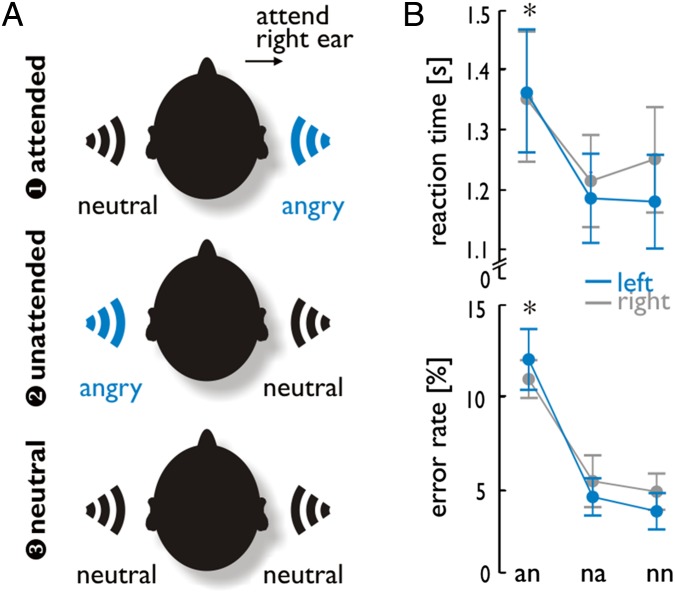
Fig. 1.
Experiment 2 included three emotion conditions, with angry voices presented in the left or right ear or neither. On an trials, an angry voice was heard on the task-relevant side, whereas on na trials, an angry voice was heard on the task-irrelevant side. On nn trials, neutral voices were presented to each ear. (A) Examples show all three conditions when attention was focused on the right ear. The same trials were also performed when attention was focused on the left ear (not shown here). (B) RTs and error rates for gender decisions on the attended voice revealed a main effect for the factor condition, indicating increased RTs and error rates during the an condition, as indicated by the asterisks.
Results
Behavioral Data.
In experiment 2, patients heard one voice in each ear simultaneously and were asked to judge the gender of the person speaking on one side (attended ear) but to ignore the voice on the other side (unattended ear). Reaction times (RTs) and error rates (%) in this task were analyzed by a repeated-measure ANOVA with the between-subject factor group (left MTL patients, right MTL patients) and the within-subject factors laterality (attend left ear, attend right ear) and condition (angry voice presented to the attended ear), angry voice presented to the unattended ear, neutral voices in both ears) (Fig. 1). There was no effect of laterality (F1,18 = 0.342, P = 0.566, η2 = 0.019) and group (F1,18 < 0.001, P = 0.999, η2 < 0.001) on RTs. However, RTs differed across conditions (F2,36 = 17.551, P < 0.001, η2 = 0.494), and Bonferroni corrected pairwise comparisons indicated longer response latencies for anger-attended trials (an) compared with both anger-unattended trials (na; P < 0.001) and neutral trials (nn; P = 0.002). There were no significant interactions between experimental factors for RTs (all F < 1.167, all P > 0.294). Similarly, error rates showed a significant difference only between conditions (F1,18 = 65.652, P < 0.001, η2 = 0.785), with more errors for an trials compared with both na (P < 0.001) and nn (P < 0.001) trials (all after Bonferroni corrected post hoc comparisons). The factors laterality and group were not significant, and no interaction between factors reached significance (all F < 1.440, all P > 0.250, all η2 < 0.063).
Anatomical Lesion Data.
We compared left and right MTL patients for lesion size, which was scored as the lesion volume divided by total brain volume for each patient. There was a tendency toward a greater lesion size in right MTL patients (Mlesion = 0.025, SDlesion = 0.0068) compared with left MTL patients (Mlesion = 0.019, SDlesion = 0.0057; t18 = 2.001, P = 0.060, independent-sample t test). We therefore tested whether the lesion size had an influence on the patient’s behavioral performance for either ear. We first scored the difference between an and nn trials, as well as between na and nn trials, separately for RTs and for error rates. A Pearson correlation between these difference scores and the lesion size did not reveal any significant correlation for RTs [all r < abs(0.058), all P > 0.883] and error rates [all r < abs(0.484), all P > 0.156], except for a trend in significance for the difference in error rates for an trials compared with nn trials (r = 0.601, P = 0.066) in left MTL patients. No effects were found for RTs [all r < abs(0.267), all P > 0.137] and error rates [all r < abs(0.487), all P > 0.154] in right MTL patients either. We also performed an additional ANOVA on RTs and error rates as reported above, but additionally taking the lesion size into account as a covariate. This analysis revealed a similar pattern of results compared with the analysis without the lesion size as a covariate (SI Results). Altogether, these data indicated that the lesion size did not influence the behavioral performance of the patients.
Finally, we tested for any structural difference in the intact amygdala using voxel-based morphometry (VBM) and an anatomical region of interest (ROI) that was defined by the healthy amygdala volume from each patient (SI Results and Fig. S1B). These VBM results did not reveal significant differences between the intact left amygdala [mean volume (Mvol) = 0.528, SDvol = 0.082] of right MTL patients and the intact right amygdala (Mvol = 0.591, SDvol = 0.141) of left MTL patients (t18 = 1.214, P = 0.240, independent sample t test).
Functional Brain Data.
Experiment 1.
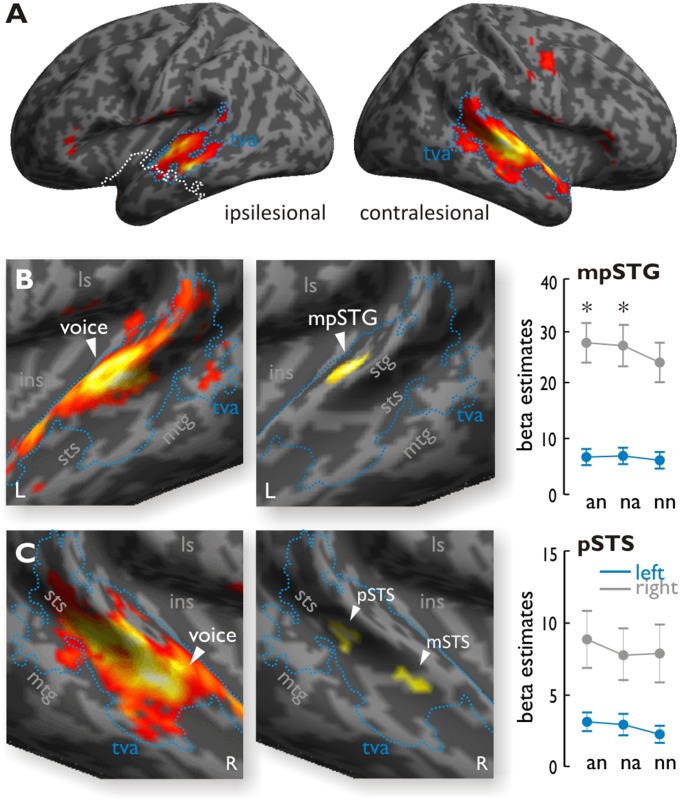
By comparing activations to human voices relative to other sounds across both patient groups, we identified bilateral temporal regions extending from the middle STG (mSTG) to posterior STG (pSTG) in the ipsilesional hemisphere and from the anterior STG (aSTG) to pSTG in the contralesional hemisphere (Fig. 2A). Unless stated otherwise, all functional maps are threshold at a combined voxel threshold of P < 0.005 and a cluster extend threshold of k = 67 corresponding to P < 0.05 corrected at the cluster level. A comparison of these voice-selective responses in right MTL patients relative to left MTL patients showed differential increases in the contralesional left midposterior STG [mpSTG; Montreal Neurological Institute (MNI) xyz: −60 −22 8] to vocal stimuli compared with nonvocal stimuli (Fig. 2B). For left MTL patients, conversely, we found greater increases in the contralesional right mSTS and the pSTS relative to right MTL patients, but both peak activations only appeared at a slightly lower threshold (P < 0.006). We combined this slightly lower threshold with a cluster extent threshold of k = 77, which corresponds to P < 0.05 corrected at the cluster level (Fig. 2C and SI Materials and Methods). Because the lesion size in some patients extended into the lateral superior temporal cortex (STC), we performed the same analysis taking into account the individual lesion size, which overlapped with the lateral STC (SI Results and Fig. S2). We found that the level of contralesional activity in the mSTS and pSTS for left MTL patients was associated with the lesion size in the ipsilesional lateral STC.
Fig. 2.
(A) Activity for vocal sounds compared with nonvocal sounds across all MTL patients in experiment 1. The blue outline denotes the temporal voice area (tva), and the white line denotes the lesion extent. (B, Left) In right MTL patients, comparing voices relative to nonvocal sounds showed extended activity in the left STG. (B, Middle) When comparing right MTL patients relative to left MTL patients, voices produced significantly higher activity in the left mpSTG. (B, Right) Activity in the functionally defined voice-selective STG, plotted across experimental conditions during the dichotic listening task (experiment 2), also showed increased activity for an and na trials compared with nn trials for the right MTL patients (as indicated by the asterisks) but not for the left MTL patients. (C, Left) In left MTL patients, comparing voices relative to nonvocal sounds showed extended activity in the right STG. (C, Middle) Comparing responses to voices in left MTL patients relative to right MTL patients activated the right pSTS and mSTS, although with a slightly lower voxel threshold of P < 0.006 and cluster extent of k = 77. These clusters were not modulated by the experimental conditions during the dichotic listening task (experiment 2). ins, insula; L, left; ls, lateral sulcus; mtg, middle temporal gyrus; R, right.
Experiment 2.
Because behavioral results did not reveal any significant difference between attending to the left ear or right ear, all an, na, and nn trials were pooled across both attention conditions. We performed a whole-brain analysis on the functional data but report here only data of the main target brain regions. A full list of all activations is provided in SI Results (Tables S2–S4).
STC.
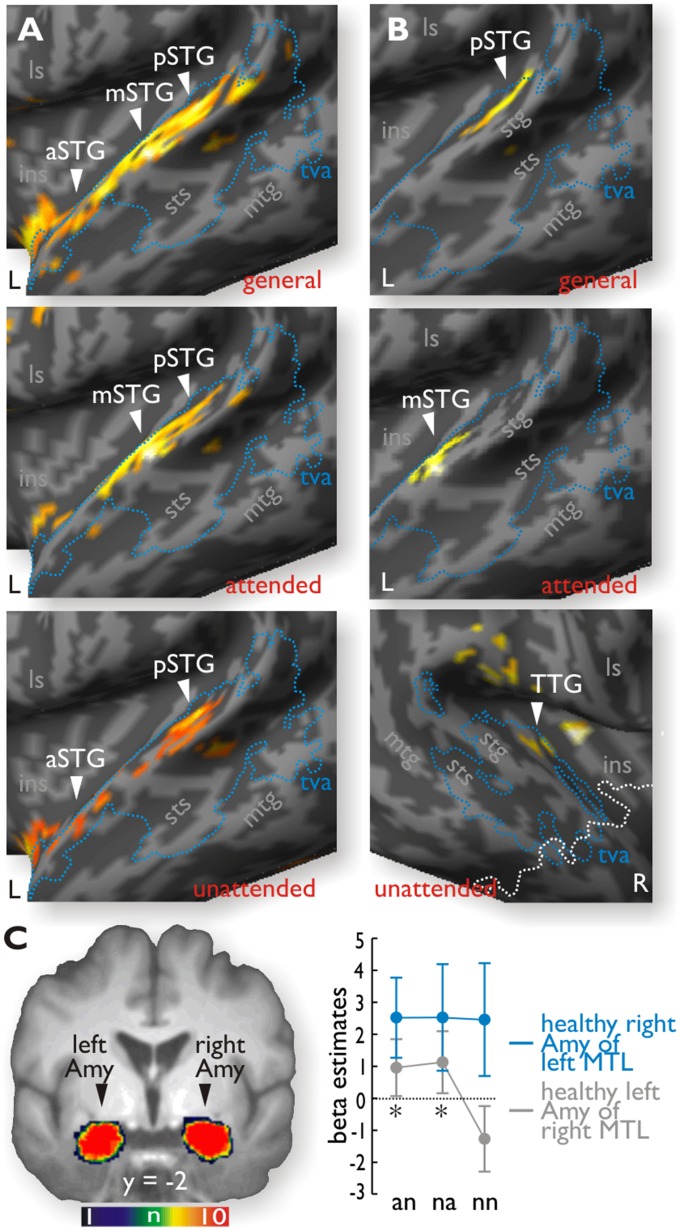
For the comparison of all anger (an + na) with nn trials (Table S2), right MTL patients showed activity in the left aSTG (MNI xyz: −50 0 −4), the left mSTG (MNI xyz: −60 −10 0), and the left pSTG (MNI xyz: −66 −36 12) (Fig. 3A). For an compared with nn trials in right MTL patients, activations peaked in the left mSTG (MNI xyz: −60 −8 0) and left pSTG (MNI xyz: −54 −22 6), whereas activity for na compared with nn trials (Table S3) peaked in the aSTG (MNI xyz: −50 0 −4) and pSTG (MNI xyz: −52 −26 6). A direct statistical comparison of right MTL patients relative to left MTL patients revealed stronger increases in the left pSTG (MNI xyz: −50 −36 16) for an trials in general, in the left mSTG (MNI xyz: −60 −10 0) especially for an trials, and in the right transverse temporal gyrus (TTG; MNI xyz: 38 −16 8) especially for na trials (Fig. 3B). Similar comparisons in left MTL patients revealed no differential increase in either the right or left auditory region for angry voices (an or na trials) relative to the nn trials, or relative to the right MTL patients. An additional analysis taking into account the lesion size in the lateral aSTC indicated that the higher activity in the contralesional left mSTG for right MTL patients was associated with the ipsilesional STC lesion size (SI Results and Fig. S3).
Fig. 3.
(A) Unlike left MTL patients, right MTL patients had significant increased activity in the left STG for angry (an + na) trials compared with neutral (nn) trials (general, Upper), as well as for an (Middle) and na (Lower) trials taken separately. These emotional increases were located in the voice-sensitive cortex (tva, blue outline; Fig. 2). (B) Direct comparison between left and right MTL patients revealed significantly increased activity in the left pSTG for all angry trials (general), in the left mSTG for an trials, and in the right TTG for na trials. The white outline indicates the maximal lesion extension in the right hemisphere. (C) Mean beta estimates extracted from the healthy amygdala (Amy) in native space revealed no modulation by the task conditions on the right side for left MTL patients but significant increases (indicated by the asterisks) on the left side for right MTL patients, with higher responses to both an and na trials compared with nn trials.
Furthermore, we also extracted the mean beta estimates of activity for voxels within the left mpSTG region, which were independently defined by increased voice sensitivity in right MTL patients compared with left MTL patients during experiment 1 (above). These data were then analyzed, using SPSS software (version 22; IBM), by a repeated-measure ANOVA with the between-subject factor group (left and right MTL patients) and the within-subject factor emotion condition (an, na, and nn trials). This analysis confirmed a significant difference between the two groups (F1,18 = 24.815, P < 0.001, η2 = 0.580), as well as a significant difference between conditions (F2,36 = 5.404, P = 0.009, η2 = 0.231). The interaction between group and conditions showed a trend toward significance (F2,36 = 2.936, P = 0.066, η2 = 0.140), but given our strong predictions for a differential effect of emotional compared with neutral voices and for group differences, we followed up this tendency by direct post hoc paired-sample t tests. These tests indicated that both an (t9 = 2.971, P = 0.016) and na (t9 = 2.703, P = 0.024) trials showed reliably higher signals than nn trials for right MTL patients, but no such effects were observed for left MTL patients (all t < 1.768, all P > 0.111) (Fig. 2B). These effects showed a relative consistency across all right MTL patients (SI Results and Fig. S4A). The right pSTS and mSTS, which showed greater response to voices in left MTL patients in experiment 1, were not modulated by experimental conditions in the dichotic listening experiment (pSTS: F2,36 = 2.730, P = 0.079, η2 = 0.132; mSTS: F2,36 = 2.341, P = 0.111, η2 = 0.115) and did not show a group-by-condition interaction (pSTS: F2,36 = 0.728, P = 0.490, η2 = 0.039; mSTS: F2,36 = 0.559, P = 0.577, η2 = 0.030) (Fig. 2C).
Amygdala.
The whole-brain analysis did not reveal significant activity in the amygdala for the left or right MTL patient groups, taken separately or together. Because the amygdala was one of our major target brain regions, we performed further ROI analyses on the mean amygdala signal extracted from a binary mask corresponding to the healthy amygdala volume in native space that was defined by an automated segmentation approach (Fig. S1B). The mean beta estimates during the dichotic listening task were subjected to a repeated-measure ANOVA with the between-subject factor group (left and right MTL patients) and the within-subject factor condition (an, na, and nn trials). Although there was no difference between left and right MTL patients (F1,18 = 1.638, P = 0.217, η2 = 0.083), the effect of condition (F2,36 = 3.012, P = 0.062, η2 = 0.143) and the interaction between condition and group (F2,36 = 2.689, P = 0.082, η2 = 0.130) both showed a trend toward significance. Given again our strong predictions according to the experimental factors and given that the amygdala was one of our major ROIs, we followed up this tendency. Follow-up post hoc comparisons using a paired-sample t test indicated that the intact left amygdala of right MTL patients showed significant increases for both an (t9 = 2.348, P = 0.043) and na (t9 = 2.375, P = 0.042) trials compared with nn trials, whereas no such effects were found for the right amygdala in left MTL patients (all t < 0.087, all P > 0.933) (Fig. 3C). These effects again showed a relative consistency across all right MTL patients (SI Results and Fig. S4B).
Inferior frontal cortex.
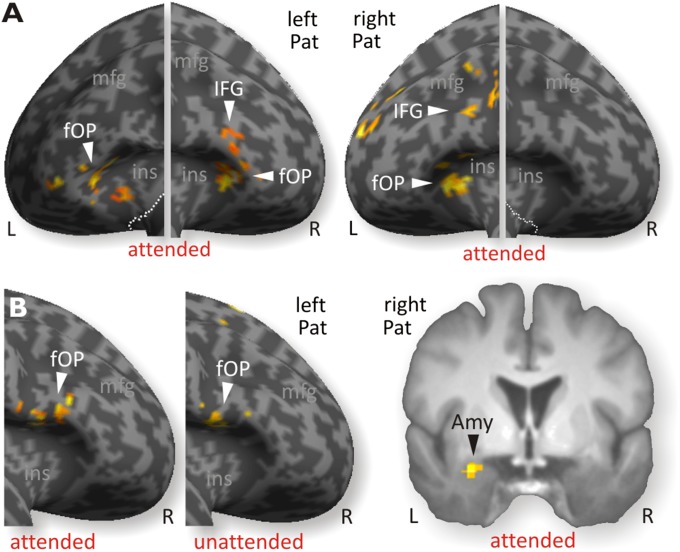
Left MTL patients showed increased activity in the left frontal operculum [fOP; Brodmann area 47, (MNI xyz: −36 30 2)] for all anger (an + na) trials compared with nn trials (Table S2), and in bilateral fOP (MNI xyz: −38 20 2 and 44 32 0, respectively) for an trials compared with nn trials (Fig. 4A, Left, and Table S3). Right MTL patients also had activity in the left fOP (MNI xyz: −44 10 32) for all angry (an + na) trials and for an trials (MNI xyz: −34 32 0) (Fig. 3A, Right). The na trials compared with nn trials (Table S3) produced no significant increase in the inferior frontal gyrus (IFG). A direct statistical comparison between left and right MTL patients in these conditions did not reveal any significant difference in IFG. In addition to these main contrasts, we performed a functional connectivity analysis using a psychophysiological interaction (PPI) approach (41), with the left fOP as the seed region, because the left fOP was the only region that showed common activity for both patient groups (Table S4). For an trials compared with nn trials, the left MTL patients showed functional connectivity with the right IFG (MNI xyz: 44 14 2; Fig. 4B, Left), whereas the right MTL patients showed no functional connectivity for this comparison. A direct comparison between groups revealed that the functional connectivity of left-to-right IFG (MNI xyz: 44 12 2) was significantly higher in left MTL patients compared with right MTL patients, but the reverse comparison of right MTL patients with left MTL patients revealed higher connectivity of the left IFG with the left amygdala (MNI xyz: −28 2 −20; Fig. 4B, Right). A similar PPI analysis for na trials, using the same left fOP as the seed region, also revealed increased functional connectivity with the opposite right IFG in left MTL patients compared with right MTL patients (Fig. 4B, Middle). An additional analysis taking into account the lesion size in the STC did not reveal any sensitivity of activity in the frontal cortex or of the functional connections to this lesion size (SI Results and Fig. S5).
Fig. 4.
(A, Left) Left MTL patients (Pat) showed activations to angry voices in bilateral IFC, especially for an trials. (A, Right) For the same trials, right MTL patients showed activity only in the left IFC. (B, Right) PPI analysis using the left fOP as a seed region revealed higher functional connectivity with the left amygdala for right MTL patients during an trials but not during na trials. (B, Left) In left MTL patients, connectivity of the left fOP increased with the right IFC only, during both an and na trials, but not with the amygdala.
Discussion
Based on the assumption that the impaired decoding of the emotional value of voices due to lesions in the amygdala leads to reduced remote influences, and thus impaired cortical processing, we acquired behavioral and functional brain data in left or right amygdala patients. First, all patients showed reduced activation to vocalizations in the ipsilesional auditory cortex. Second, although both left and right MTL patients had similar behavioral performances on the dichotic listening task, the two groups differed significantly at the neural level. Right MTL patients had enhanced activity in the left voice-sensitive auditory cortex in response to vocal emotions, whereas left MTL patients showed no such differential effect. Second, according to our hypothesis of differential emotion effects in the left and right MTL patients, we found that the intact left amygdala was modulated by angry relative to neutral voices in right MTL patients, unlike the intact right amygdala for left MTL patients. Finally, the left amygdala was functionally connected with the left IFG in right MTL patients, whereas left MTL patients exhibited functional connectivity only between bilateral IFG.
Our results thus show that deficits in sensory decoding of vocal emotions critically depend on the side of the lesioned amygdala. Right MTL patients with a healthy left amygdala showed the expected pattern of increased activity in several subregions of the right STC, overlapping with the voice-sensitive areas (42), together with preserved recruitment of the left amygdala and the left IFG. All of these regions have been previously shown to be involved in processing vocal emotions, including similar or identical experimental paradigms in healthy individuals (4, 29, 38). Accordingly, we found selective increases in the left mSTG for right MTL patients when their attention was explicitly focused on the ear where angry voices were presented, as previously found in healthy individuals using an identical experimental design (4, 38). This activity in the contralesional mSTG seemed to be associated with ipsilesional lesion size in the lateral aSTC; thus, this activation might partly reflect some contralesional activity of compensation due to ipsilesional impairments. In addition, a slightly more posterior region in the left mpSTG showed not only enhanced responses to angry voices (experiment 2) but also stronger sensitivity to human voices in right MTL patients relative to left MTL patients (experiment 1). Thus, these data seem to indicate that only the left amygdala seems to have distant influences on the processing of emotional voices in the auditory cortex.
Conversely to right MTL patients, globally higher responses to voices relative to nonvocal sounds were found in right STS regions for left MTL patients in experiment 1, but these regions were not modulated by the conditions of experiment 2, especially for the comparison of emotional with neutral voices. This finding indicates that amygdala damage in either hemisphere leads to reduced auditory analysis in the ipsilesional higher level auditory cortex in response to human vocalizations. However, only left (not right) amygdala damage appeared to abolish the emotional enhancement beyond this generally reduced response to human voices. Finally, in trials where angry voices were presented on the to-be-ignored ear, right MTL patients also showed activity in the right TTG. This finding might indicate enhanced sensory processing for acoustic features of emotional voices in low-level auditory areas driven by a remote influence of the amygdala under conditions when attention is not focused on the side of the angry voice (29, 38). Although sensory cortical activity is predominantly modulated in the ipsilateral hemisphere (38, 43), a recent effective connectivity study found that amygdala activity can also drive the contralateral hemisphere according to the emotional value of a sound (44), possibly explaining the preserved ipsilesional activity in early right auditory cortex in right MTL patients.
Unlike right MTL patients, left MTL patients did not show a significant emotional enhancement of cortical responses in the voice-sensitive areas of both hemispheres. Because right MTL patients showed a residual modulation of their intact left amygdala by angry relative to neutral voices, it is likely that this response drove the preserved sensory enhancement in the left STG, whereas no right amygdala modulation of angry relative to neutral voices was observed in left MTL patients, and hence no differential response in auditory cortex. This asymmetry, which was formally confirmed by direct interhemispheric contrasts between patient groups, suggests that the intact right amygdala in left MTL patients was unable to distinguish angry (an + na) from nn voice trials. This finding points to a relative, but not exclusive, importance of the left hemisphere in the recognition of vocal emotions superimposed on linguistic or speech-like material (13). Although not tested in the present study, future studies might investigate if nonverbal vocal emotions (45), which include no linguistic features, might more strongly rely on right hemisphere mechanisms.
Our data contrast with previous observations that right MTL lesions may lead to greater impairment in recognizing emotional stimuli (12, 17, 37). However, previous studies focused on the recognition of emotional (especially fearful) faces (12, 46). Notably, one study reported impaired recognition of emotion from words in patients with left MTL lesions (13). These data seem to suggest that amygdala lesion side might interact with the type of stimulus material. Therefore, although the present study found no right amygdala response to emotional cues embedded in speech-like stimuli, it is possible that different results would be observed for vocal expressions without any speech-like structure, such as emotional onomatopoeia. Our data furthermore contradict a recent report by Edmiston et al. (26) showing enhanced visual cortex activity in both left and right MTL patients in response to emotional visual scenes. However, given that corticoamygdala connections are similar in the visual and auditory systems (9) and involve extensive regions from early to higher level areas (9), similar modulatory influences from the amygdala are likely to exist across different sensory modalities. Our study included a sizeable sample of patients and a randomized event-related task in which we orthogonally manipulated emotional valence and overt attention (16, 18). In contrast, Edmiston et al. (26) tested a smaller sample of left MTL patients with lesions in the amygdala proper (n = 3) and with only a minor lesion overlap. More critically, they did not control for overt top-down attention effects. Emotional scenes more strongly engage attentional processing due to greater stimulus complexity and intrinsic content (47), as already shown for pictures judged as “more interesting” (27). In addition, top-down attention effects may have been amplified in the study of Edmiston et al. (26) due to long stimulus presentation time and block-wise presentation during passive viewing. Thus, an impact of amygdala dysfunction on cortical processing of emotional stimuli might be detectable in MTL patients only during well-controlled, task-dependent experimental conditions.
Another notable result in our study is that patients also differed in their pattern of activity and connectivity of bilateral IFG. The right MTL patients showed selective connectivity of the left IFG (fOP) with the intact left amygdala, especially when angry voices were presented on the to-be-attended ear, whereas only connectivity between the IFG in both hemispheres was modulated by emotion in the left MTL patients. The IFG is usually involved in the evaluation and categorization of vocal emotions (34). In the absence of enhanced sensory analysis of vocal emotions in the auditory cortex, left MTL patients might still be able to use strategic and explicit categorization processes through cognitive controlled decoding in the IFG.
Taken together, we provide previously unidentified evidence that unilateral amygdala lesions generally lead to reduced cortical processing of human vocalizations in the ipsilesional (but not contralesional) auditory cortices (experiment 1) and that the left amygdala, but not the right amygdala, has a causal functional role in enhancing the auditory cortical processing of vocal emotions (experiment 2). However, we also have to highlight some potential limitations of this study. First, we only used neutral and angry voices, and any generalization of the lesion effects on processing other types of vocalizations has to be drawn with caution. However, studies in healthy humans have shown a sensitivity of the amygdala to different types of emotions, but with a predominant sensitivity to angry voices (48). Second, in some patients, the temporal lesion extended into the lateroanterior temporal lobe, including potential voice-sensitive regions in the STG and STS. One activation peak was found in the contralesional aSTG. The homolog area in the ipsilesional hemisphere was located at the lesion border and might explain a potentially missing activation in the ipsilesional aSTG. However, the core of voice-sensitive regions is commonly located more in the mSTG and mSTS and in the pSTG and pSTS (30, 49). Third, some of the reported effects were based only on trends in significance for the interaction between experimental conditions, which were followed-up by significant post hoc tests according to our predictions. Some of the effects thus might be taken with caution, and future studies might include larger MTL patient samples to increase the statistical power.
Materials and Methods
Twenty-two patients took part in two experiments, but two had to be excluded because of poor compliance with the task instructions. The final sample consisted of 20 patients with a unilateral medial temporal lobectomy including the amygdala, 10 with left resection (five male, mean age of 42.60 y, SD = 16.53, age range: 22–66 y) and 10 with right resection (six male, mean age of 40.30 y, SD = 13.03, age range: 25–67 y). All patients (Table S1) underwent fMRI scanning several months after their lobectomy, which was performed for treatment of pharmacoresistant epilepsy. Patients were selected according to the following criteria: (i) single unilateral surgery no longer than 15 y before the experiment, (ii) no psychiatric disorders pre- or postsurgery, (iii) no other neurological disorder affecting brain function, and (iv) success of surgery with complete disappearance or important remission of epileptic attacks. Twelve patients (seven left and five right MTL patients) were treated with antiepileptic drugs during the time of their participation in the experiment. Participants were native French or German speakers, had normal or corrected-to-normal vision, and had normal hearing abilities. Participants gave written informed consent for their participation in accordance with ethical and data security guidelines of the University of Geneva. The study was approved by the local ethics committee of the University of Geneva. Additional information on materials and methods is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by the Swiss National Science Foundation (Grant 105314_124572/1 to D.G. and Grants 105314_146559/1 – SF and 140332_146633 to M.S.), the National Center of Competence in Research in Affective Sciences (Grant 51NF40-104897 to D.G. and P.V.), and the Geneva Academic Society (Foremane Fund Grant to P.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411315112/-/DCSupplemental.
References
- 1.Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. Neuroimage. 2006;31(2):906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 2.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 3.Frühholz S, Grandjean D. Amygdala subregions differentially respond and rapidly adapt to threatening voices. Cortex. 2013;49(5):1394–1403. doi: 10.1016/j.cortex.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Sander D, et al. Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. Neuroimage. 2005;28(4):848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Wiethoff S, Wildgruber D, Grodd W, Ethofer T. Response and habituation of the amygdala during processing of emotional prosody. Neuroreport. 2009;20(15):1356–1360. doi: 10.1097/WNR.0b013e328330eb83. [DOI] [PubMed] [Google Scholar]
- 6.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 7.Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J Neurosci. 1998;18(7):2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, von Kriegstein K, Friston K, Griffiths TD. Features versus feelings: Dissociable representations of the acoustic features and valence of aversive sounds. J Neurosci. 2012;32(41):14184–14192. doi: 10.1523/JNEUROSCI.1759-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das P, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26(1):141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Meletti S, et al. Impaired facial emotion recognition in early-onset right mesial temporal lobe epilepsy. Neurology. 2003;60(3):426–431. doi: 10.1212/wnl.60.3.426. [DOI] [PubMed] [Google Scholar]
- 13.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 14.Sprengelmeyer R, et al. Knowing no fear. Proc Biol Sci. 1999;266(1437):2451–2456. doi: 10.1098/rspb.1999.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott SK, et al. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;385(6613):254–257. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- 16.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 17.Benuzzi F, et al. Impaired fear processing in right mesial temporal sclerosis: A fMRI study. Brain Res Bull. 2004;63(4):269–281. doi: 10.1016/j.brainresbull.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Hadj-Bouziane F, et al. Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proc Natl Acad Sci USA. 2012;109(52):E3640–E3648. doi: 10.1073/pnas.1218406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Li H, Jin Z, Shou T, Yu H. Feedback of the amygdala globally modulates visual response of primary visual cortex in the cat. Neuroimage. 2014;84:775–785. doi: 10.1016/j.neuroimage.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Adolphs R, Tranel D. Intact recognition of emotional prosody following amygdala damage. Neuropsychologia. 1999;37(11):1285–1292. doi: 10.1016/s0028-3932(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 21.Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci. 2002;14(5):709–720. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- 22.Bach DR, Hurlemann R, Dolan RJ. Unimpaired discrimination of fearful prosody after amygdala lesion. Neuropsychologia. 2013;51(11):2070–2074. doi: 10.1016/j.neuropsychologia.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piech RM, et al. Attentional capture by emotional stimuli is preserved in patients with amygdala lesions. Neuropsychologia. 2011;49(12):3314–3319. doi: 10.1016/j.neuropsychologia.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci. 2009;12(10):1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach DR, Talmi D, Hurlemann R, Patin A, Dolan RJ. Automatic relevance detection in the absence of a functional amygdala. Neuropsychologia. 2011;49(5):1302–1305. doi: 10.1016/j.neuropsychologia.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmiston EK, et al. Enhanced visual cortical activation for emotional stimuli is preserved in patients with unilateral amygdala resection. J Neurosci. 2013;33(27):11023–11031. doi: 10.1523/JNEUROSCI.0401-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mourão-Miranda J, et al. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20(4):1955–1963. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Lang PJ, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35(2):199–210. [PubMed] [Google Scholar]
- 29.Frühholz S, Ceravolo L, Grandjean D. Specific brain networks during explicit and implicit decoding of emotional prosody. Cereb Cortex. 2012;22(5):1107–1117. doi: 10.1093/cercor/bhr184. [DOI] [PubMed] [Google Scholar]
- 30.Frühholz S, Grandjean D. Multiple subregions in superior temporal cortex are differentially sensitive to vocal expressions: A quantitative meta-analysis. Neurosci Biobehav Rev. 2013;37(1):24–35. doi: 10.1016/j.neubiorev.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Bach DR, et al. The effect of appraisal level on processing of emotional prosody in meaningless speech. Neuroimage. 2008;42(2):919–927. doi: 10.1016/j.neuroimage.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Fecteau S, Belin P, Joanette Y, Armony JL. Amygdala responses to nonlinguistic emotional vocalizations. Neuroimage. 2007;36(2):480–487. doi: 10.1016/j.neuroimage.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Reser DH, Burman KJ, Richardson KE, Spitzer MW, Rosa MG. Connections of the marmoset rostrotemporal auditory area: Express pathways for analysis of affective content in hearing. Eur J Neurosci. 2009;30(4):578–592. doi: 10.1111/j.1460-9568.2009.06846.x. [DOI] [PubMed] [Google Scholar]
- 34.Frühholz S, Grandjean D. Processing of emotional vocalizations in bilateral inferior frontal cortex. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2847–2855. doi: 10.1016/j.neubiorev.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Leitman DI, et al. “It’s Not What You Say, But How You Say it”: A Reciprocal Temporo-frontal Network for Affective Prosody. Front Hum Neurosci. 2010;4:19. doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaucousin V, et al. FMRI study of emotional speech comprehension. Cereb Cortex. 2007;17(2):339–352. doi: 10.1093/cercor/bhj151. [DOI] [PubMed] [Google Scholar]
- 37.Golouboff N, et al. Impaired facial expression recognition in children with temporal lobe epilepsy: Impact of early seizure onset on fear recognition. Neuropsychologia. 2008;46(5):1415–1428. doi: 10.1016/j.neuropsychologia.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Grandjean D, et al. The voices of wrath: Brain responses to angry prosody in meaningless speech. Nat Neurosci. 2005;8(2):145–146. doi: 10.1038/nn1392. [DOI] [PubMed] [Google Scholar]
- 39.Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- 40.Mothes-Lasch M, Mentzel HJ, Miltner WH, Straube T. Visual attention modulates brain activation to angry voices. J Neurosci. 2011;31(26):9594–9598. doi: 10.1523/JNEUROSCI.6665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 42.Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- 43.Vuilleumier P. The role of the human amygdala in perception and attention. In: Whalen PJ, editor. The Human Amygdala. Guilford; New York: 2009. [Google Scholar]
- 44.Frühholz S, Grandjean D. Towards a fronto-temporal neural network for the decoding of angry vocal expressions. Neuroimage. 2012;62(3):1658–1666. doi: 10.1016/j.neuroimage.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Milesi V, et al. Multimodal emotion perception after anterior temporal lobectomy (ATL) Front Hum Neurosci. 2014;8:275. doi: 10.3389/fnhum.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cristinzio C, N’Diaye K, Seeck M, Vuilleumier P, Sander D. Integration of gaze direction and facial expression in patients with unilateral amygdala damage. Brain. 2010;133(Pt 1):248–261. doi: 10.1093/brain/awp255. [DOI] [PubMed] [Google Scholar]
- 47.Lane RD, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35(11):1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 48.Frühholz S, Trost W, Grandjean D. The role of the medial temporal limbic system in processing emotions in voice and music. Prog Neurobiol. 2014;123C:1–17. doi: 10.1016/j.pneurobio.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Ahrens MM, Awwad Shiekh Hasan B, Giordano BL, Belin P. Gender differences in the temporal voice areas. Front Neurosci. 2014;8:228. doi: 10.3389/fnins.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.